Crossref Citations
This article has been cited by the following publications. This list is generated based on data provided by
Crossref.
Petit, Patrice X.
Susin, Santos-Antonio
Zamzami, Naoufal
Mignotte, Bernard
and
Kroemer, Guido
1996.
Mitochondria and programmed cell death: back to the future.
FEBS Letters,
Vol. 396,
Issue. 1,
p.
7.
Bogachev, A V
Murtazina, R A
and
Skulachev, V P
1996.
H+/e- stoichiometry for NADH dehydrogenase I and dimethyl sulfoxide reductase in anaerobically grown Escherichia coli cells.
Journal of Bacteriology,
Vol. 178,
Issue. 21,
p.
6233.
Papa, Sergio
Scacco, Salvatore
Schliebs, Martina
Trappe, Jörg
and
Seibel, Peter
1996.
Mitochondrial diseases and aging.
Molecular Aspects of Medicine,
Vol. 17,
Issue. 6,
p.
511.
Skulachev, Vladimir P.
1996.
Why are mitochondria involved in apoptosis? Permeability transition pores and apoptosis as selective mechanisms to eliminate superoxide‐producing mitochondria and cell.
FEBS Letters,
Vol. 397,
Issue. 1,
p.
7.
Chernyak, Boris V.
1997.
Cyclosporin A‐sensitive release of Ca2+from mitochondria in intact thymocytes.
FEBS Letters,
Vol. 418,
Issue. 1-2,
p.
131.
Budd, Samantha L.
Castilho, Roger F.
and
Nicholls, David G.
1997.
Mitochondrial membrane potential and hydroethidine‐monitored superoxide generation in cultured cerebellar granule cells.
FEBS Letters,
Vol. 415,
Issue. 1,
p.
21.
1997.
Rabbit muscle GAPDH: non‐phosphorylating dehydrogenase activity induced by hydrogen peroxide.
FEBS Letters,
Vol. 414,
Issue. 2,
p.
247.
1997.
Generation of protonic potential by the bd‐type quinol oxidase of Azotobacter vinelandii.
FEBS Letters,
Vol. 414,
Issue. 2,
p.
369.
Starkov, Anatoly A
Simonyan, Ruben A
Dedukhova, Vera I
Mansurova, Svetlana E
Palamarchuk, Larisa A
and
Skulachev, Vladimir P
1997.
Regulation of the energy coupling in mitochondria by some steroid and thyroid hormones.
Biochimica et Biophysica Acta (BBA) - Bioenergetics,
Vol. 1318,
Issue. 1-2,
p.
173.
Popov, V.N
Simonian, R.A
Skulachev, V.P
and
Starkov, A.A
1997.
Inhibition of the alternative oxidase stimulates H2O2 production in plant mitochondria.
FEBS Letters,
Vol. 415,
Issue. 1,
p.
87.
Millar, A. Harvey
and
Day, David A.
1997.
Alternative solutions to radical problems.
Trends in Plant Science,
Vol. 2,
Issue. 8,
p.
288.
Igamberdiev, Abir U
Bykova, Natalia V
and
Gardeström, Per
1997.
Involvement of cyanide‐resistant and rotenone‐insensitive pathways of mitochondrial electron transport during oxidation of glycine in higher plants.
FEBS Letters,
Vol. 412,
Issue. 2,
p.
265.
Korshunov, Sergey S.
Skulachev, Vladimir P.
and
Starkov, Anatoly A.
1997.
High protonic potential actuates a mechanism of production of reactive oxygen species in mitochondria.
FEBS Letters,
Vol. 416,
Issue. 1,
p.
15.
Papa, S.
and
Skulachev, V. P.
1997.
Detection of Mitochondrial Diseases.
p.
305.
Krasnikov, Boris F
Kuzminova, Alevtina E
and
Zorov, Dmitry B
1997.
The Ca2+‐induced pore opening in mitochondria energized by succinate‐ferricyanide electron transport.
FEBS Letters,
Vol. 419,
Issue. 1,
p.
137.
Starkov, Anatoly A
Bloch, Dmitry A
Chernyak, Boris V
Dedukhova, Vera I
Mansurova, Svetlana E
Severina, Inna I
Simonyan, Ruben A
Vygodina, Tatyana V
and
Skulachev, Vladimir P
1997.
6-Ketocholestanol is a recoupler for mitochondria, chromatophores and cytochrome oxidase proteoliposomes.
Biochimica et Biophysica Acta (BBA) - Bioenergetics,
Vol. 1318,
Issue. 1-2,
p.
159.
Malkevitch, N.V
Dedukhova, V.I
Simonian, R.A
Skulachev, V.P
and
Starkov, A.A
1997.
Thyroxine induces cyclosporin A‐insensitive, Ca2+‐dependent reversible permeability transition pore in rat liver mitochondria.
FEBS Letters,
Vol. 412,
Issue. 1,
p.
173.
Petit, Patrice X
Goubern, Marc
Diolez, Philippe
Susin, Santos A
Zamzami, Naoufal
and
Kroemer, Guido
1998.
Disruption of the outer mitochondrial membrane as a result of large amplitude swelling: the impact of irreversible permeability transition.
FEBS Letters,
Vol. 426,
Issue. 1,
p.
111.
Nicholls, David G
and
Budd, Samantha L
1998.
Mitochondria and neuronal glutamate excitotoxicity.
Biochimica et Biophysica Acta (BBA) - Bioenergetics,
Vol. 1366,
Issue. 1-2,
p.
97.
Montal, M.
1998.
Mitochondria, glutamate neurotoxicity and the death cascade.
Biochimica et Biophysica Acta (BBA) - Bioenergetics,
Vol. 1366,
Issue. 1-2,
p.
113.
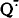 These two events should dramatically enhance non-enzymatic formation of reactive oxygen species, i.e. of
These two events should dramatically enhance non-enzymatic formation of reactive oxygen species, i.e. of 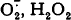 , and OHׁ, and, hence, the probability of oxidative damage to cellular components. In this paper, a concept is put forward proposing that non-phosphorylating (uncoupled or non-coupled) respiration takes part in maintenance of low levels of both O2 and the O2 reductants when phosphorylating respiration fails to do this job due to lack of ADP.
, and OHׁ, and, hence, the probability of oxidative damage to cellular components. In this paper, a concept is put forward proposing that non-phosphorylating (uncoupled or non-coupled) respiration takes part in maintenance of low levels of both O2 and the O2 reductants when phosphorylating respiration fails to do this job due to lack of ADP.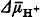 , stimulates O2 consumption and decreases the level of
, stimulates O2 consumption and decreases the level of 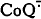 which otherwise accumulates and serves as one-electron O2 reductant. In this connection, the role of natural uncouplers (thyroid hormones), recouplers (male sex hormones and progesterone), non-specific pore in the inner mitochondrial membrane, and apoptosis, as well as of non-coupled electron transfer chains in plants and bacteria will be considered.
which otherwise accumulates and serves as one-electron O2 reductant. In this connection, the role of natural uncouplers (thyroid hormones), recouplers (male sex hormones and progesterone), non-specific pore in the inner mitochondrial membrane, and apoptosis, as well as of non-coupled electron transfer chains in plants and bacteria will be considered.

