Introduction
Although most genetic information is stored in double-stranded (dsDNA), genetic expression requires unwinding of DNA into single-stranded DNA (ssDNA). In particular, transient portions of ssDNA appear during replication, recombination or repair processes; ssDNA is more sensitive to nuclease degradation and this leads to the formation of secondary structures, which prevent previously cited processes. To solve these problems, cells need specialised ssDNA-binding proteins (SSB) that bind and stabilise ssDNA structures. These proteins usually do not share significant sequence similarity, but all contain a DNA-binding oligonucleotide binding OB fold (Su et al., Reference Su, Wang, Yagi, Shishmarev, Mason, Smith, Vandevenne, Dixon and Otting2014), consisting of a five-stranded curved β sheet capped by a helix. This specific fold is responsible not only for ssDNA binding but also often for the self-assembly of oligomeric SSB. In bacterial cells, ssDNA fragments also play crucial roles, including nucleoid and phage DNA replication or genetic recombination (Molan and Zgur Bertok, Reference Molan and Zgur Bertok2022).
In bacterial chromosome replication, ssDNA regions are necessary for initiating the process of DNA synthesis by melting the double helix, recruiting the multiprotein machinery and allowing formation of primers and replication forks (Zawilak-Pawlik et al., Reference Zawilak-Pawlik, Nowaczyk and Zakrzewska-Czerwinska2017). Therefore, proteins interacting with ssDNA and/or stabilising such DNA fragments, like the single-stranded DNA-binding protein, play important roles in facilitating the formation of nucleoprotein complexes at replication forks and maintaining their functions (Oakley, Reference Oakley2019). Bacterial proteins that interact with such DNA structures particularly affect replication of viruses, including bacteriophages, having genomes composed of ssDNA (Shulman and Davidson, Reference Shulman and Davidson2017). The transition from ss to dsDNA is a crucial step in propagation of such viruses; thus, proteins interacting with ssDNA can significantly modulate the viral replication processes.
Another process in which ssDNA is crucial is homologous recombination. DNA strand displacement and replacement occurring in this process require the formation of complexes including ssDNA and specific proteins (Piazza and Heyer, Reference Piazza and Heyer2019), and the influence of ssDNA-interacting proteins may be important in modulating recombination efficiency. The function of the bacteriophage λRed-recombination system may serve as an example of such an ssDNA-dependent process (Sharan et al., Reference Sharan, Thomason, Kuznetsov and Court2009). In summary, proteins interacting with ssDNA may influence various biological processes, and thus, one should consider that any ssDNA-binding protein might modulate chromosomal and viral replication, as well as genetic recombination.
In this work, we focused our attention on the ssDNA-binding property of the Escherichia coli Hfq protein. Hfq is an abundant protein that flexibly binds nucleic acids (NA) (Rajkowitsch and Schroeder, Reference Rajkowitsch and Schroeder2007; Vogel and Luisi, Reference Vogel and Luisi2011). Structurally, the amino-terminal region of Hfq (NTR, 65 residues) shares homologies with the Sm family of protein (Wilusz and Wilusz, Reference Wilusz and Wilusz2013) adopting the OB-like fold. Hfq-NTR is comprised of five β-strands and another Sm-proteins assembles into a cyclic oligomer to form the functional unit (Brennan and Link, Reference Brennan and Link2007). Although the mechanism by which Hfq binds NA is not completely clear, it is now established that its NTR binds RNA and DNA. Uridine-rich RNA is bound to one face of the torus called the proximal face, while the A-rich sequences bind to the opposite distal face; dsDNA is also bound on the proximal face of the ring (Supplementary Fig. S1) (Link et al., Reference Link, Valentin-Hansen and Brennan2009; Orans et al., Reference Orans, Kovach, Hoff, Horstmann and Brennan2020).
In addition to the well-characterised Sm domain, the Hfq protein also possesses a C-terminal region (CTR, 40 residues) located at the periphery of the torus (Arluison et al., Reference Arluison, Folichon, Marco, Derreumaux, Pellegrini, Seguin, Hajnsdorf and Regnier2004). Although no atomic 3D structure is known for the CTR, it has been shown to self-assemble into an amyloid structure (Fortas et al., Reference Fortas, Piccirilli, Malabirade, Militello, Trepout, Marco, Taghbalout and Arluison2015). Recently, it has been suggested that the CTR collaborates with the different RNA binding faces of Hfq, with important outcomes for some RNAs (Kavita et al., Reference Kavita, Zhang, Tai, Majdalani, Storz and Gottesman2022).
Functionally, Hfq controls a large number of bacterial functions. Among them, most are related to RNAs. Hfq was first identified as a host factor for a RNA bacteriophage but later found to play a general role in RNA metabolism (Vogel and Luisi, Reference Vogel and Luisi2011). In particular, it facilitates the pairing of small non-coding RNA (sRNA) with target mRNA, allowing gene regulation at the post-transcriptional level. Indeed, Hfq allows a tight and fast regulation of gene expression and triggers stress-relief pathways (Gottesman, Reference Gottesman2019).
Interestingly, Hfq also binds to DNA (Cech et al., Reference Cech, Szalewska-Palasz, Kubiak, Malabirade, Grange, Arluison and Wegrzyn2016) and some of the phenotypic effects appearing due to the lack of Hfq may be linked to defects in DNA-related processes. Hfq binds both linear and circular dsDNA (Takada et al., Reference Takada, Wachi, Kaidow, Takamura and Nagai1997) and a significant amount of the protein is found in the nucleoid (~20%) (Diestra et al., Reference Diestra, Cayrol, Arluison and Risco2009). Hfq binding results in the condensation of DNA through protein–protein interactions. This activity, consisting of the mediation of NA interactions and referred to as bridging, is observed for other nucleoid-associated proteins (NAPs) to form loops (Rajkowitsch and Schroeder, Reference Rajkowitsch and Schroeder2007; Wiggins et al., Reference Wiggins, Dame, Noom and Wuite2009).
The compaction of DNA by Hfq in vitro is mainly due to its CTR (Malabirade et al., Reference Malabirade, Jiang, Kubiak, Diaz-Mendoza, Liu, Van Kan, Berret, Arluison and Van Der Maarel2017a). Thus, Hfq probably plays a critical role in the architecture of the chromosome, even if this has not been established formally. If Hfq does not affect DNA supercoiling and transcription directly (Malabirade et al., Reference Malabirade, Partouche, El Hamoui, Turbant, Geinguenaud, Recouvreux, Bizien, Busi, Wien and Arluison2018), it possibly regulates them indirectly, for instance by regulating the expression of a transcriptional regulator (Majdalani et al., Reference Majdalani, Cunning, Sledjeski, Elliott and Gottesman1998).
The work reported here further explores a newly discovered property of Hfq, its ability to bind ssDNA and to drastically change ssDNA structure by promoting its alignment. Effects of Hfq on DNA transactions in which ssDNA regions are crucial have been assessed.
Methods
Details of methods can be found online as Supplementary Material.
Protein expression and purification
Wild-type (WT) and truncated Hfq forms of E. coli Hfq were purified as described previously (Taghbalout et al., Reference Taghbalout, Yang and Arluison2014; Malabirade et al., Reference Malabirade, Morgado-Brajones, Trepout, Wien, Marquez, Seguin, Marco, Velez and Arluison2017b). CTR peptide was chemically synthesised. This part of the protein cannot be purified from bacteria as it is unstable when translated alone (Taghbalout et al., Reference Taghbalout, Yang and Arluison2014). We determined that the pH ~ 5 used was the most appropriate to form the complex with DNA. Although pH 5 seems to be far from physiological conditions, it is still relevant as Gram-negative bacteria can acidify their cytosol when adapting to the vacuoles of the host macrophage, and many virulent genes belong to the Hfq regulon in these bacterial species (Kenney, Reference Kenney2019).
Fluorescence anisotropy measurements
Fluorescence anisotropy measurements were collected as described previously (Geinguenaud et al., Reference Geinguenaud, Calandrini, Teixeira, Mayer, Liquier, Lavelle and Arluison2011).
Optical microscopy of ssDNA-Hfq/CTR/NTR complexes
DNA in the single-stranded form was prepared by alkali-induced denaturation of double-stranded λ-DNA (Basak et al., Reference Basak, Liu, Qureshi, Gupta, Zhang, De Vries, Van Kan, Dheen and Van Der Maarel2019). Then, Hfq (29.8 μM) was added to the solution with concentration of one hexamer-Hfq per 200 bases. A similar procedure was used to make the ssDNA with Hfq-CTR and Hfq-NTR. However, the molar concentration of CTR used was six times higher than Hfq, that is, 6 CTR for 200 bases of DNA. For fluorescence imaging, 1 h before imaging, ssDNA was stained with YOYO-1 at a concentration of one YOYO-1 dye per four bases.
Synchrotron radiation circular and linear dichroism
Complex between dA59 and Hfq-CTR was prepared as described previously (El Hamoui et al., Reference El Hamoui, Yadav, Radiom, Wien, Berret, Van Der Maarel and Arluison2020). Synchrotron radiation circular dichroism (SRCD) measurements were carried out at DISCO/SOLEIL Synchrotron (proposal 20200007). Samples were loaded into a CaF2 circular cell (24 μm path length). Due to the origin of absorption, spectra of mixed samples could not be standardised to ∆ε and spectra are presented in mdeg maintaining the same molar ratios for all presented samples. Synchrotron radiation linear dichroism (SRLD) measurements were carried out in the same cell by collecting triplicates every 90° from 0 to 270°. For the data acquisition, the modulator phase was set to λ× 0.608 doubling the lock-in amplifier frequency in order to measure only LD absorption.
Couette flow SRLD
Couette flow SRLD measurements were performed at the AU-CD beamline on the ASTRID2 Synchrotron (proposal ISA-21-102), as detailed in Wien et al. (Reference Wien, Martinez, Le Brun, Jones, Vronning Hoffmann, Waeytens, Berbon, Habenstein and Arluison2019). Due to the much larger path length of the Couette flow cell used for SRLD measurements (0.5 mm) compared to the path length used for SRCD measurements (0.024 mm), the complex between CTR and the dA59 had a very strong LD signal under flow conditions; the samples were diluted (1/36) compared to the concentrations used for SRCD.
Fourier transform infrared spectroscopy
For Fourier transform infrared (FTIR) analysis, the same solutions used for SRCD analysis were lyophilised and re-dissolved in D2O (5 μL). FTIR measurements were performed as detailed in Geinguenaud et al. (Reference Geinguenaud, Calandrini, Teixeira, Mayer, Liquier, Lavelle and Arluison2011).
Transmission electron microscopy imaging
3.65 nM circular ssDNA molecules extracted from ΦX174 virions was incubated with 100 nM CTR in Tris–HCl 10 mM pH 7.5 EDTA 1 mM for 10 min at 20 °C. 5 μl was then deposited onto positively functionalised grids covered with a thin carbon film (Beloin et al., Reference Beloin, Jeusset, Revet, Mirambeau, Le Hegarat and Le Cam2003). Grids were washed with aqueous 2% (w/v) uranyl acetate, dried and observed in annular darkfield mode using a Zeiss 902 EM. Veletta CCD camera is controlled by iTEM software (Olympus Soft Imaging).
E. coli strains and bacteriophages
E. coli WT strain MG1655 (Jensen, Reference Jensen1993) was used as the hfq + positive control. Its Δhfq and ΔCTR derivatives were constructed as described in Gaffke et al. (Reference Gaffke, Kubiak, Cyske and Wegrzyn2021). Quantification of Hfq and its CTR-truncated form was by Western blotting and confirmed by dot blotting. For propagation of bacteriophage M13, hfq +, Δhfq and ΔCTR derivatives of E. coli strain Hfr3000 (Bachmann, Reference Bachmann1972) were constructed by P1 transduction. Bacteriophages M13 (Salivar et al., Reference Salivar, Tzagoloff and Pratt1964), λcI857S7(am) (Goldberg and Howe, Reference Goldberg and Howe1969), called λS(am) in this work, and λb519imm21susP (Wegrzyn et al., Reference Wegrzyn, Wegrzyn, Konieczny, Bielawski, Konopa, Obuchowski, Helinski and Taylor1995), called λP(am) in this work, were used. E. coli strain TAP90 (Patterson and Dean, Reference Patterson and Dean1987) was used for propagation and titration of phages λcI857S7(am) and λb519imm21susP.
Bacteriophage M13 development and efficiency of phage λ recombination
Development of phage M13 phage and λ recombination efficiency were tested according to the previously described method (Mosberg et al., Reference Mosberg, Lajoie and Church2010).
Results
Hfq binds, coats and spreads ssDNA
The potential of Hfq to bind to ssDNA was investigated. We chose (dA)n sequences because Hfq has the highest affinity for A-rich sequences (Folichon et al., Reference Folichon, Arluison, Pellegrini, Huntzinger, Regnier and Hajnsdorf2003; Geinguenaud et al., Reference Geinguenaud, Calandrini, Teixeira, Mayer, Liquier, Lavelle and Arluison2011). Indeed, A-tracts are over-represented and distributed throughout the E. coli genome in phased A-repetitions (~ 12 nucleotides), organised in approximately 100 nucleotides-long clusters (Tolstorukov et al., Reference Tolstorukov, Virnik, Adhya and Zhurkin2005). Titrations of dA7, dA20 and dA59 with Hfq gave Kd values of 3.5 ± 0.2 μM, 183 ± 8 nM and 166 ± 16 nM, respectively (Supplementary Fig. S2). A weaker affinity was measured for the CTR and dA59, about 4.2 ± 0.3 μM, thus suggesting that Hfq can bind dA59 using both its NTR and CTR regions, as previously observed for longer dsDNA (Jiang et al., Reference Jiang, Zhang, Guttula, Liu, Van Kan, Lavelle, Kubiak, Malabirade, Lapp, Arluison and Van Der Maarel2015).
The coating of ssDNA by Hfq and its truncated forms was then tested. For this purpose, different approaches have been used. First DNA in its single-stranded form was prepared by alkali-induced denaturation of λ dsDNA. The ssDNA molecules were subsequently complexed with the protein following a buffer exchange according to the method previously reported (Basak et al., Reference Basak, Liu, Qureshi, Gupta, Zhang, De Vries, Van Kan, Dheen and Van Der Maarel2019). Prior to imaging using fluorescence microscopy, the complexes were stained with YOYO-1 dye. The fluorescence intensity is relatively weak, because for ssDNA the dye is side-bound and cannot intercalate. Two different types of experiments have been done: stretched on a surface combed and confined in a nanochannel. First, the coated molecules were analysed on a flat surface (Allemand et al., Reference Allemand, Bensimon, Jullien, Bensimon and Croquette1997). The images are shown in Fig. 1a; the corresponding measured average stretches are also presented. As shown, the results are almost identical for Hfq, CTR or NTR. The ssDNA molecules are combed to a similar length of 25 ± 2 μm (0.52 nm/base), irrespective of the protein or its truncated forms. This confirms that both the NTR and CTR bind and coat ssDNA. A second type of experiment was done using a nanofluidic device to mimic a confined environment. In this experiment, ssDNA coated with Hfq was brought inside a rectangular channel with a cross-sectional diameter of 125 nm using an electric field. Once the complexes are inside the channel, they were allowed to relax for 2 min before imaging. The stretch of the complexes in the longitudinal direction of the channel was measured and is shown in the histogram in Fig. 1b. The corresponding histogram pertaining to the combing experiment has also been included. The mean stretch of the nano-confined complexes was found to be 15 ± 2 μm. In a rigid origami structure, ssDNA takes a maximal stretch 0.68 ± 0.02 nm/base (Roth et al, Reference Roth, Glick Azaria, Girshevitz, Bitler and Garini2018). Accordingly, the stretch per unit contour length (relative stretch) of the complexes inside the channel amounts 0.46 ± 0.06. In the case of naked dsDNA confined to the same channel, the relative stretch was reported to be 0.528 ± 0.005 (Yadav et al., Reference Yadav, Rosencrans, Basak, Van Kan and Van Der Maarel2020). It must be emphasised that bare ssDNA without protein coating is notoriously difficult to linearise with molecular combing and cannot be stretched inside a nanochannel due to strong intramolecular hybridisation. We conclude that the relative stretches of ssDNA coated with full-length Hfq and naked dsDNA confined to the 125 nm channel are similar, which indicates that the bending rigidity (persistence length) of the coated ssDNA filament is about the same as that of bare dsDNA. In contrast, previous reports with another method allowing imaging of naked ssDNA pointed to lower values with a wide variation for ssDNA extension, that is, from 0.18 nm/base to 0.36 nm/base (Hansma et al., Reference Hansma, Sinsheimer, Li and Hansma1992; Woolley and Kelly, Reference Woolley and Kelly2001), thus ssDNA extension by Hfq and its CTR (0.52 nm/base) is significantly more. Extension of ssDNA by Hfq and/or its CTR also exceeds the extension following coating of ssDNA with a cationic-neutral polypeptide copolymer (0.32 nm/base; Basak et al., Reference Basak, Liu, Qureshi, Gupta, Zhang, De Vries, Van Kan, Dheen and Van Der Maarel2019). Note that in these experiments, the coated ssDNA molecules are aligned through the application of flow (combing) or confinement to a long and narrow channel. Although some intermolecular aggregation was observed, these aggregates generally break up due to the alignment procedure. Quantitative information regarding intermolecular bridging could, hence, not be obtained from these essentially single-molecule stretching experiments.

Fig. 1. ssDNA coating by Hfq. (a) Montages of ssDNA coated with Hfq or its truncated forms. Molecules are stained with YOYO-1 and stretched on the flat surface by molecular combing. Scale bar 5 μm. The corresponding measured extension for combed molecules is also given. Note that the molar concentration of CTR is kept six times higher than hexameric-HFq to maintain the stoichiometric ratio. (b) Histogram of ssDNA molecules imaged in combing (orange, 28 molecules) and inside nanofluidic channel (blue, 39 molecules). The average extension for the combing experiment is 25 ± 2 μm and in the nanofluidic channel is 15 ± 2 μm. (c) TEM evidence of Hfq-ssDNA binding. ΦX174 ssDNA virions were incubated in the presence of CTR. Before incubation, virions ssDNA is difficult to visualise (sub-panel a1). CTR binding to ssDNA ΦX174 allows the spreading of some region of the DNA, while others are strongly bridged (sub-panels b1 and b2). In this case, the CTR causes the association of several ΦX174 that cannot be differentiated and the length of the viral DNA cannot be measured. Scale bars: 200 nm.
The effect of Hfq-CTR was then confirmed with Transmission electron microscopy (TEM). This experiment was done with circular viral DNA (single-stranded) and not linear molecules (Fig. 1c). Circular ssDNA molecules were deposited on a carbon surface previously functionalised with positive charges and positively stained. TEM clearly confirms the capability of CTR to spread some regions of the viral DNA in conjunction with folding of other regions by intra- or intermolecular bridging interaction (Fig. 1c, sub-panels b1 and b2). Note that the relative alignment, parallel or antiparallel, of ssDNA portions in the bridged regions cannot be determined.
Hfq changes ssDNA structure and allows DNA alignment
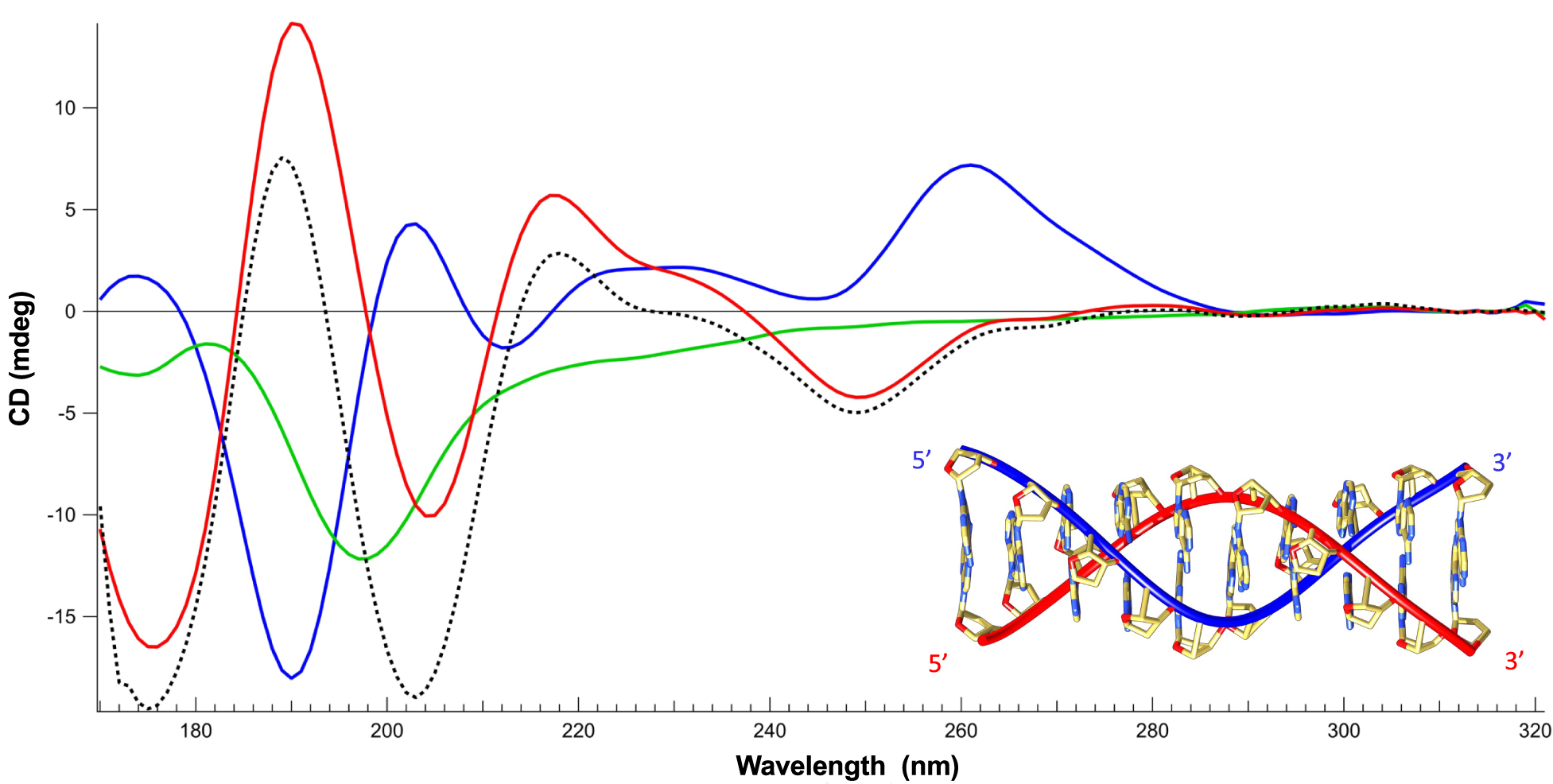
Next, the effect of Hfq-CTR on ssDNA has been investigated using SRCD. We identified significant spectral changes over the whole spectrum (Fig. 2). Assuming that the protein is restructuring (amyloid formation) upon DNA binding (Malabirade et al., Reference Malabirade, Partouche, El Hamoui, Turbant, Geinguenaud, Recouvreux, Bizien, Busi, Wien and Arluison2018), we also identified that the ssDNA structure changes. We note a positive band at 180 nm and a negative band at 190 nm both indicative of left-handed NA (Wien et al., Reference Wien, Geinguenaud, Grange and Arluison2021). In contrast, a positive band around 185 nm and a negative one between 200 and 210 nm are indicative of right-handed NAs (Wien et al., Reference Wien, Geinguenaud, Grange and Arluison2021). The spectral change identified could be correlated with base-tilting of A-rich sequences (Edmondson and Johnson, Reference Edmondson and Johnson1985; Wien et al., Reference Wien, Martinez, Le Brun, Jones, Vronning Hoffmann, Waeytens, Berbon, Habenstein and Arluison2019). Furthermore, the influence of Hfq on the positive CD signal around 270 nm may be influenced by base pairing and stacking (Holm et al., Reference Holm, Nielsen, Hoffmann and Nielsen2010; Wien et al., Reference Wien, Geinguenaud, Grange and Arluison2021).

Fig. 2. Structure characterisation of ssDNA complexed to Hfq-CTR by SRCD spectroscopy. Spectra of DNA in the absence (red) and presence of CTR (blue). CTR alone (green). The dotted spectrum represents the theoretical sum of individual spectra of the DNA and CTR. The measured spectrum of the complex is significantly different compared to the DNA + peptide theoretical spectra, indicating an conformational change of the complexed ssDNA. Note that the same analysis with the full-length protein was impractical due to the low solubility of the protein. Inset: model of parallel DNA (Gleghorn et al., Reference Gleghorn, Zhao, Turner and Maquat2016).
One possible explanation for the spectral inversion observed could be due to a change in helical parameters. To test this possibility, we used FTIR spectroscopy to analyse sugar puckering. When only C3’-endo sugars are present, characteristic absorption bands located at 865 and 820 cm−1 are observed, corresponding to the A-form. A contribution around 840 cm−1 is indicative of a C2’-endo conformation (B-form) (Wien et al., Reference Wien, Geinguenaud, Grange and Arluison2021). The formation of a Z-like form is excluded as it would give a triplet at 970, 951 and 925 cm−1 (Banyay et al., Reference Banyay, Sarkar and Graslund2003). The A-form would give a triplet at 977, 968 and 952 cm−1 and the B-form would give a singlet at 970 cm−1. As shown in Fig. 3a, we clearly see that the d-ribose stays in C2’-endo since we observe the typical bands near 840 and 970 cm−1. We thus conclude that ssDNA complexed to Hfq remains in B-form.
A possibility could be that the pH 5 used in our conditions could result in the formation of A+ adenine and subsequently in a parallel helix (Gleghorn et al., Reference Gleghorn, Zhao, Turner and Maquat2016). FTIR analysis of our complex confirms this hypothesis: the band at 1658 cm−1 is absent in dA59 alone, but the shift from 932 to 947 cm−1 and the net decrease of the band intensity at 1080 cm−1 show that a parallel helix is formed by dA59 when bound to CTR (Fig. 3b) (Taillandier and Liquier, Reference Taillandier and Liquier2002). This result is confirmed using SRCD as dA59 complexed to CTR exhibits a spectrum similar to that of poly(dA) parallel helix (Holm et al., Reference Holm, Munksgaard Nielsen, Vronning Hoffmann and Brondsted Nielsen2012). We thus conclude the CTR induces the formation of ds parallel helix from ss dA59. This is not the case for ssDNA alone; thus, the CTR promotes the formation of this parallel helix.

Fig. 3. (a) FTIR transmission spectrum of ssDNA in the presence of Hfq-CTR. The ribose stays in C2’-endo since we observe the typical bands at 840 and 970 cm−1. (b) FTIR transmission spectrum of ssDNA in the presence of CTR. The band observed at 1658 cm−1, absent in dA59 alone, the shift from 932 to 947 cm−1 and the net decrease of the band intensity at 1089 cm−1 indicates that a parallel helix is formed by dA59 when bound to CTR.
Next, as the formation of such a parallel helix could result in the alignment of ssDNA, we analysed the possible alignment of the ssDNA molecule by CTR. Indeed, we previously observed that the CTR amyloid region has a propensity to align DNA (Wien et al., Reference Wien, Martinez, Le Brun, Jones, Vronning Hoffmann, Waeytens, Berbon, Habenstein and Arluison2019). As shown in Fig. 4, a clear alignment by the CTR:ssDNA complex can also be observed under Couette flow conditions. The alignment without rotation in a flow cell was also confirmed by SRLD using a CD cell and a CD cell rotation chamber (Fig. 4c). Alignment of the complex in the CD sample may occur upon loading between short path lengths; hence, linear dichroism (LD) signals emerge. These signals, if strong enough, spill into the CD signal via LD-CD cross talk (Sutherland, Reference Sutherland, Wallace and Janes2009) and distort the CD spectrum. In order to eliminate the LD contribution acquisition of CD spectra at four angles (0°-90°-180°-270°) were averaged.

Fig.4. LD signal (a) and absorbance spectra (b) of the complex dA59:CTR (blue), dA59 (red) and Hfq-CTR (green). Spectra were measured with a sample path length of 0.5 mm and rotation speed of the Couette flow cell of 3000 rpm. (c) SRLD analysis of the same complex measured in a classic 0.024 mm path length cell, rotating the cell holder every 90°. The overall shape of the spectra is conserved with maxima and minima in the same positions compared to (a). Amplitude differences are most likely due to differences of the experiments, with a less perfect alignment of the sample in the classic cell.
Shown in Fig. 4a are the SRLD spectra of CTR, ssDNA and finally of the complex CTR:dA59. The LD signal is effectively zero for both Hfq-CTR and ssDNA compared to the very strong LD signal of the complex. This shows that although the two components do not align under flow conditions, the complex readily aligns. In contrast to the LD signal observed for dsDNA complexes with Hfq-CTR (Wien et al., Reference Wien, Martinez, Le Brun, Jones, Vronning Hoffmann, Waeytens, Berbon, Habenstein and Arluison2019), we observe that the LD signal is positive for all wavelengths including the wavelength range from 240 to 300 nm where the DNA signal dominates. This leads to a surprising conclusion: where long DNA normally aligns along the flow direction giving rise to negative LD signals (Dicko et al., Reference Dicko, Hicks, Dafforn, Vollrath, Rodger and Hoffmann2008), the parallel dsDNA formed by dA59 are incorporated into the polymers of the complex in such a way that they are aligned perpendicular to the length of the amyloid strand. The positive signal at 195 nm is in agreement with the β-sheet secondary structure aligned perpendicular to the amyloid fibrils and thus the flow (Wien et al., Reference Wien, Martinez, Le Brun, Jones, Vronning Hoffmann, Waeytens, Berbon, Habenstein and Arluison2019) since β-strands have a transition dipole moment near 195 nm aligned perpendicular to the direction of the strand (Nordén et al., Reference Nordén, Rodger and Daffron2010).
In conclusion, our results clearly show that in vitro the Hfq protein and in particular its CTR amyloid region are able to bind to ssDNA, cover, spread and bridge it, and to allow the alignment of ssDNA molecules. Because ssDNA is an essential intermediate in many DNA metabolic processes, we then analysed the effect of Hfq in vivo for two processes where ssDNA is formed. Hfq could indeed be part of the ssDNA-binding SSB protein family in prokaryotic cells, which bind to ssDNA, stabilising and protecting intermediate states of DNA recombination and replication.
This could particularly be the case for A-rich sequences found throughout the E. coli genome and when pH decreases during host infection (Tolstorukov et al., Reference Tolstorukov, Virnik, Adhya and Zhurkin2005; Kenney, Reference Kenney2019). Furthermore, it could prevent ssDNA chemical attack or be involved in removal of secondary structures that could impair ssDNA-related processes (Molan and Zgur Bertok, Reference Molan and Zgur Bertok2022).
Hfq influences replication of bacteriophage M13 and genetic recombination
To evaluate if Hfq influences biological processes in which ssDNA regions are involved, we have tested efficiencies of M13-bacteriophage replication which has an ssDNA genome and genetic recombination between genomes of λ bacteriophage (Red-recombination system).
When testing development of the M13 bacteriophage, we found that its efficiency is significantly more effective in the Δhfq mutant relative to WT control (Fig. 5a). Since effects observed in mutants completely devoid of Hfq might be caused by the absence of various activities of this protein, we repeated these experiments using a mutant with deletion of the Hfq CTR while leaving intact the NTR. In such a mutant, replication of the M13 phage was significantly less efficient relative to the WT counterpart, as shown by slower phage development and lower burst size (Fig. 5a).

Fig. 5. (a) Development of bacteriophage M13 in E. coli. The presented results indicate mean values from three experiments with error bars indicating SD. Symbols (#) and (*) indicate statistically significant differences (p < 0.05 in the t-test) between results obtained for hfq + and Δhfq, and hfq + and ΔCTR, respectively. (b) Efficiency of recombination between λ bacteriophage genomes in E. coli. The presented results show mean values from three experiments with error bars indicating SD. Value of 100% represents fraction of λP +S + recombinants appearing after infection of the hfq + host cells with λcI857S7(am) and λb519imm21susP phages which was equal to 0.17 ± 0.04%. Symbols (*) indicate statistically significant differences (p < 0.05 in the t-test) between results obtained for hfq + and either Δhfq or ΔCTR hosts.
To test the efficiency of genetic recombination, we have employed λ bacteriophage mutants. Nonsense (amber, or shortly am) λ mutants in genes P or S can propagate only in suppressor E. coli hosts (supE or supF, respectively) but not in WT bacteria. When WT, ∆hfq or ∆CTR cells were infected simultaneously with both phage mutants, Red-recombination could occur. Culture growth was timely stopped and phage lysates were prepared after one single lytic development cycle was reached. Thus, phage progeny contained parental phages and recombinants, including λP(am)S(am) and λP +S + variants. The latter phages could be easily distinguished from parental viruses because of their ability to form plaques on the WT host. By comparing phage titers on WT and supE supF strains, we found that the fraction of recombinant λP +S + phages after propagation in control (hfq +) cells was equal to 0.17 ± 0.04%, which was significantly higher than fractions of revertants (spontaneous P + and S + reverse mutants, which appear among mutant phages), achieving a frequency of 0.001% and < 0.0001% for λP(am) and λS(am), respectively. When testing efficiency of recombination in Δhfq and ΔCTR hosts, evident influence of the absence of either whole Hfq or its CTR could be observed. Interestingly, the fraction of λP +S + recombinants was about 2-fold lower after propagation of phages in the Δhfq host than in WT bacteria, while estimated efficiency of recombination was over two times higher in the ΔCTR host relative to the WT counterpart (Fig. 5b). Therefore, a lack of Hfq results in λ phage recombination inhibition, while in the presence of only Hfq-NTR, this process is more efficient in E. coli.
As the absence of the CTR could influence Hfq stability and its abundance (Arluison et al., Reference Arluison, Folichon, Marco, Derreumaux, Pellegrini, Seguin, Hajnsdorf and Regnier2004), quantification of Hfq and its CTR-truncated form was performed; no significant differences were observed (Gaffke et al., Reference Gaffke, Kubiak, Cyske and Wegrzyn2021). Therefore, we conclude that differences in efficiencies of phage M13 replication and phage λ genetic recombination between WT bacteria and hfq mutants arise from dysfunctions of Hfq in the mutant cells.
Discussion
The results presented in this paper show that, in addition to its role in RNA metabolism, Hfq binds to ssDNA and may therefore play an important role in genetic processes involving ssDNA, including recombination and replication. In vivo, the Hfq protein has been discovered as a host factor for bacteriophage replication (Franze de Fernandez et al., Reference Franze De Fernandez, Hayward and August1972). Further studies indicated then that this protein mediates various RNA transactions (Vogel and Luisi, Reference Vogel and Luisi2011; Sobrero and Valverde, Reference Sobrero and Valverde2012).
Although early studies suggested that Hfq interacts with ssRNA, subsequent experiments indicated that this protein can interact with DNA (Cech et al., Reference Cech, Szalewska-Palasz, Kubiak, Malabirade, Grange, Arluison and Wegrzyn2016). In this report, we demonstrate that Hfq interacts also with ssDNA. Note that the role of Hfq in ssDNA binding and remodelling strengthens the general role of bacterial NAPs in similar processes, such as for HU and H-NS/StpA family (Shiraishi et al., Reference Shiraishi, Ogata, Hanada, Kano and Ikeda2007; Kamashev et al., Reference Kamashev, Balandina, Mazur, Arimondo and Rouviere-Yaniv2008; Yang et al., Reference Yang, Kong, Sun, Kong and Shi2019).
We show here that the ssDNA-binding properties of Hfq are mainly due to its CTR. We observe that CTR binding to ssDNA allows its alignment by forming a parallel helix. This is not the case for ssDNA alone; thus, the CTR promotes the formation of this parallel helix probably by changing the pKA of adenines.
Interestingly, Hfq-CTR has also a propensity to juxtapose two DNA molecules and this is highly suggestive of a role in modulation of efficiency of recombination and/or transformation, as it was formerly shown for another “dsDNA bridging/ssDNA binding” protein, DprA (Mortier-Barriere et al., Reference Mortier-Barriere, Velten, Dupaigne, Mirouze, Pietrement, Mcgovern, Fichant, Martin, Noirot, Le Cam, Polard and Claverys2007). To test if such interaction can play a physiological role we have performed efficiency assays for processes that require ssDNA. Our finding that two processes are affected, either positively (M13 replication) or negatively (λ recombination) in the Δhfq mutant might suggest that this is the case. However, any secondary effects of Hfq-mediated changes in regulatory RNA functions could not be excluded in such experimental systems. Therefore, we have also used the ΔCTR mutant in which only the NTR of Hfq is present. Surprisingly, we found that effects of the ΔCTR mutation on both M13 replication and λ recombination are more pronounced than in the case of Δhfq, and opposite to those observed in cells completely lacking Hfq. These results corroborate the conclusion that ssDNA binding by Hfq has a physiological relevance. We have also demonstrated that in vivo Hfq-CTR stimulates replication of bacteriophage M13 genome, while inhibiting Red recombination. We propose that Hfq-mediated stimulation of M13-replication might relate to changes in ssDNA conformation facilitating alignment of ssDNA in parallel helices, and then the formation of the replication complex, similarly to the mechanism occurring in the Qβ bacteriophage replication. On the other hand, covering ssDNA regions by Hfq during genetic recombination may impair this process due to lower availability of recombining sequences during the exchange process of strands between two DNA molecules.
Recombination efficiency is also controlled by Hfq-CTR which inhibits recombination. Mutant Δhfq cells lacking both the NTR and CTR regions recombine less efficiently in contrast to only CTR deficient cells. WT cells containing Hfq are not substantially more efficient in recombination than Δhfq. Therefore, we hypothesise that effects of the absence of CTR in Δhfq cells might be hidden due to the inability of the NTR to perform its functions. This includes interactions with sRNA and ultimately regulation of gene expression. This definitely makes Hfq an important player to consider in bacterial chromosome structure and gene expression control.
Open Peer Review
To view the open peer review materials for this article, please visit http://doi.org/10.1017/qrd.2022.15.
Acknowledgements
We are very grateful to C. Lavelle (MNHN, Paris), P. Dupaigne (IGR, Villejuif, France) and O. Pietrement (U. of Dijon, France) for their contribution to TEM measurements at an early stage of this work and for many fruitful discussions.
Authors’ contributions
K.K. constructed plasmids and E. coli mutant strains and performed in vivo experiments (replication and recombination assessment). J.v.d.M. and I.Y. did optical microscopy of ssDNA complexes. F.W., V.A., N.C.J. and S.V.H. did SRCD, SRLD and OCD measurement and analysis. A.C. and E.L.C. did TEM measurements. F.G. did FTIR measurements. G.W. and V.A. concepted the study and supervised the work. All authors participated in the design, interpretation of the studies and analysis of the data and review of the manuscript.
Supplementary Materials
To view supplementary material for this article, please visit http://doi.org/10.1017/qrd.2022.15.
Financial support
This research was supported by Singapore Ministry of Education Academic Research Fund (Tier 1 R-144-000-451-114 and Tier 2 MOE-T2EP50121–0003 grants), National Science Center (Poland) (2016/21/N/NZ1/02850 to KK) and University of Gdansk (531-D020-D242–21 to GW). SRCD measurements on DISCO beamline at the SOLEIL Synchrotron were performed under proposal 20200007. SRLD measurements on ASTRID2 synchrotron radiation facility (Aarhus University, Denmark) were performed under proposal ISA-21-102. Campus France is gratefully acknowledged for their financial support of this work through PHC Polonium with Poland 27701VG. This study contributes to the IdEx Université de Paris ANR-18-IDEX-0001. This work was supported by a public grant overseen by the French National research Agency (ANR) as part of the ‘Investissement d’Avenir’ program, through the ‘ADI 2019’ project funded by the IDEX Paris-Saclay, ANR-11-IDEX-0003-02.
Conflict of interest
The authors declare no potential conflicts of interest with respect to the research, authorship and/or publication of this article.








Comments
Comments to Author: 1. The general reader would benefit from the inclusion of drawings (a new Figure 1?) summarizing the structure of the Hfq hexamer while in contact with nucleic acids. This would allow the contributions of the NTR and the CTR to nucleic acid interaction to be appreciated more easily.
2. Lines 80-81. In order to provide a more comprehensive, comparative survey of Hfq and other RNA binding proteins from Escherichia coli, please cite Rajkowitsch L and Schroeder R (2007) Dissecting RNA chaperone activity. RNA 13: 2053-2060.
3. Lines 107-109. "If Hfq does not affect DNA supercoiling and transcription directly it
4. possibly regulates them indirectly, for instance by a transcriptional regulator expression (Malabirade et al., 2018)." Please clarify the meaning of "by a transcriptional regulator expression". Do you mean: by affecting the expression of a transcription regulator?
5. Line 122. We determined that the pH~5 used was the most appropriate to form the complex with DNA. This is not the physiological pH of the bacterial cytosol (which is closer to neutral pH). Given that the CTR fragment of Hfq is being discussed, please comment on the pH discrepancy in terms of physiological relevance of the acidic pH value. The point is relevant because pathogenic strains of Gram-negative bacteria can acidify the cytosol when adapting to the vacuole of the host macrophage and many virulence genes belong to the Hfq regulon in these bacterial species.
6. Line 164. In what way is E. coli strain MG1655 an hfq+ 'variant'? Do you mean that it is a positive control for the hfq and CTR deletion mutants?
7. Lines 181-186. The selection of the 59-mer A-tract was made on the grounds of experimental convenience. It would be helpful to link this selection to the in vivo situation by citing examples of known Hfq binding regions in bacteria with this sequence. Is there even one biological example?
8. Lines 223-229. The experiments described here seem to involve Phi-X, which is described variously as a 'virion' and a 'plasmid' in the legend to Figure 1C (lines 552-557). This element was not introduced in the Materials and Methods section devoted to bacteriophage (lines 163-176). Please describe Phi-X and carefully define the terms 'virion' and 'plasmid' as used by the authors in this manuscript.
9. Lines 220-229. Intra- and intermolecular bridging is introduced. This topic should be summarized in the Introduction, and the paper by Rajkowitsch & Schroeder (2007) RNA 13: 2053-2060, cited.
10. The finding that Hfq binds, coats and spreads ssDNA is perhaps not surprising (lines 180-229). However, it is interesting that the CTR and NTR do the same. The CD experiments (lines 231-290) reveal that Hfq promotes alignment of A-tracts and that these have a B-form structure (line 250).
11. The conclusions drawn in lines 283-290 about the SSB-like nature of Hfq are based on a limited number of experiments with a 59-mer A-tract studied at pH 5 in vitro. Given that Hfq has a well-established biological role as an RNA chaperone, such conclusions about its wider role(s) in the bacterium must await more experimentation. At present, it would be safer to characterize these as speculation rather than firm conclusions.
12. Lines 292-325. Biological experiments with bacteriophage revealed effects of Hfq and its CTR on molecular processes associated with lambda and M13 phage. However, no mechanistic insights have emerged so far, so we are left with just generalized observations about correlations between Hfq, its CTR and the life cycles of two phages.
13. The role of Hfq in ssDNA binding and modeling is reminiscent of the role of bacterial nucleoid-associated protein HU in similar processes (Kamashev et al. 2008. Nucleic Acids Research 36: 1026-1036). HU has roles in both RNA and DNA metabolism, in the latter case, interacting with both ds- and ssDNA. This provides an interesting point of comparison between two heavily investigated proteins involved in genome metabolism. Some commentary on this topic would add to the scholarly value of the present paper.
14. The authors have used sophisticated in vitro methods to study a difficult system. They have been very frank in describing the limits of their findings. Despite the general lack of specific, mechanistic insights at the present time, the work does provide evidence that the ssDNA binding activity of Hfq has physiological relevance at some level. Therefore, it provides a platform for deeper analysis of these molecular processes in the future.