INTRODUCTION
Synchronized demographic and behavioral patterns among distinct and geographically separate populations is a well-known natural phenomenon that has been demonstrated across animal and plant populations. The study of spatial synchrony has thus become a key topic in population ecology. “Spatial synchrony” refers to coincident changes in the abundance or adaptive response of geographically disjunct populations (Liebhold et al., Reference Liebhold, Koenig and Bjørnstad2004). Three primary mechanisms have been offered to explain such synchrony: (1) dispersal or migration among populations, (2) trophic interactions with populations of other species that are themselves spatially synchronous or mobile, and (3) spatially correlated environmental influences (Liebhold et al., Reference Liebhold, Koenig and Bjørnstad2004). This last phenomenon, known as the “Moran effect,” reflects upon the tendency of spatially separated populations to fluctuate in synchrony when exposed to similar environmental conditions (Moran, Reference Moran1953). The Moran effect is often thought to be the result of synchronous weather or climate influences acting on spatially disjunct populations (Moran, Reference Moran1953; Koenig, Reference Koenig2002; Rosenstock et al., Reference Rosenstock, Hastings, Koenig, Lyles and Brown2011; Kahilainen et al., Reference Kahilainen, Nouhuys, Schulz and Saastamoinen2018).
For prehistoric humans, Shennan et al. (Reference Shennan, Downey, Timpson, Edinborough, Colledge, Kerig, Manning and Thomas2013) were the first to identify synchrony in 14C date-based human population proxies across mid-Holocene Europe. This synchrony was attributed to migration and population growth, induced by the introduction of agriculture 8000–6000 cal yr BP. Recently, Freeman et al. (Reference Freeman, Baggio, Robinson, Byers, Gayo, Finley, Meyer, Kelly and Anderies2018) argued that synchronous patterns in 14C time series observed across the globe during the Holocene were the result of intensified networks of trade and migration within continents, while convergent cultural evolution toward more energy-consuming political economies with higher carrying capacities account for global synchrony. However, as Freeman et al. (Reference Freeman, Baggio, Robinson, Byers, Gayo, Finley, Meyer, Kelly and Anderies2018) admit, they omit climate change as the driving force behind the observed synchrony, despite it being the explanation most commonly used in ecology. This is critical, as climate can influence human growth rates either directly (extreme events) or indirectly by affecting environmental productivity and, consequently, food availability. We suggest that evaluating the role of climate change in driving synchronous human demographic and adaptive responses requires analyses sensitive to regionally specific ecological conditions.
Here, we compare Holocene hunter-gatherer ecodynamics in two northern European regions: western Finland and northern Norway. We investigate the role of climate in controlling coastal hunter-gatherer population trends and changes in adaptive strategies between the two regions. We show that population size and adaptive strategies change synchronously between western Finland and northern Norway. These changes coincide with climate changes and consequent changes in food availability. Thus, our results highlight the role of environmental factors in creating spatial synchrony in long-term human population dynamics across space.
REGIONAL SETTING
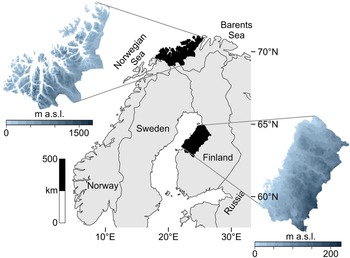
The data catchment areas of our study are the coasts of northern Troms and western Finnmark Counties, constituting the northwesternmost margin of Norway (69°–71° latitude), and the Ostrobothnian coast in western-central Finland (63°–65° latitude) (Fig. 1).
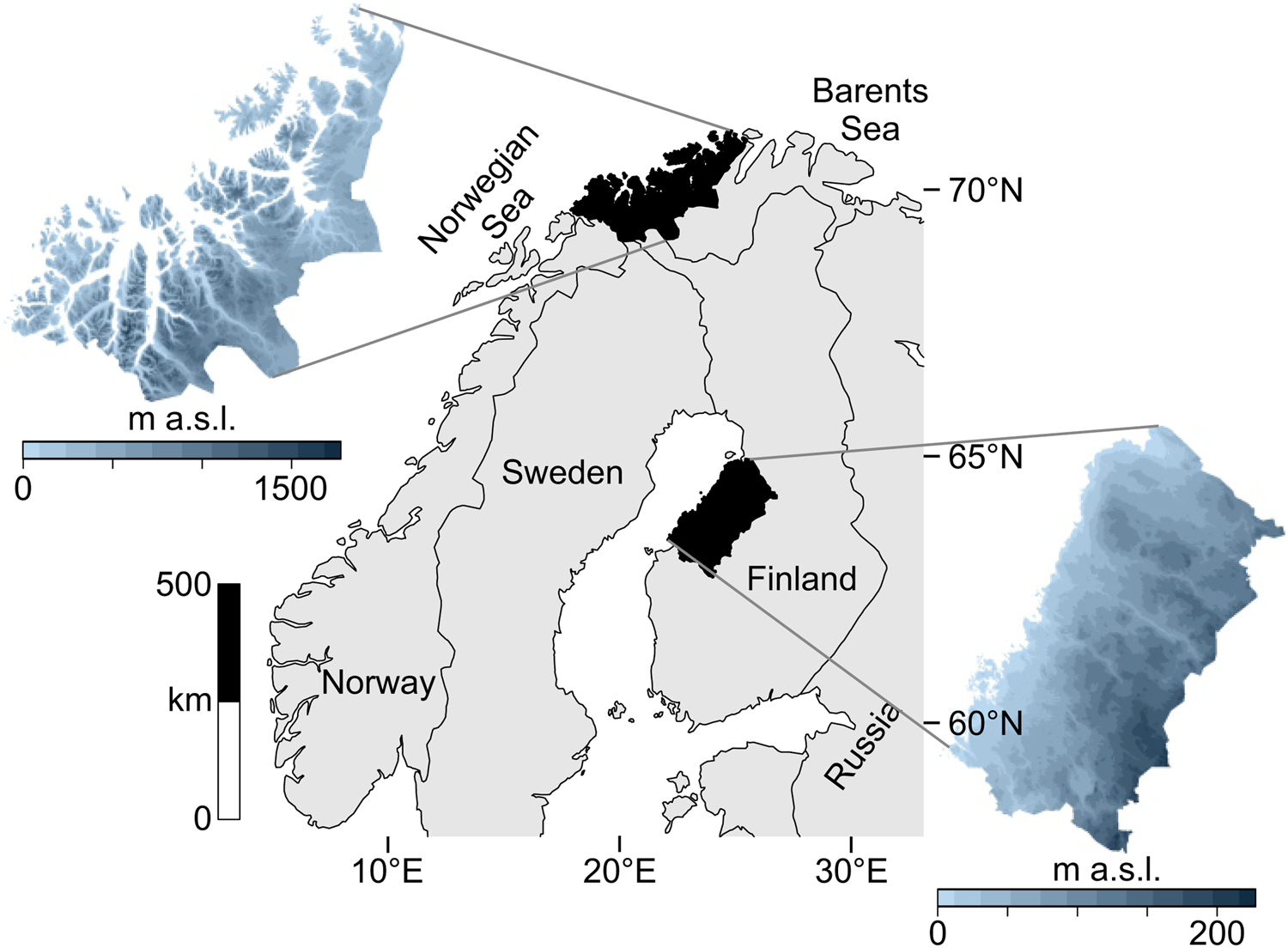
Figure 1. Map of the two study regions within northern Europe.
The study areas occupy northern coastal ecotones while simultaneously being different systems in terms of ecology and geography. These areas are positioned along different aquatic systems: northern Norway is on the oceanic interface of the North Atlantic and the Barents Sea, where upwelling, salinity, and significant tidal actions produce a highly productive coast. Western Finland is adjacent to the more enclosed Gulf of Bothnia in the Baltic Sea, marked by relatively low salinity and minimal tides. Also, the topography of these areas differ: western Finland is a flat continuous coastline, while northern Norway is a rugged, mountainous coastline scattered with islands and deep-cutting fjords.
By the time of the mid-Holocene, the two areas had quite different ecological systems. The Finnish area has a significantly more productive terrestrial ecosystem compared with that of northern Norway, primarily due to latitudinal differences. Major changes occurred in the terrestrial environment during the mid-Holocene, as the previously species-rich mixed forest of the Finnish terrestrial system became increasingly dominated by spruce (Picea abies). This turned the forest ecosystem into a modern boreal taiga dominated by spruce (Picea abies) and pine (Pinus sylvestris) (Seppä et al., Reference Seppä, Alenius, Bradshaw, Giesecke, Heikkilä and Muukkonen2009a).
A recent compilation of a large set of pollen cores from across northern Norway indicates a patchwork of vegetation cover, structured by both the inland/coast axis and the west/east axis, in which the outer coastal area of northwestern Norway was characterized by birch (Betula) forest cover exceeding current conditions (Sjögren and Damm, Reference Sjögren and Damm2019). This likely impacted the biogeography of key terrestrial mammals with a shift from postglacial large herds of migratory ecotype reindeer (Rangifer tarandus) to smaller herds of sedentary ecotype reindeer (R. tarandus) (Hood, Reference Hood, Skandfer, Blankholm and Hoodin press, p.23).
Another important factor in area selection is the fact that Fennoscandia hosts archaeological records of continuous hunter-gatherer populations throughout the Holocene. These records demonstrate shared adaptive characteristics between the areas with reliance on marine subsistence technologies at an early stage. What is more, there are some indications of participation in extensive interaction spheres, as evidenced by shared material culture traits such as slate technology, ceramics, rock art, imported amber, and early metal products (Damm, Reference Damm2006; Nordqvist et al., Reference Nordqvist, Herva, Ikäheimo and Lahelma2012; Ramstad et al., Reference Ramstad, Axelsson and Strinnholm2015). However, very little evidence exists to determine the magnitude of interaction between the areas. The assumed connections must therefore be seen as highly tentative, and we stress that there is more evidence indicating separate rather than shared culture-history in these areas.
The areas have some similarities in postglacial colonization history, but also exhibit important differences. Following the deglaciation of the final Pleistocene, coastal areas of the Fennoscandian/Baltic shield became increasingly accessible for colonization by marine flora and faunas. This process is thought to have triggered a significant incentive for humans to colonize the postglacial coastal landscape of northernmost Europe. This entailed a radical economic shift: From terrestrially oriented foraging societies of the late-glacial Ahrensburgian and Butovo/Veretye groups on the Eurasian plain, moving north and west and developing the maritime adaptations quintessential to the Scandinavian Mesolithic (Schmitt et al., Reference Schmitt, Larsson, Schrum, Alekseeva, Tomczak and Svedhage2006; Bang-Andersen, Reference Bang-Andersen2012, Reference Bang-Andersen2013; Schmitt, Reference Schmitt2015; Schmitt and Svedhage, Reference Schmitt and Svedhage2015; Dolukhanov et al., Reference Dolukhanov, Kosheleva, Lisitsyn, Subetto, Kotlyakov, Velichko and Vasil'ev2017). The colonization of Norway at the termination of the Younger Dryas (11,700 cal yr BP) occurred along a coastal route requiring seafaring vessels and the know-how of a marine-oriented economy (Bjerck, Reference Bjerck2017). The case is somewhat different in Finland, which was colonized via a terrestrial route. The Finnish case is most in line with the model suggesting maritime adaptations originated in Upper Paleolithic river resource utilization and were later adapted to larger water bodies, allowing people to move into the marine niche on the oceanic coasts (Vasil'evskii et al., Reference Vasil'evskii, Bland, Gokhman, Workman and Workman1998; see also; Cziesla, Reference Cziesla2007, Reference Cziesla, Persson, Riede, Skar, Breivik and Jonsson2018; Terberger et al., Reference Terberger, Floss, Heinzelmann, Kotula, Serangeli, Pastoors and Auffermann2013). At the Pleistocene/Holocene transition, most of present-day Finland was submerged due to glacio-isostatic loading, yet the ensuing isostatic uplift rapidly transformed the area from a postglacial coast into a patchwork of rivers, lakes, and wetlands. The archaeological record also testifies to aquatic economies from the very onset. Complex technologies used for aquatic resource exploitation are evident already from the early Holocene, including the spectacularly well-preserved Antrean fishnet dated to 10,500 cal yr BP. During the mid-Holocene, massive stationary fishing structures, such as weirs and lath screen traps recovered from multiple estuaries, offer extensive evidence of marine-oriented facilities requiring substantial investment (Koivisto, Reference Koivisto2012; Koivisto and Nurminen, Reference Koivisto and Nurminen2015; Butler et al., Reference Butler, Koivisto, Brumfeld and Shahack-Gross2019; Groß et al., Reference Groß, Zander, Boethius, Dreibrodt, Grøn, Hansson and Jessen2018; Koivisto et al., Reference Koivisto, Latvakoski and Perttola2018). The different routes to maritime adaptations underline the comparative relevance of the cases and provide pertinent insight into the evolution of full-fledged maritime adaptations.
Data quality is also a vital factor in area selection. Both areas have been intensively investigated archaeologically, including large-scale excavations and surveys. Together with excellent paleoenvironmental records, the two regions offer robust testing grounds for evaluating changing human ecodynamics.
MATERIALS AND METHODS
Human population size proxies
We reconstruct human population trends in the two areas using temporal frequency distributions of archaeological materials. We consider the time span from the early Holocene colonization at ~12,000 cal yr BP to about 2000 cal yr BP, at which point farming achieved a more permanent foothold and changes in settlement patterns and economy ensued in northern Fennoscandia. Before this, farming made minimal impact on both areas, particularly in northern Norway.
For western Finland, we use the temporal distribution of 754 shoreline-dated sites as the basis of the population reconstruction (Tallavaara and Pesonen, Reference Tallavaara and Pesonen2018). A gradual and well-established shoreline displacement due to postglacial isostatic uplift provides high-resolution dating on the basis of elevation above sea level. As with radiocarbon dates, we assume that variation in the number of sites reflects relative changes in past population size. The sites have primarily been identified through LiDAR mapping, and the current sample exclusively consists of sites positively confirmed as archaeological remains by field surveys. Included site types range from open-air sites to pithouse sites of variable sizes to row-house sites and megastructures. Despite a potentially lower chronological resolution, we argue that this approach is justifiable, as it substantially boosts sample size in an area containing few radiocarbon-dated sites. Further, the approach helps overcome investigation biases, as all identifiable site types are included regardless of the presence of radiocarbon dates. Thus, this site-based proxy sidesteps many of the sampling biases inherent in radiocarbon-based population proxies. This approach also takes advantage of the favorable isostatic properties of the area. Western Finland is positioned near the weight center of the Fennoscandian ice cap, resulting in isostatic uplift of more than 200 m over the past 12,000 yr. Given a mostly flat topography, the isostatic rebound of the area provides ideal conditions for high-resolution shoreline dating. Virtually identical trends have been established between the regional site-based reconstruction and the summed probability distribution (SPD), which is based on radiocarbon dates covering the total area of Finland (Tallavaara and Pesonen, Reference Tallavaara and Pesonen2018). This strengthens the reliability of the site-based proxy.
For northern Norway, the reconstruction of population dynamics is based on the SPD method of radiocarbon-dated site-occupation events (Shennan and Edinborough, Reference Shennan and Edinborough2007; Williams, Reference Williams2012; cf. Ramsey, Reference Ramsey2017). This method is premised on the proportional relation between population size and datable components of the archaeological record (Rick, Reference Rick1987; see Haynes, Reference Haynes1969; Kirch, Reference Kirch1980). This so-called dates-as-data premise implies that smaller populations leave behind a smaller sample of archaeologically visible traces compared with larger populations. Major efforts have been made to test this premise (Surovell and Brantingham, Reference Surovell and Brantingham2007; Surovell et al., Reference Surovell, Byrd Finley, Smith, Brantingham and Kelly2009; Shennan, Reference Shennan2013; Timpson et al., Reference Timpson, Colledge, Crema, Edinborough, Kerig, Manning, Thomas and Shennan2014). Following the results in Edinborough et al. (Reference Edinborough, Porčić, Martindale, Brown, Supernant and Ames2017), the method has demonstrated its usefulness in reconstructing paleodemographic fluctuations. For the current study, archaeological radiocarbon dates were collected for the coast of northwestern Norway, which contains the densest and most recently produced radiocarbon record in northern Norway. The data set (N = 735) exclusively comprises radiocarbon dates from secure archaeological contexts, made on terrestrial carbon (data set available at: https://dataverse.no/dataset.xhtml?persistentId=doi:10.18710/AV9R5X). These have been further vetted for taphonomic, investigative, and sampling biases (Jørgensen, Reference Jørgensen2018). The dates were then structured into 503 bins of 200 yr to control for overrepresentation of more intensively investigated sites. Further details on auditing measures of the current data set are presented in Jørgensen (Reference Jørgensen2018). Although the population proxies for our two study areas are derived from different source data, we have opted for this strategy, as it produced samples of comparable size.
Paleoenvironmental data
Holocene environmental changes are represented by eight paleoecological and paleoclimatic proxies. We selected available proxies related to the productivity of terrestrial and marine environments and, consequently, to food availability for hunter-gatherers. Somewhat different environmental proxy types represent the two areas. This is the result of regional differences in depositional and geomorphic qualities, as well as unequal conditions for preservation of paleoenvironmental proxy data. The paleoenvironmental proxies discussed in this paper are summarized in Table 1.
Table 1. Climate records employed for paleoenvironmental review.
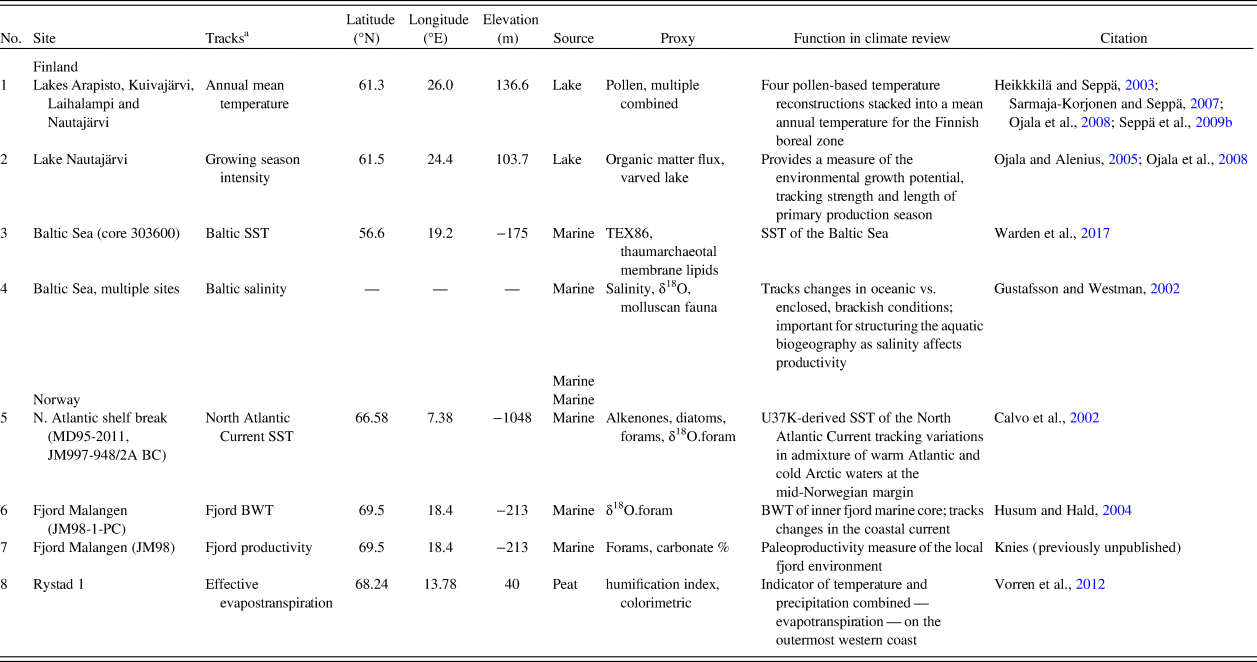
a BWT, bottom water temperature; SST, sea-surface temperature.
Prehistoric human population dynamics in western Finland are compared with: (1) a measure of annual mean temperature, which is a stack of four pollen-based temperature reconstructions across southern and central Finland; (2) the strength and length of the growing season, which is based on organic matter flux in a varved lake (Ojala and Alenius, Reference Ojala and Alenius2005; Ojala et al., Reference Ojala, Alenius, Seppä and Giesecke2008); (3) Baltic Sea surface temperature (SST) reconstruction, derived from TEX86-paleothermometer measurements (Warden et al., Reference Warden, Moros, Neumann, Shennan, Timpson, Manning, Sollai, Wacker, Perner, Häusler, Leipe, Zillén, Kotilainen, Jansen, Schneider, Oeberst, Arz and Damsté2017); and (4) Baltic Sea salinity levels (‰) based on the compilation of several proxies (Gustafsson and Westman, Reference Gustafsson and Westman2002). Salinity is important in tracking changes in oceanic versus the enclosed, brackish conditions of the Baltic Sea. This has ecological implications, as salinity levels structure aquatic biogeography and affect the productivity of the Baltic Sea.
For northern Norway, the SST of the North Atlantic Current collected at the offshore shelf break tracks variation in mixing of warm Atlantic and cold Arctic waters at the mid-Norwegian margin (Calvo et al., Reference Calvo, Grimalt and Jansen2002). Ocean mixing is a significant factor in structuring marine biogeography and for inferring large-scale oceanographic and environmental conditions. Two proxies of inner coastal aquatic conditions in northern Norway are included, as the fjord biome is of great importance to the human populations in the area. Bottom water temperatures (BWT) of a major fjord system (Malangen fjord) in the study area track changes in the coastal current (Husum and Hald, Reference Husum and Hald2004). In addition, we contribute a new paleoproductivity measure of the same local fjord environment. The fjord productivity proxy is made up of previously unpublished data, courtesy of Jochen Knies at the Norwegian Geological Survey. The percentage of carbonate is used as a direct marker of productivity in the fjord as it relates to the abundance of calcium/chalk-dependent zooplankton, which in turn is the foundation of the marine trophic pyramid. This assumption is justified, as the relative proportion of terrigenous-free (biogenic) carbonate has been shown to be a highly suitable indicator of changes in paleoproductivity in the area (Knies et al., Reference Knies, Hald, Ebbesen, Mann and Vogt2003, pp. 1–2; cf. Gardner et al., Reference Gardner, Dean and Dartnell1997).
We also include a humification index from the outermost western coast of Norway. The peat humification index is a combined indicator of temperature and precipitation—evapotranspiration—that also reflects changes in terrestrial productivity (Vorren et al., Reference Vorren, Jensen and Nilssen2012).
Maritime resource exploitation data
To explore potential synchrony between adaptive strategies, population size, and environment, we assembled multiple indicators of marine resource use.
To track changes in the subsistence/adaptive strategies in western Finland, we calculate two closely related measures: the proportion of seal bones in archaeofaunal assemblages in coastal sites (seal NISP (Number of Identified Specimens)/total NISP) and the index of seal bones relative to terrestrial mammals (seal NISP/(seal NISP + terrestrial mammal NISP)) (Grayson, Reference Grayson1984). Although not a direct quantitative measure of seal consumption, we assume that changes in the proportion of seal bones reflect changes in the importance of seals in human diet. As a secondary premise, we assume that such variation indirectly reflects adaptive adjustments following either environmental or technological changes. The archaeofaunal data consist of 37,810 burnt bone fragments from 72 archaeological assemblages across the Finnish coast. These data were extracted from the archives of osteological reports compiled by Pirkko Ukkonen and Kristiina Mannermaa at the Finnish Museum of Natural History and from osteological reports at the National Board of Antiquities. The faunal record was attributed to broad chronological periods based on time constraints given by associated radiocarbon dates or typological artifact attribution: Early Mesolithic (11,000–8500 cal yr BP), Late Mesolithic (8500–7200 cal yr BP), Early Sub-Neolithic (7200–6000 cal yr BP), Middle Sub-Neolithic (6000–5400 cal yr BP), and Late Sub-Neolithic (5400–3500 cal yr BP).
Due to poor preservation of organic remains, there is no representative archaeofaunal sample to draw on from the Norwegian area, and we had to devise an alternative measure of marine resource use. To map changes in marine resource use, we assembled a “slate index,” premised on the strong affinity between maritime adaptive strategies and the use of slate tools. The slate index tracks the abundance of slate tools relative to other lithic industries, based on the averaged frequencies of slate versus cryptocrystalline lithic materials from a selection of reliably dated site assemblages. The data set consists of 37 securely dated lithic assemblages covering the entire local Stone Age chronology, with more than 22,000 lithic objects. Importantly, most of the assemblages stem from multiphase sites of significant occupation history. This factor helps control for variation in site function. As the ecological properties of a single coastal site are assumed to be more or less stable, any major variation in lithic assemblage composition through time is assumed to reflect changes in subsistence strategies.
Based on the near-universal reliance on slate tools among circumpolar maritime hunter-gatherers (Fitzhugh, Reference Fitzhugh1974), we assume that slate tools provide a reliable indication of maritime resource exploitation. There have been multiple attempts at explaining the strong prevalence and assumed superiority of slate tools for maritime economic purposes (Gjessing, Reference Gjessing1953; Dumond, Reference Dumond1968; Ritchie, Reference Ritchie1969; Fitzhugh, Reference Fitzhugh1974; Clark, Reference Clark1980, Reference Clark1982; Morin, Reference Morin2004; Graesch, Reference Graesch2007; Dinwiddie, Reference Dinwiddie2014). As a basic premise, we follow several arguments and empirical demonstrations (Clark, Reference Clark1979; Wilhelmsson, Reference Wilhelmsson1996; Nuñez, Reference Nuñez1998; Morin, Reference Morin2004) that slate technologies can reduce handling costs and facilitating mass harvesting of marine resources (sensu Madsen and Schmitt, Reference Madsen and Schmitt1998), and thus alter the energy budget and ranking of marine/terrestrial resources. As a result, slate technology could significantly boost food security and survivorship, and hence population numbers, among maritime hunter-gatherers. In northern Norway, slate tools have an almost exclusively coastal distribution, supporting our assumption that slate tools were used primarily as a maritime technology and thus are a relevant proxy for marine resource exploitation. Despite lower sampling density of inland sites potentially contributing to this picture, a review of existing data suggests two patterns: (1) There is literally no evidence for slate tool production in the interior, indicating import (Hood, Reference Hood1992, p. 521). (2) In the rare cases of locally procured material, inland slate tools appear to be of a much more silicified raw material and subject to a different reduction sequence, occasionally even made by recycling greenstone tools (see Rigajokka site [Helskog, Reference Helskog1974, pp. 4–5]) — thus not really slate at all.
RESULTS
Figure 2 shows the reconstructed population dynamics/trajectories for northern Norway and western Finland and reveals a clearly synchronous pattern between the two regions. A major feature in both reconstructions is the prominent boom-and-bust cycle between 6500/6000 and 5000 cal yr BP. However, in northern Norway the highest population levels apparently occur ca. 300 yr earlier than in western Finland. In addition to this major population boom-and-bust, the population proxies further indicate minor, synchronous declines at 8200 and 7000 cal yr BP.

Figure 2. Comparative figure of reconstructed population trends of the two areas. Blue bars mark synchronous, negative fluctuations. Red bar marks synchronous, positive fluctuation. Dotted, vertical line illustrates the lag in timing of the most significant population cycle between the areas.
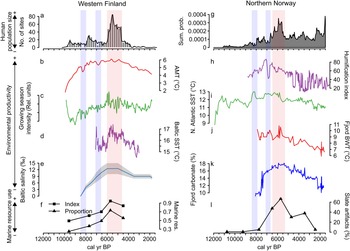
Figure 3 further shows a correspondence between long-term human population dynamics and environmental variability in both areas. In the Finnish data set, proxies covering both marine and terrestrial productivity show increasing trends culminating around 6000 cal yr BP, concurrent with the prominent population peak (Fig. 3b–e). This is particularly evident in the marked correspondence between the reconstructed population trend, growing season intensity (Fig. 3c), and the Baltic Sea SST (Fig. 3d). The subsequent population decline coincides with declining late Holocene productivity (see also Tallavaara and Seppä, Reference Tallavaara and Seppä2012). Furthermore, population dips observed in both areas at around 8200 and 7000 cal yr BP coincide with shorter-duration downturns in temperature and growing season intensity (Fig. 3b–d).
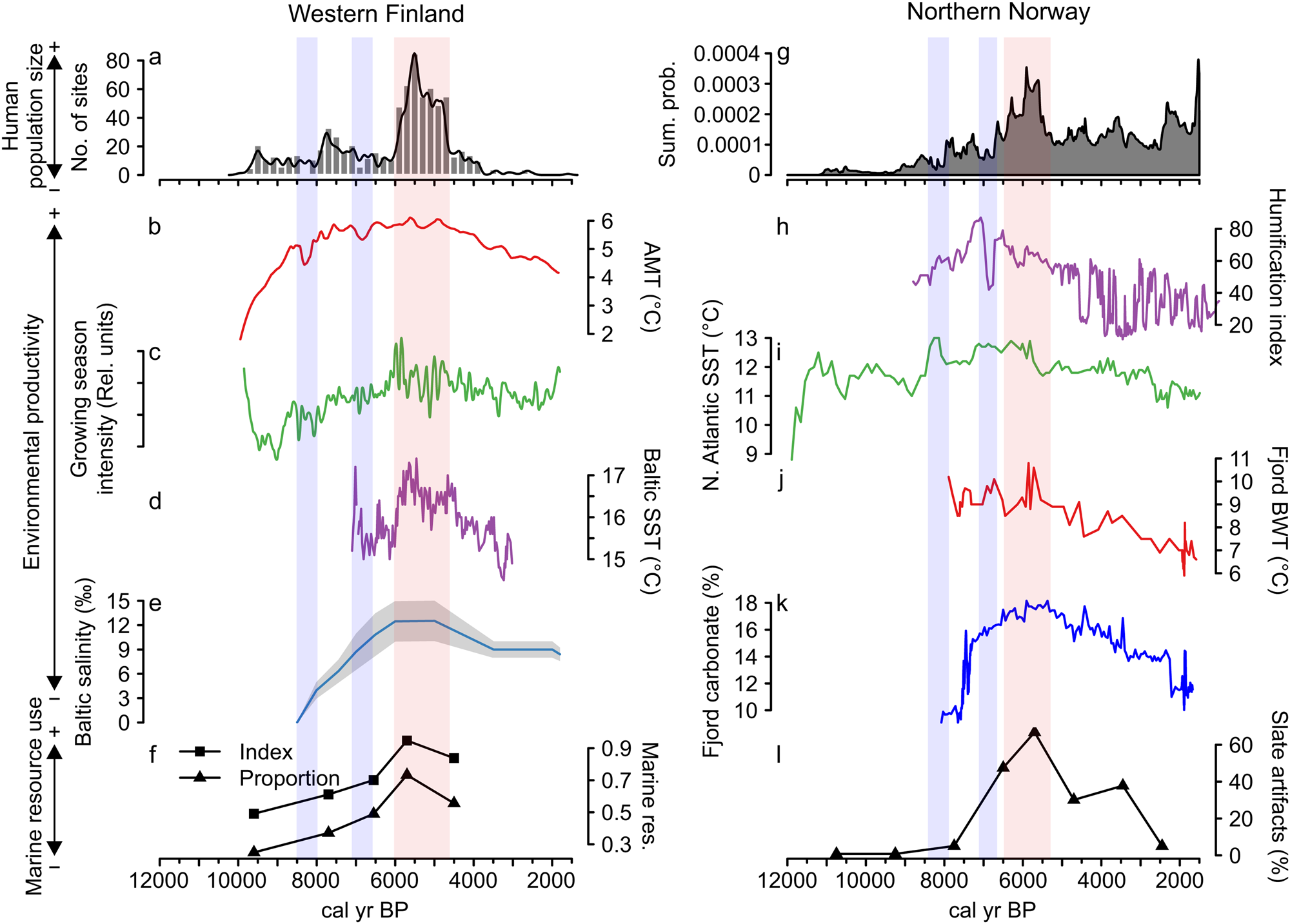
Figure 3. Combined figure of reconstructed population trends, climate proxy comparisons, and marine resource exploitation indicators: (a) Finnish area population reconstruction; (b) Finnish area annual mean temperature (AMT); (c) Finnish area growing-season intensity; (d) Baltic Sea surface temperature (SST); (e) Baltic Sea salinity; (f) index and proportion of seal bones in Finnish faunal assemblages; (g) Norwegian area population reconstruction; (h) Norwegian evapotranspiration; (i) North Atlantic coastal current temperature (SST); (j) Norwegian fjord bottom water temperature (BWT); (k) Norwegian fjord productivity (carbonate %); and (l) proportion of slate tools in coastal, northern Norwegian lithic assemblages.
The pattern is similar in the Norwegian study area, where marine proxies (Fig. 3i–k) show peaking SSTs and marine productivity around 6000 cal yr BP. The North Atlantic Current conveyed higher quantities of warm Atlantic water during the mid-Holocene and the coastal water temperature and marine productivity peaked in the major fjord system within the study area (Fig. 3i). This indicates a mild climate with increased Atlantic water in the fjord system that drove the production of carbonate (either produced in situ or transported with the Atlantic water).
In accordance with the Finnish data, temperatures and productivity declined after 6000 cal yr BP. The evapotranspiration reconstruction (Fig. 3h) shows a slightly different pattern, as the highest values occur between 7500 and 6500 cal yr BP. Nevertheless, very stable conditions are recorded around the 6000 cal yr BP population peak, while a general climate shift toward highly variable conditions occurred with the transition to the late Holocene.
In northern Norway, the population decline at 8200 cal yr BP coincide with the Storegga tsunami, caused by the massive submarine landslide in the Norwegian Sea (Romundset and Bondevik, Reference Romundset and Bondevik2011). Furthermore, the 7000 cal BP downturn corresponds to the Tapes transgression (Sørensen et al., Reference Sørensen, Bakkelid and Torp1987; Romundset et al., Reference Romundset, Bondevik and Bennike2011). Thus, taphonomic loss of archaeological material may be responsible for the declines in the Norwegian population proxy (see also Jørgensen, Reference Jørgensen2018, 5). However, this is most likely an insufficient cause, as the population declines at 8200 and 7000 cal yr BP perfectly mirror the Finnish settlement data, in which no such taphonomic loss is observed. This suggests that these specific declines in northern Norway most likely are actual demographic events. Although low amplitude, these apparent population dips correspond to a hiatus in settlement sites in Finland and reduced site numbers in Norway.
Considering the precise synchrony of these events between western Finland and northern Norway, it is of interest that the main population event appears to occur slightly earlier in northern Norway, with a more gradual buildup and more abrupt collapse compared with the Finnish population cycle. The slight variation in dating of these events may be the result of the methods used to reconstruct population dynamics. This has been indicated previously, as a similar age shift in the highest population levels between different population proxies has been observed in the Finnish data (Tallavaara and Pesonen, Reference Tallavaara and Pesonen2018). Another possibility is that the timing of the main population cycle corresponds to different timings of the most favorable environmental conditions in the separate areas. This is supported by the identification of a latitudinal gradient in the timing and duration of the peak Holocene thermal maximum occurring earlier in the higher latitudes of Fennoscandia (Eldevik et al., Reference Eldevik, Risebrobakken, Bjune, Andersson, Birks, Dokken and Drange2014, p. 228). Future efforts should aim at discriminating between methodological and climatic effects in explaining this lag, as well as further issues of data resolution.
In addition to the correspondence between population and environmental proxies, proxies indicating marine resource use also correlate with population and environmental proxies in both areas. Marine resource use increases along with increasing population size and environmental productivity until around 6000 cal yr BP and declines as population size and productivity proxies decrease. The Finnish archaeofaunal record (Fig. 3f) shows that during the boom phase of the mid-Holocene population event, seal bones make up more than 70% of the coastal archaeofaunal assemblages. The trend of seal exploitation intensity corresponds to both the growth and decline phases of the population trajectory.
In Norway, the use of slate intensified from 7000 cal yr BP and became the dominant lithic industry by the time of the population peak (Fig. 3l). By this time, slate concentrations often constituted as much as 80% of coastal assemblages. We assume that this reflects a change in adaptive strategies toward more intensified use of marine resources in the region. A shift away from slate in favor of a more expedient technology based on local quartz occurred simultaneous with the population decline. Slate was still important for some time after the 5500 cal yr BP population decline, but the slate component is reduced from 70%–80% to about 30%. In addition to the slate index, several other characteristics of the Norwegian archaeological record support the idea of increased marine resource use during the population boom. From 7000 cal yr BP, larger coastal sites consisting of multiple pit houses emerge. Despite there being some indications of pit-house construction occurring before this period, this represented a marked change in settlement longevity (Damm et al., Reference Damm, Skandfer, Jørgensen, Sjögren, Vollan and Jordan2019; Gjerde and Skandfer, Reference Gjerde, Skandfer and Blankholm2018), indicating increased locational investment in coastal sites and a shift in coastal settlement pattern and organization. Furthermore, recent investigations of differences in coast and inland human presence clearly demonstrate an almost complete lack of inland occupation concurrent with the major population peak at the coast (Jørgensen and Riede, Reference Jørgensen and Riede2019; Hood et al., Reference Hood, Blankholm, Skandfer, Skandfer, Blankholm and Hoodin press). This corroborates the previous impression that major population packing occurred on the coast and that activity in the interior was minimal at this time (Hood, Reference Hood2012). Given the significant difference in magnitude between inland and coastal settlements, packing does not seem a sufficient explanation. We suggest actual population growth followed coastal packing, although this is in need of further enquiry.
Highlighting this, the archaeological and rock art records suggest technological and organizational intensification of marine resources through the introduction of more efficient hunting/processing tools and (most likely) cooperative hunting strategies (Gjerde, Reference Gjerde2018). Dietary investigations of the only mid-Holocene human individual currently known from northern Norway (Måløy Island) demonstrate a spectacularly high intake of marine protein (Günther et al., Reference Günther, Malmström, Svensson, Omrak, Sánchez-Quinto, Kılınç and Krzewińska2018, S1, 12). Discriminating the isotopic signature of marine mammal protein from that of migratory cod is difficult due to comparable trophic levels (Schulting et al., Reference Schulting, Budd and Denham2016), but migratory cod is by far the most dominant species in the region's faunal record during the time of the population boom (Olsen, Reference Olsen1967; Utne, Reference Utne1973; Engelstad, Reference Engelstad1983; Renouf, Reference Renouf1989). Tentatively, this may indicate adaptive adjustments toward lower-ranked fish resources. Systematic diachronic sampling of biochemical dietary proxies may help resolve these issues in the future.
DISCUSSION
Our main finding is the clear spatial synchronicity in demographic trends and adaptive strategies between two geographically separate human populations. Our results also strongly suggest that this synchronicity is related to the variability in both terrestrial and marine productivity, which themselves are correlated between the two areas. While the details of these human ecodynamics and the pathways toward increased populations and maritime adaptations differ between the two focus regions, the outcomes are comparable. This suggests that the long-term demographic trajectories in both areas were ultimately regulated by climate and its downstream effect on both terrestrial and marine productivity and hence food availability for hunter-gatherers. The high productivity of the mid-Holocene would have increased the environmental carrying capacity, and in concert with highly stable climatic conditions, offered unprecedented potential for human population growth. This seemingly mechanistic climate forcing of human populations is further supported by the synchronous decline in population numbers and environmental productivity after 5500 cal yr BP, as well as by short-term declines at 8200 and 7000 cal yr BP. Thus, our results apparently demonstrate Moran effects in action among human populations. The implication being that climate has the potential to synchronize long-term human population trajectories among foraging economies. Future research would need to investigate to what extent this relation also holds for food-producing populations.
Although our results suggest that climate is the most likely explanation for the spatial synchrony between the northern Norwegian and western Finnish hunter-gatherer populations, other mechanisms may still be in play. The trend correspondence between population size, climate, and adaptive strategies highlights the more generalized problem of what should be ascribed causal primacy among demographic, environmental, and technological factors in bringing about synchronous adaptive strategies: Did marine resource exploitation vary independently of population size, or did the maritime specialization result from changes in population size, thus being density dependent? The latter option fits the concept of marine resources becoming attractive only when population packing restricts terrestrial hunting capabilities, creating an imbalance between human population growth and its (assumed) preference for a terrestrial resource base (Binford, Reference Binford2001, pp. 188, 210; Kelly, Reference Kelly2013). This is thought to follow from the high handling and initial investment costs in aquatic resource exploitation in order to turn a profit, exemplified by the development of boats, specialized fishing equipment and marine hunting gear, as well as bulk processing and storage (Osborn, Reference Osborn1977; see also Yesner et al., Reference Yesner, Ayres, Carlson, Davis, Dewar, Morales and Hassan1980; Steffian et al., Reference Steffian, Saltonstall and Kopperl2006; Fitzhugh, Reference Fitzhugh, Max Friesen and Mason2016). Yet maritime intensification would also require some other factor responsible for the initial population growth necessary to achieve sufficient population packing to mitigate the higher investment costs.
In our case, however, this seems problematic. First, human population growth and marine resource exploitation appear to increase alongside a coupled marine–terrestrial productivity increase. One might point to the significantly fewer trophic levels in high-latitude, terrestrial ecosystems as a possible limitation to terrestrially based human population growth (cf. Steele, Reference Steele1985; Carr et al., Reference Carr, Neigel, Estes, Andelman, Warner and Largier2003; Steele et al., Reference Steele, Brink and Scott2019). The abundance of ungulates is strictly regulated by density-dependent mechanisms in boreal forests (Bergerud et al., Reference Bergerud, Luttich and Camps2012, p. 102), and is arguably less resilient in the face of human overexploitation than marine resources (Minc and Smith, Reference Minc, Smith, Halstead and O'Shea1989; Gunderson, Reference Gunderson2000). It is therefore not clear whether continued terrestrial growth results in a linear increase in resource abundance relevant to human economic exploitation. This is an unresolved issue for future research to consider, yet current data do not support scarce terrestrial resources as the driving factor of the regime shift in marine exploitation. Further lacking support is the possibility of a significantly earlier terrestrial productivity peak driving the shift toward intensified marine economies (particularly in light of a wider range of terrestrial proxies from northern Norway [Balascio and Bradley, Reference Balascio and Bradley2012; Wittmeier et al., Reference Wittmeier, Bakke, Vasskog and Trachsel2015; Sjögren and Damm, Reference Sjögren and Damm2019]). Second, the intensity of marine resource use appears to decline along with declining terrestrial (and marine) productivity. Third, if marine resources are secondary to terrestrial resources, it would make it difficult to explain how aquatic resources could support the population growth observed in our data or how some of the highest population densities in the ethnographic record are found among maritime-adapted hunter-gatherers. For now, we cannot resolve the causal relationship between technological change and population growth. The fact that increase and decrease of marine resource use follow the trends in environmental productivity nevertheless suggests that adaptive changes in our study areas were ultimately subordinate to climate changes.
An alternative to endemic population growth in ecological terms is dispersal between populations, which is another common factor causing spatial synchrony and may pertain to our case as well, for example, through source-sink dynamics (Kawecki, Reference Kawecki and Gaggiotti2004). Agriculture was broadly adopted across northern parts of continental Europe, southern Scandinavia, and the British Isles ca. 6000 cal yr BP. This created an unparalleled population boom roughly synchronous to the pattern observed in the population proxies from western Finland and northern Norway. Intuitively, this might suggest that the mid-Holocene population peak in our study area relates to agricultural expansion, either directly through incoming farmers contributing to the population growth or indirectly by displacing hunter-gatherers into northern “foraging refugia,” as suggested for central Europe (Silva and Vander Linden, Reference Silva and Vander Linden2017). The direct influence of farmers is problematic, however, as solid evidence for agriculture in our study areas is significantly younger than the 6000 cal yr BP population event (Sjögren, Reference Sjögren2009, p. 707; Sjögren and Arntzen, Reference Sjögren and Arntzen2013; Lahtinen et al., Reference Lahtinen, Oinonen, Tallavaara, Walker and Rowley-Conwy2017; cf. Mökkönen, Reference Mökkönen2009). Indirect influences of agriculture are equally problematic. First, the hunter-gatherer population in northern Norway was already growing some 500 yr before agriculture was introduced to southern Scandinavia. The same pattern of pre-agricultural population growth is evident when reviewing the population reconstruction of Holocene Finland in its entirety (Tallavaara et al., Reference Tallavaara, Pesonen and Oinonen2010; Tallavaara and Seppä, Reference Tallavaara and Seppä2012). Second, displacement of hunter-gatherers from south to north would neither explain the remarkable population decline after 6000 cal yr BP or short-term declines at 8200 and 7000 cal yr BP.
In the case of observed synchronicity among human populations, an additional synchronizing factor of social interactions through trade and networks has been proposed (Freeman et al., Reference Freeman, Baggio, Robinson, Byers, Gayo, Finley, Meyer, Kelly and Anderies2018). The dissemination of improved subsistence technologies could tentatively drive synchronous demographic and adaptive strategies within our study areas. If so, cultural diffusion might facilitate the observed shift in marine exploitation regime while also contributing to population growth. This is particularly pertinent for two technological industries in the area: slate tools and early pottery.
The slate index (Fig. 3i) demonstrates strong correspondence with population dynamics in Norway. Assuming that slate tools are superior in marine resource processing, one might expect slate industries to be of comparable importance among the coastal population of the Finnish area. No such quantitative data set or overview for Finland currently exists. However, there are some similarities in slate technology that may suggest social networking in action between Finland and Norway (cf. Äyräpää, Reference Äyräpää1950; Huurre, Reference Huurre1983). Such is demonstrated by the long (100–150 mm) and slender (10–15 mm) Pyheensilta/Nyelv lance points occurring in both areas. A review of a large set of lance points, including a depot containing points at various stages of completion (Hesjedal et al., Reference Hesjedal, Damm, Olsen and Storli1996, p. 70), demonstrates remarkable standardization in production technique and morphometric qualities. The standardized breadth and hafting characteristics of Pyheensilta points, as well as the frequent resharpening of broken distal ends, reflect optimal characteristics for effective marine hunting. Maritime technologies are strongly associated with multicomponent and replaceable components, given the complexity of hunting on water and the need for quick replacement/repair of hunting gear—a “maintainable” characteristic within an otherwise mostly “reliable” technology (sensu Bleed, Reference Bleed1986). We therefore suggest that the Pyheensilta/Nyelv lances provide a telling example of shared marine subsistence technology. We equally maintain that the adoption of common technologies followed similar adaptive strategies to shared environmental conditions.
The other significant change with potential ramifications for the synchronous mid-Holocene population and marine boom-and-bust cycles is the introduction of ceramic technology. Ceramics dispersed throughout northern and eastern Fennoscandia around 7200 cal yr BP in the form of Early Comb Ware—concurrent with the uptake of slate technology in northern Norway. The demographic impact of ceramic technologies is, tentatively, the enhancement of the nutritional uptake of various foodstuffs through cooking, which may reduce child mortality (Jordan and Zvelebil, Reference Jordan and Zvelebil2010, p. 54). Interestingly, the beginning of pottery production in our study areas roughly coincides with the beginning of the mid-Holocene population growth and increase in marine resource use proxies, when Finnish sites (<6000 cal yr BP) are characterized by large quantities of pottery (Nuñez, Reference Nuñez1990; Pesonen and Leskinen, Reference Pesonen, Leskinen, Jordan and Zvelebil2009). Although it has been suggested that the uptake of pottery was related to the intensification of marine resources, lipid analyses of food crusts on pottery walls suggest a wide range of resources were processed in the vessels (Cramp et al., Reference Cramp, Evershed, Lavento, Halinen, Mannermaa, Oinonen, Kettunen, Perola, Onkamo and Heyd2014; Pääkkönen et al., Reference Pääkkönen, Bläuer, Evershed and Asplund2016; Papakosta and Pesonen, Reference Papakosta and Pesonen2019).
Crucially, major discrepancies in the uptake and maintenance of ceramic technologies in the area go against subsistence technologies as a causal factor in the observed synchrony. In Finland, pottery was in use throughout prehistory, despite the reduced importance of marine resources and the population decline after 5000 cal yr BP. In northern Norway, however, pottery did not disperse beyond the very easternmost region and was likely a short-lived effervescence based on the short duration and small number of ceramics recovered, with a complete lack of later Comb Ceramic phases (cf. Skandfer, Reference Skandfer2003; Hood and Helama, Reference Hood and Helama2010). There are potential functional reasons for this discrepancy, beyond the greater geographical proximity of the Finnish area to dispersive centers of ceramic technology in Eurasia. The eco-setting of western Finland, which was likely more conducive to year-round habitation, in combination with the evidently strong emphasis on estuarine/riverine fisheries, meant that populations could benefit from ceramics for bulk processing and storage. In Norway, there is to date no evidence to support surplus production of riverine/estuarine resources throughout the Stone Age (see Engelstad, Reference Engelstad1984; Renouf, Reference Renouf1986, p. 10). However, mass processing and storage through passive technologies such as preservation through air-drying of stockfish has deep roots in Norway (Perdikaris, Reference Perdikaris1999; Star et al., Reference Star, Boessenkool, Gondek, Nikulina, Hufthammer, Pampoulie and Knutsen2017). The climatic conditions required for such preservative techniques are very specific to the northern Norwegian coast and are not met in the Finnish area. Although archaeologically elusive, we see no reason why the basic innovation of leaving fish to dry by itself would not have been practiced already during the mid-Holocene. If so, the appeal of pottery may have been negligible to the Norwegian population.
We cannot exclude the effects of migration, social interactions, or cultural diffusion. It is conceivable that the adoption of new and potentially improved subsistence technologies occurring simultaneously across northern Europe contributed to the growth phase of the 6500/6000 cal yr BP population cycle. The explanatory power of subsistence technology, however, is undermined by the fact that the population decline occurred independently of changes in subsistence technologies in our study areas and by the fact that both population growth and decline phases coincided with environmental changes. We therefore believe that the observed synchronicity in the long-term population dynamics is better explained by climate-induced variability in environmental productivity acting over large areas, albeit at much larger temporal scales than typically observed in ecological research. This result is at odds with the conclusion of Freeman et al. (Reference Freeman, Baggio, Robinson, Byers, Gayo, Finley, Meyer, Kelly and Anderies2018), who found that environmental variability made no discernible impact on population synchrony. Instead, they suggested that societies dependent on organic sources of energy appear no more synchronous with solar energy fluctuations than fossil fuel–based economies. However, their conclusions are hampered by the use of sunspot data as a measure of environmental variability. Although solar energy is the primary driver of Earth's climate, the influence of solar activity cycles on climatic variability appears to be limited at best (George and Telford, Reference George and Telford2017; Schurer et al., Reference Schurer, Tett and Hegerl2014; Telford et al., Reference Telford, Rehfeld and St. George2015; Turner et al., Reference Turner, Swindles, Charman, Langdon, Morris, Booth, Parry and Nichols2016).
Instead, net primary productivity (NPP) is the crucial driver of energy availability for the immediate-return, organic economies most typical of hunter-gatherers (Tallavaara et al., Reference Tallavaara, Eronen and Luoto2018), as opposed to economies reliant on stored energy reserves (Kander et al., Reference Kander, Malanima and Warde2013). NPP is controlled by temperature and precipitation, which can be correlated across distances of up to 5000 km, but not globally (Koenig, Reference Koenig2002). Therefore, there is no justification for using any single record of climate or energy availability, such as Greenland ice cores or sunspot data, when analyzing synchrony among prehistoric populations. In addition, taphonomic loss of archaeological material must be taken into account, as the exponential-like shape prevalent across the mean trends of human proxy records may well be influenced by taphonomic processes (Surovell and Brantingham, Reference Surovell and Brantingham2007; Surovell et al., Reference Surovell, Byrd Finley, Smith, Brantingham and Kelly2009).
Consequently, Freeman et al. (Reference Freeman, Baggio, Robinson, Byers, Gayo, Finley, Meyer, Kelly and Anderies2018) do not properly address environmental variability or energy availability as a potential driver of synchrony. However, they demonstrate that spatial synchrony decreases with distance between proxy records. Importantly, the adjacent U.S. states Arizona and New Mexico could make for a convincing case in which synchrony is best explained by social interaction and cultural diffusion. However, geographical affinity also implies being subjected to similar environmental parameters. Without further investigation of archaeological and environmental records at the regional scale, spatial proximity is not in itself a sufficient condition to come to a conclusion about the causes of synchronicity. We therefore reiterate Koenig's (Reference Koenig2002, p. 288) argument, stating that “patterns of spatial autocorrelation in environmental factors should be carefully considered before concluding that synchrony in any particular system is driven by some factor beyond environmental correlation.”
Despite some indications that both foraging and early farming communities were equally susceptible to climate change (Bevan et al., Reference Bevan, Colledge, Fuller, Fyfe, Shennan and Stevens2017; Warden et al., Reference Warden, Moros, Neumann, Shennan, Timpson, Manning, Sollai, Wacker, Perner, Häusler, Leipe, Zillén, Kotilainen, Jansen, Schneider, Oeberst, Arz and Damsté2017), hunter-gatherer populations are generally assumed to be more directly controlled by NPP (Tallavaara et al., Reference Tallavaara, Eronen and Luoto2018). Still, hunter-gatherers relying on marine resources may take a hybrid form through delayed-return systems, as bulk processing and storage of energy for lean-season consumption is a common characteristic of many northern, maritime groups (Fitzhugh, Reference Fitzhugh, Max Friesen and Mason2016). Such delayed-return economies help overcome the limitations imposed by the direct consumption characterizing organic economies. Either way, the archaeological record suggests that the maritime adaptations under study could only mitigate low-amplitude annual variations and at best delay specific returns on an interannual scale. This is not sufficient to significantly boost carrying capacities or mitigate increased variation in resource abundance like modern economies, which are basically extreme delayed-return systems relying on nuclear or fossil fuels (and therefore unsuitable as a comparative case). The limited and short-term mitigation capabilities of pre-industrial economic systems in significantly delaying returns would explain the inability of the populations to avoid decline along with reduced environmental productivity before 5000 cal yr BP.
It seems that convergent cultural evolution toward more energy-consuming economies becomes important after the adoption of intensified agriculture relying on active niche construction and yielding reliable surpluses. Consequently, we suggest that intensified economies and social interaction networks have limited impact on long-term hunter-gatherer population trajectories beyond what is already proscribed by external, environmental drivers.
CONCLUSION
This paper reviewed environmental productivity in relation to subsistence strategies in aquatic settings to unpack the drivers of synchrony between separate human populations. We presented a case study of two northern European subregions and demonstrated significantly synchronous trends across demographic, adaptive, and environmental parameters. Based on an evaluation of different hypotheses, we suggested that the synchronous human ecodynamic trends across Holocene coastal Fennoscandia were a result of shared variability in environmental productivity. Considering that the population trajectories of the two separate areas display remarkable synchronicity and that these follow attendant climate variability in a lockstep manner, the results lend support to the notion that changes in environmental productivity more or less directly induce changes in hunter-gatherer population size. The peak in productivity during the mid-Holocene would have drastically increased the environmental carrying capacity and so provided unprecedented human demographic growth potential. In addition, the long-term stability of the environment during the mid-Holocene may also have been a contributory factor to the observed human ecodynamics, dampening the amplitude of fluctuations that might otherwise be difficult to mitigate with short-duration, delayed-return, risk-reduction measures (Riede et al., Reference Riede, Høye, Tejsner, Veldhuis and Willerslev2018).
Our results further demonstrate that major economic changes correspond to demographic and environmental dynamics as evidenced by a suite of marine resource exploitation proxies. It is striking that both populations developed similar adaptive strategies, relying heavily on marine resources. Unpacking the causal mechanisms behind this regime shift toward intensive marine exploitation is beyond our ability at this point. The possibility that population growth was driven primarily by a change in subsistence technology, however, is undermined by the fact that the population decline apparently occurred independent of changes in subsistence technologies in our study areas and by the fact that both population growth and decline phases coincide with environmental changes. More detailed technological investigations are needed in order to properly understand these relations.
Future research should aim at establishing the extent to which the mid-Holocene productivity increase was coupled between marine and terrestrial environments and determining the human implications of a potential imbalance in marine versus terrestrial ecosystem responses to large-scale climate change. If the productivity increase was actually stronger in the marine environment, it may provide a working hypothesis as to why we observe economic, technological, and social-organizational shifts in mid-Holocene northeastern Fennoscandia. However, the paleoproductivity proxies presented here suggest a coupled response between marine and terrestrial ecosystems.
Another venue for further exploration is potential threshold effects operational in maritime adaptations. It is conceivable that the profitability of marine resource exploitation increases nonlinearly, given all its costs (high handling and initial investments), whenever marine productivity increases above some critical level. The pathways responsible for steering ocean–atmospheric interactions are multifaceted (Wunsch, Reference Wunsch2005; Yu and Weller, Reference Yu and Weller2007) and may imply more complex climatic drivers of marine productivity compared with terrestrial productivity (Bromley et al., Reference Bromley, De Saussure, Clipp and Wright1967; Behrenfeld et al., Reference Behrenfeld, O'Malley, Siegel, McClain, Sarmiento, Feldman, Milligan, Falkowski, Letelier and Boss2006; Meehl et al., Reference Meehl, Arblaster, Fasullo, Hu and Trenberth2011; Holt et al., Reference Holt, Schrum, Cannaby, Daewel, Allen, Artioli and Bopp2016; Schmitt, Reference Schmitt2018). It is necessary to identify and model various ecosystem components and thermal thresholds to evaluate this properly. Yet thresholds imply sharp changes in resource use between different system states, while our data indicate rather gradual changes in marine resource use in both areas. Identifying and distinguishing between thresholds versus gradual changes is fundamentally problematic in past ecodynamic systems due to issues of sampling and resolution. Yet the fact that the intensification and deintensification of maritime economies occurred over a multimillennial period clearly indicate gradual developmental trends.
Although a previous study found only minimal evidence for environmental variability as a cause of synchronicity (Freeman et al., Reference Freeman, Baggio, Robinson, Byers, Gayo, Finley, Meyer, Kelly and Anderies2018), the Fennoscandian archaeological record clearly demonstrates the important role of spatially correlated environmental influences—Moran effects—in creating spatial synchrony among hunter-gatherer populations. The implication is, contrary to Freeman et al. (Reference Freeman, Baggio, Robinson, Byers, Gayo, Finley, Meyer, Kelly and Anderies2018), that intensified economies and social interaction networks have limited impact on long-term hunter-gatherer population trajectories beyond what is already proscribed by external, environmental drivers.
ACKNOWLEDGMENTS
This paper benefited from input from several people. We would especially like to thank Jochen Knies at the Norwegian Geological Survey, who very generously shared and allowed us to use his Malangen Fjord paleoproductivity data. We are grateful for the opportunity to present and discuss this work at the SARG 2018 conference at Alta, Norway, which provided useful input. We thank Felix Riede, Charlotte Damm, Bryan Hood, and three anonymous reviewers for their insightful feedback. In addition, we thank the editors at Quaternary Research for generously sharing thoughtful reviews that undoubtedly helped improve this paper. This work was supported by the Stone Age Demographics project funded by the Research Council of Norway (grant no. 261760) and through a doctoral fellowship funded by UiT—The Arctic University of Norway.
MT would like to acknowledge financial support from Kone Foundation and Academy of Finland.