1. Introduction
Over many years, the intensive use of fossil fuels in transport and energy production sectors has led to environmental degradation due to the high emission of CO2 into the atmosphere (Dijs et al Reference Dijs, van der Windt, Kaihola and van der Borg2006; Guo et al Reference Guo, Liang, Umar and Mirza2022; Liaquat et al Reference Liaquat, Masjuki, Kalam, Varman, Hazrat, Shahabuddin and Mofijur2012). According to (ICPP 2013) the combustion of fossil fuels for energy production, transport, and heating are the primary sources of CO2 emission from human activities. Aiming to reduce the amount of CO2 in the atmosphere, several countries are focused on using the liquid fuels to produce energy from renewable resources that are environmentally friendly (De Souza et al Reference De Souza, Scherer, Cáceres, Caires and M’Peko2013).
Although bioenergy provides a greener alternative to fossil fuels, this method of energy production is still rare worldwide as it requires more efforts in research and production (Anyaoha and Zhang Reference Anyaoha and Zhang2021; Casau et al Reference Casau, Dias and Matias2022). Therefore, in Poland, as in other European countries, fuel blends have been put into use with the aim of reducing the net flux of carbon into atmosphere, and the research on the content of biocomponents within these blends has been undertaken (Dobrzyńska et al Reference Dobrzyńska, Szewczyńska, Pośniak, Szczotka, Puchałka and Woodburn2020; Howaniec and Smoliński 2014; Verger et al Reference Verger, Azimov and Adeniyi2022).
Fuel blends involve the mixtures of fossil fuels with fuels derived from the contemporary biosphere in varying percentages to obtain a fuel that minimize the harmful effects while optimizing certain useful properties. They are relatively new to the market in comparison to pure fossil fuels (Culp Reference Culp2017). The blending of liquid fuels must be carried out in a controllable and reproducible manner and requires knowledge of the fuel components that compose the blend mixtures, the reason for the blending, and the expected properties of the fuel blends (Alves and Poppi Reference Alves and Poppi2016).
Knowledge of the content of liquid fuels that are expected to be on the market is important in various sectors for defining fuel quality (Sulek et al Reference Sulek, Kulczycki and Malysa2010; Haverly et al Reference Haverly, Fenwick, Patterson and Slade2019; Morone and D’Amato Reference Morone and D’Amato2019). The characteristics of the fuel blend content are important factors which are used to avoid the interference of nonlinear blending effects on values of the parameters studied (Castillo et al Reference Castillo, Castro and Mahalec2017). A directive of the European Union Parliament (Directive 2009/30/EC 2009) aims to reduce the emission of greenhouse gases by 6% from road transport, causing the rapid implementation of strategies to produce new hybrid fuels that satisfy these requirements. Furthermore, this directive stated that liquid fuels must contain at least 10% fuel of biogenic origin by 2020 (Krajcar Bronić et al Reference Krajcar Bronić, Barešić, Horvatinčić and Sironić2017). As a result, in the past few years, the biobased content of fuels has increased in most European countries.
One of the strategies developed was an increase in the blending of petroleum-based diesel with biobased fatty acid methyl esters (FAMEs) (Idoeta et al Reference Idoeta, Pérez, Herranz and Legarda2014; Schuurman et al Reference Schuurman, Fogassy and Mirodatos2013). FAMEs produced from animal fat or vegetable oil (by the transesterification of triglycerides) have physical properties closer to fossil diesel fuels, compared to pure vegetable oils. However, although the production of FAMEs is straightforward, they are not suitable for various uses as they can cause problems in the engine, such as internal diesel injector deposits, poor evaporation process and poor fuel-air mixing (Urzędowska and Stępień Reference Urzędowska and Stępień2016; Zhang et al Reference Zhang, Deng, Pham, Zuo, Peng and Yin2018). Moreover, due to the fact that the net energy value of these biofuels has been questioned, this blending method showed barriers that limited its potential for mass use and also had some negative impacts on the environment (Pimentel and Patzek Reference Pimentel and Patzek2005; Schuurman et al Reference Schuurman, Fogassy and Mirodatos2013; Serrano-Ruiz and Dumesic Reference Serrano-Ruiz and Dumesic2011).
For this reason, paraffinic hydrotreated vegetable oil (HVO) has been developed because of its suitable characteristics compared to those of FAMEs. Although HVO has a complicated production process and is expensive, its production from triglyceride-rich waste material is less complicated compared to diesel production, and when added to petrodiesel, the resulting products are extremely stable (Idoeta et al Reference Idoeta, Pérez, Herranz and Legarda2014; Türck et al Reference Türck, Singer, Lichtinger, Almaddad, Türck, Jakob, Garbe, Ruck and Krahl2021).
The use of fuel blends composed of HVO and other fuels such as petroleum-diesel have many advantages due to the low sulfur and aromatic content in HVO, that lead to lower emission of aromatics, SO2, particulates and NOx. HVO combustion decreases greenhouse gas emissions more than fuels derived from petroleum (Vrtiška and Šimáček Reference Vrtiška and Šimáček2016).
The production of fuel blends led to the need for techniques to determine the content of their biocomponents precisely and accurately. Radiocarbon measurement is one way to quantify the contents of biocomponents in fuel blends. Contrary to plants, the primary recipients of the atmospheric 14CO2, with the highest level of 14C that represent the modern 14C level, fossil fuels are devoid of 14C due to their old age (Culp Reference Culp2017; Hajdas et al Reference Hajdas, Ascough, Garnett, Fallon, Pearson, Quarta, Spalding, Yamaguchi and Yoneda2021; Libby Reference Libby1961).
Measurements of radiocarbon content in fuel blends are carried out with the methods listed in American Society for Testing and Materials (ASTM) standard D6866, with the most recent version released in 2022. These are currently the standard test methods used to determine the biobased contents in liquid, gaseous, and solid samples using the radiocarbon analysis (Alves and Poppi Reference Alves and Poppi2016; Culp et al Reference Culp, Cherkinsky and Ravi Prasad2014; Idoeta et al Reference Idoeta, Pérez, Herranz and Legarda2014; Oinonen et al Reference Oinonen, Hakanpää-Laitinen, Hämäläinen, Kaskela and Jungner2010).
One of the most accurate and precise ASTM D6866-22 methods for 14C determination is accelerator mass spectrometry (AMS). For the AMS method, the liquid samples are prepared using tin capsules for liquids and combusted to produce CO2 which is graphitized to obtain graphite for 14C measurements. The main advantages of the AMS technique are greater precision and the use of a small sample size (Baranyika et al Reference Baranyika, Piotrowska, Kłusek, Michczyński and Pawlyta2022; Culp et al Reference Culp, Cherkinsky and Ravi Prasad2014; Haverly et al Reference Haverly, Fenwick, Patterson and Slade2019). The second method is liquid scintillation counting (LSC).
The preparation of sample for LSC measurements requires various procedures. In the carbamate or LSC-A method, organic carbon is converted into CO2. During this process, the sample is combusted in a special apparatus under a controlled environment to form CO2 (Horvatinčić et al Reference Horvatinčić, Barešić, Bronić and Obelić2004). In the LSC-B or benzene method, the investigated sample is converted through three main steps such as carbide formation, hydrolyzation and trimerization to produce benzene for 14C measurement (Horvatinčić et al Reference Horvatinčić, Barešić, Bronić and Obelić2004). A third one is the LSC-C method that does not requires any pretreatment of sample, but it simply involves a mixture of investigated sample with a relevant scintillator to form a scintillation cocktail (Norton et al Reference Norton, Cline and Thompson2012; Krištof and Kožar Logar Reference Krištof and Kožar Logar2013; Doll et al Reference Doll, Plymale, Cooper, Kutnyakov, Swita, Lemmon, Olarte and Wang2021).
To report precise and accurate 14C measurement results, it is necessary to correct for isotope fractionation which occurred in the process of the sample preparation and 14C measurements (Baranyika et al Reference Baranyika, Piotrowska, Kłusek, Michczyński and Pawlyta2022; Maruccio et al Reference Maruccio, Quarta, Braione and Calcagnile2017). For the LSC, isotope ratio mass spectrometry (IRMS) is required for stable carbon isotope measurements used for the corrections (Prasad et al Reference Prasad, Culp and Cherkinsky2019). On the contrary, during the AMS analysis the fractionation is determined and incorporated into the data reduction (Wacker et al Reference Wacker, Synal and Marcus2010).
Nowadays, 14C measurements are used in the Gliwice Radiocarbon and Mass Spectrometry Laboratory to investigate the biocarbon content in liquid and solid samples by AMS and LSC methods (Baranyika et al Reference Baranyika, Piotrowska, Kłusek, Michczyński and Pawlyta2022; Gill et al Reference Gill, Michczyński, Michczyńska, Piotrowska, Kłusek, Końska, Wróblewski, Nadeau and Seiler2022). The aim of this study is to test and verify methods for determining the 14C content in liquid fuel blends of known bio- to fossil fuel ratios. The results of this study will contribute to the accreditation of the tested methods in the Gliwice Laboratory.
2. Materials and Methods
To investigate the precision and results accuracy in the Gliwice Radiocarbon and Mass Spectrometry Laboratory, both LSC and AMS methods are used for 14C measurements.
2.1. Blend Sample Preparation
To obtain samples for radiocarbon measurements, six different fuel blends were produced from a sample of HVO and a petrodiesel sample of infinite 14C age (ON/UF-BC; see Table 1) in the Gliwice Radiocarbon and Mass Spectrometry Laboratory. These samples were submitted in 2018 by an external oil company in high quantity, which allowed to use them for testing purposes for years (Baranyika et al Reference Baranyika, Piotrowska, Kłusek, Michczyński and Pawlyta2022).
Table 1 Blend samples prepared from archive liquid fuels (HVO and ON/UF-BC). Carbon content is a result from elemental analysis (EA), and benzene conversion efficiency was calculated based on these numbers

Moreover, ON/UF-BC diesel has been extensively used as a background sample for liquid fuels. The liquids were transferred to 20-mL vials using the 230 mm-long glass Pasteur pipettes. The mass of HVO and ON/UF-BC was adjusted to obtain the required composition and provide a total of 15g for each blend.
2.2. LSC Method for Radiocarbon Measurement in Fuel Blends
In radiocarbon research laboratories, the extensive use of LSC is well established for the quantitative determination of biocomponents in samples by means of radiocarbon measurements.
Benzene was synthesized from each of the eight samples according to the benzene method (LSC-B). To enable a slow conversion of fuel samples to lithium carbide (Li2C2) without explosion, each liquid fuel blend, background and HVO sample was mixed with purified silica sand. The sand was pre-heated to remove organic impurities at 500 oC for 12h in a muffle oven. 7 g of fuel and 7g of lithium reacted at 700 °C to form Li2C2. Once cooled, the generated Li2C2 was hydrolyzed to obtain acetylene (C2H2), which was purified by being passed through a mixture of 40 g of potassium dichromate (99.9%), 100 mL sulfuric acid (95%), and 1000 mL H2O. Purified C2H2 was trimerized to benzene using a chromium catalyst on silica gel (5.5% Cr2O3) purchased from “ATOM KOMPLEXPRYLAD” Llc research and production enterprise in Kyiv, Ukraine.
The overall efficiency of converting fuel sample to benzene (CE) reported in Table 1 was calculated as follows:
where: m C6H6 – mass of obtained benzene; CC6H6 – carbon content of benzene, which is 93.3%; mf – mass of combusted fuel; Cf – carbon content of fuel as analyzed by EA. The uncertainties were calculated according to the guide to the expression of uncertainty in measurement (BIPM et al 2008)
To remove water, the prepared benzene sample was stored with sodium for 24 hours (Gill et al Reference Gill, Michczyński, Michczyńska, Piotrowska, Kłusek, Końska, Wróblewski, Nadeau and Seiler2022). The dried benzene sample was kept in a laboratory freezer for 30 days at -20 °C to remove contamination with radon (222Rn). Afterwards the benzene was mixed with 26 mg of Butyl-PDB scintillator (Packard Canberra Company) to obtain the scintillation cocktail for 2 mL geometry. A Quantulus-1220 alpha and beta low-level LSC spectrometer was used for radiocarbon measurements (Pazdur et al Reference Pazdur, Fogtman, Michczyński and Pawlyta2003). In order to achieve appropriate precision, the sample measurement times using the LSC technique were 36 hours (Blend-10, Blend-20, Blend-30), 64 hours (HVO, Blend-15) and 72 hours (Blend-5, Blend-7.5), respectively. The total measurement time was divided into partial measurements of 30 or 40 minutes. For determination of machine background, 24-hour-long measurements were carried out. This procedure allowed for the control of the long-term stability of the measurements.
2.2.1. CO2 Sample Preparation and δ 13C Determination by IRMS
Normally, radiocarbon data is corrected for isotopic fractionation that has occurred naturally, as well as that caused by sample preparation and measurement (Fahrni et al Reference Fahrni, Southon, Santos, Palstra, Meijer and Xu2017; Stuiver and Polach Reference Stuiver and Polach1977).
To verify the fractionation of isotopes during sample processing, the IRMS method was used (Baranyika et al Reference Baranyika, Piotrowska, Kłusek, Michczyński and Pawlyta2022; Prasad et al Reference Prasad, Culp and Cherkinsky2019). For ON/UF-BC and HVO samples, after benzene preparation, 5 µl of benzene was combusted in a glass tube with copper oxide (CuO) at 560 °C for 24 h. After combustion, the glass tube was cracked, and the CO2 was purified in a vacuum line. For IRMS measurement, CO2 was subsampled with a syringe and introduced to an Isoprime continuous flow mass spectrometer. In this experiment, two reference materials, ANU-sucrose (5 mg) and NBS-22 (5 µl) were combusted with CuO and processed the same way for IRMS measurement (Baranyika et al Reference Baranyika, Piotrowska, Kłusek, Michczyński and Pawlyta2022; Coplen et al Reference Coplen, Brand, Gehre, Gro, Meijer, Toman, Verkouteren, Survey, Biogeochemistry and Box2006). The 13C/12C ratios expressed as δ 13C for reference materials were used to calculate the δ 13C for fuel samples by linear regression. The continuous flow isotope ratio mass spectrometer IsoPrime precision is 0.1 ‰ for δ 13C, based on repeated measurements for reference materials.
To obtain the δ13C values for fuel blend samples the mass-balance equation was used:
Where: δ 13 C HVO and δ 13 C bg are the stable carbon composition of HVO and ON/UF-BC, and f HVO and f bg are the respective fractions of these components, so that f HVO +f bg =1.
The radiocarbon activities determined with use of LSC method were corrected for isotope fractionation using the obtained δ 13C values, according to the equation below (Stuiver and Polach Reference Stuiver and Polach1977):
A SN is the corrected 14C activity of the sample, A S represents the measured (uncorrected) 14C activity. Hereafter, the A SN values are reported in pMC unit and referred to as 14C or radiocarbon content.
2.3. Preparation of Graphite Samples for AMS Measurement
To obtain precise and accurate results for radiocarbon measurement by AMS in Gliwice Laboratory, it is necessary to prepare graphite targets.
In this study, after mixing the fuel blend samples from the HVO and background liquid fuels, there was no additional chemical processing before combustion. However, using an adjustable micropipette (0.1–2 µL), a volume of 2 µL for each blended liquid fuel was subsampled into a tin capsule for liquid samples and weighed with a Sartorius microbalance (precision = 0.001 mg) to obtain the mass used for combustion. The tin capsules were sealed using a sealing press and combusted in an Elemental Analyzer (EA, Vario Micro Cube model from ElementarTM) at high temperature (950ºC), with ca. 42 mL (1.186 mmol) of oxygen dosing, to produce CO2, which was transferred to a graphitization reactor. The obtained CO2 was reduced to graphite with hydrogen (H2) on an iron powder (325 mesh, purity 97%) catalyst from ALDRICH chemistry company using an AGE-3 graphitization system (Němec et al Reference Němec, Wacker and Gäggeler2010). The efficiency of carbon conversion from sample to graphite measured by (Němec et al Reference Němec, Wacker and Gäggeler2010) was ca. 92%. To obtain the radiocarbon content in fuel blends, the produced graphite targets, and reference samples (Oxalic Acid II, NIST 4990C) (Mann Reference Mann1983; Stuiver Reference Stuiver1983), were measured using a MICADAS accelerator mass spectrometer (Synal et al Reference Synal, Stocker and Suter2007). The measured currents and counts were recalculated to give radiocarbon concentrations using the BATs software (Wacker et al Reference Wacker, Synal and Marcus2010). The results were normalized using δ 13C simultaneously measured by AMS and not corrected for date of sampling. The obtained F14C were recalculated by multiplication by 100 to get the reported radiocarbon measurement results in pMC unit (Stenström et al Reference Stenström, Skog, Georgiadou, Genberg and Johansson2011).
2.3.1. Reproducibility Test for Radiocarbon Measurements
To investigate the accuracy of the results for AMS measurements, a reproducibility test was conducted for one of the blends and the background sample. Multiple aliquots of fuel blend-15 and ON/UF-BC were graphitized on different days to produce graphite targets for radiocarbon measurement using the MICADAS accelerator mass spectrometer. To determine the reproducibility, the calculated average radiocarbon content for all aliquots was compared with the previous result, and they were analyzed statistically. For LSC, the measurement of the radiocarbon content in blend-15 was performed two times. Additionally, the chi-square statistical test was used to investigate the reproducibility of the measurement results for the 10 aliquots of blend-15.
3. Results and Discussion
The radiocarbon results are presented in Table 2. For radiocarbon measurements, the first parameter of interest for both the LSC and AMS laboratories was the background sample (fuel of purely fossil origin, ON/UF-BC, Table 1). The radiocarbon content of the background sample was first determined to test for sample contamination. The results of the radiocarbon measurements for the background sample showed 1.30 ± 0.11 pMC. The LSC machine background used for correction was 0.3598 ± 0.0052 cpm (counts per minute). This value is a weighted average calculated from 14 independent 24-hour-long background measurements. The consistency of individual measurements was confirmed using the χ2 test. For AMS, the nine measurements conducted yielded an average of 0.41 ± 0.18 pMC for the background sample, which is slightly higher than an average 14C content for a phthalic anhydrite blank (ca. 0.35 pMC). The difference between the LSC and AMS measurements was 0.89 ± 0.27 pMC. Therefore, given the combined uncertainty and the expected uncertified biocarbon content (0%), the measurement results for both LSC and AMS methods were not reproducible. The value obtained for ON/UF-BC sample were used for background correction for all subsequent AMS analysis.
Table 2 Measurement results of radiocarbon content (in pMC with 1-sigma uncertainty) in fuel blends and the background sample for both LSC and AMS methods and isotope fractionation (δ 13 C, measured for ON/UF-BC and HVO, calculated for blend samples – see text for methodology)
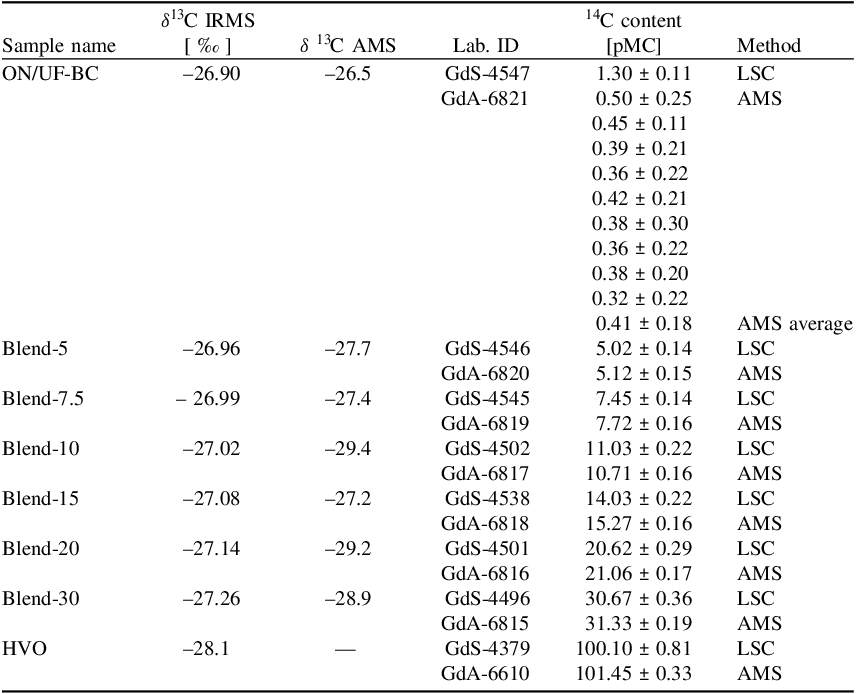
For HVO fuel, the measurement result of 14C content using LSC method gives 100.10 ± 0.81 pMC. As shown in Figure 1 and 2, this result was in agreement with AMS result (101.45 ± 0.33 pMC).

Figure 1 Radiocarbon content of the tested fuel blends (Bld) and the components (HVO and ON/UF-BC) used for their production for both methods (LSC and AMS). 2-sigma error bars are not visible at the given scale.

Figure 2 Comparison of radiocarbon content results in six fuel blends for LSC and AMS using the regression method. 2-sigma error bars are invisible due to low uncertainty values.
The radiocarbon content measured for blend-5 using the LSC and AMS methods showed 5.02 ± 0.14 pMC and 5.12 ± 0.15 pMC, respectively. The measurement results for both methods showed good reproducibility with 0.1 pMC difference, which was less than the combined uncertainty (0.21). This study was aimed at testing the reproducibility of the sample preparation procedure, and in this regard the measurement results for the LSC and AMS methods were equivalent and in good agreement with each other, given the calculated uncertainty (difference 0.10 ± 0.21 pMC).
The radiocarbon content in blend-7.5 determined by the LSC method was 7.45 ± 0.14 pMC, while the AMS method gave 7.72 ± 0.16 pMC. Given the calculated uncertainty, the measurement results using both methods were reproducible and agreed with each other (difference of 0.27 ± 0.21 pMC) and with the expected uncertified biocontents (7.5%).
Analyses of blend-10 for radiocarbon content were also conducted. The measurement using LSC, 11.03 ± 0.22 pMC, was in good agreement with the value of 10.71 ± 0.16 pMC from the AMS measurement, with a difference of 0.32 ± 0.27 pMC. Given the combined uncertainty, both measurement results were reproducible and agreed with each other.
For blend-20, the two subsamples measured by LSC and AMS gave 20.62 ± 0.29 pMC and 21.06 ± 0.17 pMC, respectively. The difference was 0.44 ± 0.34 pMC, and the expected value of the uncertified biocarbon content was 20%. The difference was satisfactory, and the values agreed with each other, given the combined uncertainty.
Similarly, the radiocarbon content for blend-30 using the LSC method was 30.67 ± 0.36 pMC. This measurement result was reproducible and in fair agreement with the 31.33 ± 0.19 pMC (with 0.66 ± 0.41 pMC difference) obtained using the AMS method and expected uncertified biocontents (30%).
The consistency of the results for blend-15 using both LSC and AMS methods was also verified. The results were 14.03 ± 0.22 pMC and 15.27 ± 0.16 pMC for LSC and AMS, respectively. The combined uncertainty was ± 0.27. However, compared to the other samples, the difference (1.24 pMC) between the methods increased. To confirm the results of the LSC measurement for this blend-15, the same experiment was repeated. Surprisingly, the resulting value of 13.46 ± 0.19 pMC was slightly lower compared to the previous value. The calculated mean was 13.75 ± 0.21 pMC. At present, there is not enough information about the possible factors that might have affected the 14C measurements of this sample, as it was kept for 30 days in a laboratory freezer before measurement. Also, blend-15 sample was used for AMS reproducibility test, giving very similar results (see Table 3 and Figure 3).
Table 3 Radiocarbon content results for the reproducibility test using the MICADAS system


Figure 3 Graphical presentation of the radiocarbon content for the reproducibility test from the MICADAS system in our laboratory. Error bars show 2-sigma uncertainties. Solid line shows the average, dotted lines show the double standard deviation from the average, and the dashed line shows the 3% acceptability limits as provided by ASTM D6866-22 standard.
The results of the radiocarbon content for all the samples are reported in Figure 1 versus the HVO content. The results showed good reproducibility between LSC and AMS. All the values were within the range of 2-sigma uncertainty bars, which showed that both the sample preparation methods and the subsequent radiocarbon measurements produced reliable results. As the 14C content in HVO is ca. 100 pMC, the results for all other fuel blends follow a linear trend.
As shown in Figure 2, the results for the six fuel blends using both LSC and AMS were also compared using a regression plot. The linear regression equation (y =ax + b; a=1.022 ± 0.025, b=0.07 ± 0.43) with R2= 0.9975 showed a good correlation between the LSC and AMS measurement results. From Figure 2, the distribution of the measured values of radiocarbon content in relation to the regression line indicated a good reproducibility between the AMS and LSC results due to the observed linearity, with only one exception of blend-15, where the measurement results for the two methods were distributed further apart compared to other samples.
Additionally, the measured results of radiocarbon concentration in 10 subsamples of blend-15 with the same volume (2 μl) are listed in Table 3.
The major purpose of conducting additional measurements was to investigate the reproducibility of the MICADAS system in the Gliwice laboratory. The calculated mean of the 10 results showed 15.12 ± 0.10 pMC. Compared to the previous measurement of the radiocarbon content in blend-15 (15.27 ± 0.16), both results were reproducible, given the calculated uncertainty (difference 0.15 ± 0.19 pMC). The results of the reproducibility test are also presented in Figure 3. They were within a 2-sigma range, which proved that from the sample preparation using a tin capsule for liquids, through the sealing procedure and the combustion in the elemental analyzer, to the graphitization by AGE, reproducible results were shown from the MICADAS system in our laboratory. To confirm the reproducibility of the measurement results by MICADAS, a chi-square (χ2) statistical test was performed, and the results are reported in Table 3. The computed χ2 value was 7.72, and the critical value (CV) was 21.67 (with a confidence level, α = 0.01). Therefore, due to the χ2 value being less than the CV, there was no significant difference between the reported results for all 10 aliquots, meaning all the results were reproducible and agreed with each other.
Conclusions
The main purpose of this study was to test the best sample preparation and measurement methods in the Gliwice Radiocarbon and Mass Spectrometry Laboratory. Using LSC and AMS methods, the preparation of fuel blend samples was tested. The measurement results from both methods showed good reproducibility for sample processing as shown in Figure 1. The minor differences between AMS and LSC may result from the different carbon conversion efficiency during the benzene production, although the corrections were made for fractionation effect. However, due to the increase of observed change for blend-15 during the reproducibility test using the LSC method, this sample will be subjected to further studies for sample preparation and measurement in the future to deduce appropriate explanations for this small change. For AMS, the sample preparation using a tin capsule for liquids and a sealing press was effective. The experimental results of the radiocarbon content were corrected for isotope fractionation processes (13C/12C ratio measured by IRMS) that occurred during the preparation and measurement. Furthermore, the investigation of the reproducibility test for blend-15 using the AMS method showed that the results for the radiocarbon content measured by the MICADAS accelerator were reliable.
Acknowledgments
This research was supported by a Silesian University of Technology subsidy for maintenance and development of research potential (14/020/BK_22/0021) and a young scientists project (14/020/BKM22/0022). The authors thank all the staff from the Gliwice 14C and AMS laboratory at the Institute of Physics - Centre for Science and Education.









