Consumption of fruits and vegetables has been associated with low incidence and mortality rate from various degenerative diseases including CVD and cancer( Reference Genkinger, Platz and Hoffman 1 , Reference Heidemann, Schulze and Franco 2 ). One of the major factors behind the protective mechanisms attributed to fruits and vegetables is their high content of vitamins and phytochemicals with antioxidant activity, such as ascorbic acid, tocopherols, carotenoids and flavonoids. Antioxidant intake has been indicated to possess greater daily variation than macronutrient intake and may not be adequately captured by commonly used 3 d or 7 d food records (FR)( Reference Willett 3 , Reference Davis 4 ). Alternatively, FFQ can act as an economical and practical method for monitoring dietary antioxidant variation in large populations because of the relatively low participant burden and ease in data processing( Reference Andersen, Veierod and Johansson 5 ).
A number of FFQ have been developed to estimate antioxidant intake; nevertheless, most FFQ have focused solely on certain antioxidants, such as vitamin C, vitamin E, carotenoids( Reference Stiegler, Sausenthaler and Buyken 6 , Reference Cena, Roggi and Turconi 7 ), isoflavones( Reference Patterson, Kristal and Tinker 8 ) and catechins in tea( Reference Hakim, Hartz and Harris 9 ), and none of them efficiently captures the intake of vitamins and flavonoids comprehensively. Therefore, there is a need for a brief FFQ to effectively capture a wide range of antioxidant intake. Simply combining food items from previous FFQ may not be appropriate, since the resulting instrument may still lack certain important food items for certain antioxidant nutrients such as flavonoids or impose a considerable burden on participants because of its substantial detail( Reference Somerset and Johannot 10 ). A potentially useful approach to identify a comprehensive food list for assessing both vitamins and flavonoids is the use of dietary total antioxidant capacity (TAC), which can provide an integrated measurement of individual antioxidants( Reference Serafini and Del Rio 11 ). Dietary TAC has been inversely associated with the risk of CVD( Reference Puchau, Zulet and de Echavarri 12 ), gastric cancer( Reference Puchau, Zulet and de Echavarri 12 , Reference Serafini, Bellocco and Wolk 13 ) and stroke( Reference Rautiainen, Larsson and Virtamo 14 , Reference Del Rio, Agnoli and Pellegrini 15 ), supporting that it may serve as a useful tool for investigating the health effects of antioxidants. Dietary TAC estimation in a representative US population has been reported via a newly validated ‘theoretical’ approach( Reference Yang, Chung and Chung 16 ) which is not constrained by the limited food items in previously built TAC databases.
Accordingly, our research group has developed a brief FFQ to assess short-term antioxidant intake including both vitamins and flavonoids with antioxidant properties along with TAC estimates. The aims of the present study were to validate this newly developed FFQ against 30 d FR and corresponding plasma antioxidant concentrations and to test the reliability of this assessment instrument in healthy college students in Connecticut, USA.
Materials and methods
Participants and study design
A total of seventy-seven apparently healthy, non-smoking college students aged 18–25 years from the University of Connecticut in Connecticut, USA were recruited. Written informed consent was obtained from all participants. On the initial visit, a screening of anthropometric measurements (height, weight), blood pressure, lipid and glucose profile (Cholestech LDX; Cholestech Corporation, Hayward, CA, USA) and a medical history survey were performed to check the eligibility of participants. Participants who had chronic diseases such as diabetes mellitus, CVD, kidney disease, autoimmune disease, cancer and malnutrition or digestion problems were excluded. The first brief FFQ (FFQ1) was administered to eligible participants by an expert dietitian and instructions were provided for a 30 d FR. At the second visit of 30 d apart, a 12 h fasting venous blood sample was taken to determine plasma antioxidant concentrations and the second FFQ (FFQ2) was administered. In the present study, sixty volunteers were retained for the month of study with a dropout rate of 22 %.
Identification of major antioxidant food sources
Inclusion of items in the FFQ was determined by identifying the regularly consumed food sources contributing most to TAC in the American diet, which was documented in our recent publication( Reference Yang, Chung and Chung 16 ). In brief, a flavonoid/proanthocyanidin database was created based on recent US Department of Agriculture (USDA) data sets: the USDA database for the flavonoid content of selected foods (2007 update)( 17 ), the USDA–Iowa State University database on the isoflavone content of foods (2008 update)( 18 ) and the USDA proanthocyanidins database released in 2004( 19 ). To calculate the antioxidant intake from food sources, we matched the food consumption data of 8809 US adults in the National Health and Nutrition Examination Survey (NHANES) 1999–2000( 20 ) and 2001–2002( 21 ) with the flavonoid/proanthocyanidin database. Daily individual flavonoid/proanthocyanidin intake from selected foods was determined by multiplying the content of the individual flavonoids or proanthocyanidins (mg aglycone equivalent/100 g food) by the daily consumption (g/d) of the selected food item. Data on individual participants’ daily dietary intakes of antioxidant vitamins were available in the NHANES 2001–2002( 20 , 21 ). Participants in the NHANES were questioned specifically about their use of vitamin and mineral supplements. To determine TAC scores of food items or dietary supplements, a ‘theoretical’ method was used in the present study( Reference Yang, Chung and Chung 16 ). The antioxidant power of individual antioxidant nutrients, expressed as vitamin C equivalent (VCE) measured by 2,2′-azino-bis-3-ethylbenzthiazoline-6-sulfonic acid (ABTS) assay, was documented in our previous study( Reference Floegel, Kim and Chung 22 ). Concisely, antioxidant capacities of forty-three major antioxidant nutrients were measured by the ABTS assay conducted according to Kim et al. ( Reference Kim, Chun and Kim 23 , Reference Kim, Lee and Lee 24 ). These antioxidants included thirty flavonoids, four proanthocyanidins, seven carotenoids, two forms of vitamin E and vitamin C. The ABTS assay utilizes quantitative concepts in reference to the familiar vitamin C to measure both hydrophilic and lipophilic antioxidant activities, and its weight-based expression enables researchers to link weight-based food consumption data to estimate TAC( Reference Kim, Chun and Kim 23 , Reference Kim, Lee and Lee 24 ). Therefore, individual antioxidant intake from diet and supplements was determined by multiplying the content of the individual antioxidant (flavonoids, proanthocyanidins, carotenoids, vitamin C and vitamin E) by the daily consumption of each selected food item. Sum of individual antioxidant intake was then calculated by summing individual antioxidant levels from all food sources reported in NHANES and dietary supplement use. Antioxidant capacity of each antioxidant consumed daily was calculated by multiplying the consumption data of each antioxidant by its respective antioxidant capacity. TAC score from the specific foods consumed daily was assessed by summing individual antioxidant capacities. The ‘theoretical’ method was validated and applied in several previous studies( Reference Floegel, Kim and Chung 22 , Reference Wang, Yang and Lee 25 , Reference Wang, Yang and Lee 26 ). Estimation of theoretical TAC in fifty popular antioxidant-rich food items in the US diet by this approach was proved to be highly correlated with the TAC values of matching food items determined analytically by ABTS and 1,1-diphenyl-2-picrylhydrazyl assays (r = 0·83 and r = 0·70, respectively)( Reference Floegel, Kim and Chung 22 ). It was also found to be positively associated with TAC values of forty-four food items from the USDA oxygen radical absorbance capacity database (r = 0·48)( Reference Floegel, Kim and Chung 22 ). According to dietary TAC estimation of the American diet by this method, teas, dietary supplements, fruit and fruit juices, and wine were the major foods or food groups contributing to TAC based on the 24 h dietary recall( Reference Yang, Chung and Chung 16 ).
Development of the FFQ
The food list for the FFQ was extracted according to the percentage of TAC contributed by each food as follows:
The top food items contributing most to TAC in this food list were selected to cover at least 83 % of the cumulative TAC, and were translated into seventy questions. The final FFQ contained eight food groups (fifteen vegetables and vegetable products, eighteen fruits, twenty-one beverages, two breads and cereals, six dairy and eggs, four oils and seasonings, two sweets and desserts, and two others such as nuts or seeds). Since the TAC scores of vitamin C, α-tocopherol and β-carotene from dietary supplements contributed almost 25 % of the TAC from diet and supplements in Americans according to the previous study( Reference Yang, Chung and Chung 16 ), vitamin C, α-tocopherol, β-carotene and multivitamins were included in the four dietary supplement questions with dosage information nested. Because limited information is available on supplementary flavonoid composition and the flavonoid intake from supplements was documented to be less than 2 % in US adults( Reference Yang, Chung and Chung 16 , Reference Chun, Floegel and Chung 27 ), flavonoid intake from supplements was not included. Food frequency was coded as daily, weekly and monthly, and from 0 to 7 occasions such as none, once monthly and daily, and the FFQ was intended to cover the previous month's consumption of food and supplements. Portion sizes were estimated using three different scales (small, medium and large). Photographic figures for a medium serving size were included to illustrate the portion size.
Estimation of antioxidant intake and total antioxidant capacity from the FFQ
To calculate antioxidant intake and TAC from data collected by the FFQ, a ‘medium’ serving size of each food item in the FFQ was set as one ‘unit’ and a ‘unit’ antioxidant database was created through combining the dietary nutrient profile for individual food items from the Nutrition Data System for Research (NDSR) software release 2010 (University of Minnesota, Minneapolis, MN, USA) with the Flavonoid and Proanthocyanidin Provisional Table developed by the Nutrition Coordinating Center (NCC; University of Minnesota). This NCC provisional table provided a way for NDSR users to link the USDA data with NDSR data via NDSR food identification numbers( 28 ). Frequency of intake on the FFQ was converted proportionally to daily units. Consequently, the daily antioxidant intake from food was calculated as follows:
Vitamin C, α-tocopherol and β-carotene intakes from dietary supplements were determined from the addition of single-nutrient supplements and from multivitamin use. The default dietary supplements database in NHANES 2007–2008 was applied to explore the strength of the supplements( 29 ). The mean dose of multivitamins per day was calculated by the frequency of intake. TAC from food or from dietary supplements was obtained by multiplying the daily antioxidant intake from food or dietary supplements by the individual antioxidant capacity analysed through ABTS assay.
30 d Food records
The 30 d FR was chosen as the reference method because it is reliable in measuring antioxidant intake with a high day-to-day variation( Reference Willett 3 ) and because its measurement errors are usually not correlated with those in FFQ( Reference Marks, Hughes and van der Pols 30 ). The study dietitian trained the participants to include all foods, beverages and dietary supplements consumed during the thirty consecutive days and reviewed the records to check for errors or omissions every day. Dietary intake data were collected and analysed by using the NDSR and the NCC Flavonoid and Proanthocyanidin Provisional Table. Dietary supplement data were estimated through NDSR updated with an NCC enhanced version of the NHANES Dietary Supplement Database 2007–2008( 28 ). TAC from diet and TAC from supplements were obtained by multiplying antioxidant profiles by the antioxidant capacities. Antioxidants estimated from 30 d FR were divided by 30 to generate daily average intake data.
Plasma antioxidant and total antioxidant capacity measurement
A 12 h fasting blood sample for plasma antioxidant analysis was collected in Vacutainer tubes containing heparin sodium at the second visit. Samples were centrifuged immediately at 3000 g for 10 min at 4°C. Plasma was separated and stored at −80°C until further measurements. Plasma vitamin C and uric acid were measured on deproteinized plasma by HPLC with UV detection as described by Ross( Reference Ross 31 ). In order to preserve vitamin C, an aliquot of plasma was deproteinized with 10 % (w/w) perchloric acid. This sample was then centrifuged (15 000 g , 5 min, 4°C) and the supernatant was kept at –80°C until analysis. Plasma α-tocopherol and γ-tocopherol were analysed using HPLC( Reference Leonard, Bruno and Paterson 32 ). The slightly modified method described by Karppi et al. ( Reference Karppia, Nurmia and Olmedilla-Alonsob 33 ) was used for carotenoid analyses. Plasma TAC was determined by the ABTS assay developed by van den Berg et al. ( Reference van den Berg, Haenen and van den Berg 34 ) and modified by Kim et al. ( Reference Kim, Chun and Kim 23 , Reference Kim, Lee and Lee 24 ). Lipid profiles including total cholesterol, TAG and glucose were measured using the Cobas C111 analyser (Roche Diagnostics, Indianapolis, IN, USA).
Statistical analyses
All statistical analyses were carried out with the SAS statistical software package version 9·2. Descriptive statistics were computed to describe sociodemographic characteristics, daily antioxidant intakes estimated from the 30 d FR and the two FFQ, daily TAC from food and supplements, plasma antioxidant/TAC levels and lipid profiles. Antioxidant intakes, TAC from diet and TAC from both diet and supplements were reported in the same units as the 30 d FR and FFQ. Crude data from the FFQ and 30 d FR were not log-transformed since the distribution test through residual and goodness-of-fit analyses did not show improvement of normality. Spearman rank correlation coefficients were calculated between the dietary nutrients estimated from FFQ2 and the 30 d FR (validity) and between dietary nutrients estimated from FFQ1 and FFQ2 (test–retest reliability). Spearman rank correlation coefficients were also examined between the respective plasma and dietary nutrients after adjusting for age, BMI, ethnicity, gender and (except for vitamin C) plasma cholesterol and plasma TAG. Attenuated correlation coefficients were not corrected given that a relatively large repetitive FR was collected( Reference Willett 3 ). Percentage agreement was assessed by calculating the percentages of participants classified into the same or adjacent tertiles of antioxidant intake by the 30 d FR and FFQ2 or by FFQ1 and FFQ2. Misclassification was reported as the percentage of participants categorized into the extreme opposite tertiles. Significance was set to a value of P < 0·05 for two-sided testing.
Ethical approval
The study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects were approved by the University of Connecticut Institutional Review Board.
Results
The majority of college students who participated in the present study were non-Hispanic white (Table 1). Fifteen participants reported taking a daily multivitamin. Eight students took a daily supplement containing vitamin C only and none of the students reported ever taking supplements with either vitamin E only or β-carotene only. The levels of plasma α-tocopherol, carotenoids (α-carotene, β-carotene, β-cryptoxanthin, lutein+zeaxanthin, lycopene) and TAC are also shown in Table 1.
Table 1 Demographic characteristics and plasma antioxidant concentrations: college students aged 18–25 years who completed a 30 d FR and two FFQ (n 60), Connecticut, USA
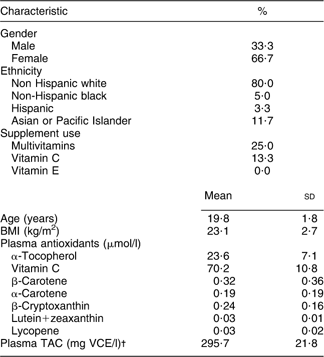
30 d FR, 30 d food record; TAC, total antioxidant capacity; VCE, vitamin C equivalent; ABTS, 2,2′-azino-bis-3-ethylbenzthiazoline-6-sulfonic acid.
†Plasma TAC was measured by ABTS assay and expressed as mg VCE/l.
Daily antioxidant intakes
Mean total energy intake was 8694 (sd 2791) kJ/d (2078 (sd 667) kcal/d) based on the 30 d FR (Table 2). Daily consumption of vitamin C, vitamin E, carotenoids, flavonoids and proanthocyanidins estimated from FFQ2 accounted for 78·0 % (78·5 % including vitamin C supplements), 46·7 % (53·6 % including vitamin E supplements), 79·9 %, 111·2 % and 30·1 % of those estimated from the 30 d FR, respectively. TAC from diet and TAC from both diet and supplements estimated by FFQ2 were almost equal to those estimated from the 30 d FR (104·8 % and 102·8 %, respectively; Table 2).
Table 2 Daily dietary antioxidant intakes and TAC estimated from the 30 d FR and FFQ2: college students aged 18–25 years who completed a 30 d FR and two FFQ (n 60), Connecticut, USA

30 d FR, 30 d food record; TAC, total antioxidant capacity; VCE, vitamin C equivalent.
†‘Diet+supplement’ means the antioxidants were estimated from both food and dietary supplements.
Validity
To test the validity, daily antioxidant intakes or TAC values from diet and from diet and supplements estimated from FFQ2 were compared with those obtained from the 30 d FR (Table 3). Spearman rank correlations to estimate validity fell within the range of 0·29 to 0·80 (P < 0·05) except for γ-tocopherol and β-cryptoxanthin, while TAC from diet and TAC from diet and supplements were highly correlated between FFQ2 and the 30 d FR (r = 0·67 and 0·71, respectively; P < 0·0001). The proportion of participants categorized in the same or adjacent tertile of dietary intake averaged 53 % or 37 %, respectively (Table 3). Therefore, on the basis of all antioxidants, on average 9 % were misclassified into the opposite tertiles.
Table 3 Spearman rank correlation coefficients and cross-classification between FFQ2 and the 30 d FR (validity) and between FFQ2 and FFQ1 (reliability): college students aged 18–25 years who completed a 30 d FR and two FFQs (n 60), Connecticut, USAFootnote †

30 d FR, 30 d food record; TAC, total antioxidant capacity.
Spearman's correlation coefficient between antioxidant intakes estimated from 30 d FR and FFQ2, or from FFQ1 and FFQ2, was significantly different from zero: *P < 0·05, **P < 0·01, ***P < 0·001.
† FFQ1 and FFQ2 were administered at 1-month interval.
‡ ‘Diet+supplement’ means the antioxidants were estimated from both food and dietary supplements.
Plasma and dietary antioxidants and TAC were compared (Table 4). Significant Spearman rank correlation coefficients were observed between dietary carotenoids estimated from FFQ2 (α-carotene, β-carotene, β-cryptoxanthin, lutein and zeaxanthin from diet) and their corresponding plasma levels in the range of 0·27 to 0·50 (P < 0·05). Adjustment for age, gender, BMI, plasma cholesterol and plasma TAG did not appreciably change the significance of these correlations. Neither TAC from diet nor TAC from diet and supplements was correlated with plasma TAC level. These associations found with FFQ2 were comparable to those from the 30 d FR, while the plasma–diet correlation regarding α-tocopherol was relatively stronger in the 30 d FR than in FFQ2.
Table 4 Spearman rank correlation coefficients between dietary antioxidants or TAC estimated from FFQ2 or the 30 d FR and corresponding plasma antioxidant or TAC levels: college students aged 18–25 years who completed 30 d FR and two FFQs (n 60), Connecticut, USA

30 d FR, 30 d food record; TAC, total antioxidant capacity.
†Models were adjusted for age, gender, ethnicity, BMI and (except for vitamin C) plasma cholesterol, and plasma TAG. For associations between antioxidants from diet only and plasma concentrations, dietary supplement use was adjusted.
‡Models were adjusted for age, gender, ethnicity, BMI and plasma uric acid. For associations between TAC from diet only and plasma concentrations, dietary supplement use was adjusted.
Test–retest reliability
Correlation coefficients to evaluate the reliability of the FFQ by comparing the two FFQ collected at 1-month interval were moderate to high (r = 0·39–0·86; P < 0·01; Table 3). The two FFQ categorized the majority of participants into the same (average 62 %) or adjacent (average 32 %) tertile of antioxidant intake, while only 6 % on average were misclassified into the opposite tertile (Table 3).
Discussion
In the present study, most dietary vitamins and flavonoids estimated from the newly developed brief antioxidant FFQ were significantly correlated with those estimated from the 30 d FR and plasma biomarkers, which suggested the FFQ to effectively capture major antioxidant intake in these college students residing in Connecticut, USA. Thus the developed FFQ could be used to assess a comprehensive range of antioxidant intakes during a short period in epidemiological or clinical settings or to simply monitor variations of antioxidant intakes in intervention trials.
In theory, during the development of an FFQ for a wide range of antioxidants, the selection of foods not only incorporates those food items that are rich in specific antioxidants, but also involves regularly consumed antioxidant sources, as well as those that may substantially contribute to dietary antioxidant variations( Reference Somerset and Johannot 10 ). The present study identified such food sources that coincide with the aforementioned criteria through ranking dietary TAC scores from food items consumed most in the USA. Food items high in dietary TAC thus represented an antioxidant source that was either commonly consumed or richest in certain or total antioxidants. Furthermore, the new ‘theoretical’ approach to assess dietary TAC was used in the present study, which added diverse individual antioxidant capacities of food items consumed daily( Reference Yang, Chung and Chung 16 ). It was validated by positive linking with TAC values obtained from the USDA oxygen radical absorbance capacity database( Reference Floegel, Kim and Chung 22 ).
Daily intakes of carotenoids, vitamin C, flavonoids and TAC estimated from the 30 d FR and FFQ were generally comparable, with a difference of 10 % to 30 %. However, daily intakes of vitamin E and proanthocyanidins estimated from the FFQ were considerably lower than those estimated from the 30 d FR. Previous studies documented that dietary vitamin E in the US diet was derived mainly from grains, fat, oils and dressings, meat, poultry and fish( Reference Chun, Floegel and Chung 27 ), while proanthocyanidins were abundant in legumes and wines but not in vegetables and fruits( Reference Wang, Chung and Song 35 ). Our FFQ was based on the food items most contributing to the TAC. Since the antioxidant capacities of various fats, meat or legumes are much lower than those of vegetables and fruits, the exclusion of the food items mentioned above may partially explain the underestimation.
However, different from the FR or dietary recall which is used for evaluating the absolute intake quantitatively, the FFQ is usually used to rank individuals from low to high intakes for associating dietary patterns with health outcomes( Reference Andersen, Veierod and Johansson 5 ). In the present study, correlation coefficients for most individual antioxidant intakes and TAC values were above 0·4, which is considered reasonable and acceptable in FFQ validation studies( Reference Willett 36 , Reference Jain 37 ). Furthermore, correlation coefficients for antioxidant intakes in the present FFQ were comparable to those previously measured by either whole-food FFQ or brief FFQ for specific antioxidants. For instance, vitamin C correlation was relatively low but within the range of 0·27 to 0·71 reported by previous studies using FR as a reference( Reference Andersen, Solvoll and Johansson 38 – Reference Date, Fukui and Yamamoto 43 ) and was similar to or higher than those in FFQ with 1-month reference period. Correlation coefficients for carotenoid subclasses were generally higher than those from preceding FFQ( Reference McNaughton, Marks and Gaffney 44 , Reference Satia, Watters and Galanko 45 ). Additionally, since the correlation analysis for testing validity has been questioned for its failure in measuring agreement( Reference Willett 3 , Reference Bland and Altman 46 ), cross-classification was used in the present study to bridge this gap( Reference Labonte, Cyr and Baril-Gravel 47 ). Cross-classification for the current FFQ indicated reasonable agreement across tertiles of antioxidant intake between the 30 d FR and FFQ and an acceptable low misclassification percentage, which further suggested that our FFQ could provide a similar ranking of antioxidant intake as did the 30 d FR. The agreement or misclassification percentages were in accordance with the validation studies conducted by Dunn et al.( Reference Dunn, Datta and Kallis 48 ) and Stiegler et al. ( Reference Stiegler, Sausenthaler and Buyken 6 ). To sum up, the correlation analysis along with the agreement categorization provided sufficient data to judge the overall ability of the FFQ on dietary antioxidant estimation and emphasized the reasonable validity of this new instrument.
Biochemical indicators of dietary intake are another useful approach to weigh FFQ validity, although this method is still prone to random and systematic errors( Reference Stiegler, Sausenthaler and Buyken 6 ). In the present study, associations between questionnaire-derived antioxidant intakes and biomarkers were comparable to or stronger than those reported by the previous studies applying biomarkers( Reference Andersen, Solvoll and Johansson 38 , Reference McNaughton, Marks and Gaffney 44 , Reference Satia, Watters and Galanko 45 , Reference Dixon, Subar and Wideroff 49 – Reference Malekshah, Kimiagar and Saadatian-Elahi 51 ). For instance, the correlation between diet and plasma α-tocopherol level was as low as those reported previously( Reference Andersen, Solvoll and Johansson 38 , Reference McNaughton, Marks and Gaffney 44 , Reference Satia, Watters and Galanko 45 , Reference Dixon, Subar and Wideroff 49 – Reference Malekshah, Kimiagar and Saadatian-Elahi 51 ). The weak correlation was probably attributable to measurement errors of the FFQ as addressed by Dixon et al. ( Reference Dixon, Subar and Wideroff 49 ), including under-reporting, poor assessments of fats and oils, and high variability of vitamin E content in the food composition databases. The positive diet–plasma relationships for several carotenoids were in accordance with those reported by other FFQ validation studies( Reference Satia, Watters and Galanko 45 , Reference Dixon, Subar and Wideroff 49 ) and quantifications of such a relationship remained within the range of previously reported correlation coefficients: from 0·31 to 0·56 for α-carotene( Reference McNaughton, Marks and Gaffney 44 , Reference Satia, Watters and Galanko 45 , Reference Dixon, Subar and Wideroff 49 , Reference Hodge, Simpson and Fridman 50 ), from 0·22 to 0·33 for β-carotene( Reference McNaughton, Marks and Gaffney 44 , Reference Satia, Watters and Galanko 45 , Reference Dixon, Subar and Wideroff 49 , Reference Hodge, Simpson and Fridman 50 ), from 0·28 to 0·62 for β-cryptoxanthin( Reference Satia, Watters and Galanko 45 , Reference Dixon, Subar and Wideroff 49 , Reference Hodge, Simpson and Fridman 50 ) and from 0·15 to 0·24 for lutein/zeaxanthin( Reference Satia, Watters and Galanko 45 , Reference Dixon, Subar and Wideroff 49 , Reference Hodge, Simpson and Fridman 50 ). However, the diet–plasma correlation for lycopene was not within the range of 0·12 to 0·42( Reference Satia, Watters and Galanko 45 , Reference Dixon, Subar and Wideroff 49 , Reference Hodge, Simpson and Fridman 50 ), which might be attributable to the exclusion of certain mixed dishes that are rich in lycopene such as pizza or pasta from the current FFQ, although tomato sauces and ketchup were included. Nevertheless, validation regarding vitamin C varied and did not produce consistent correlations( Reference Block, Woods and Potosky 40 ). The use of vitamin C as a surrogate marker is limited due to its instability and ‘threshold’ effect( Reference Padayatty and Levine 52 , Reference Jenab, Slimani and Bictash 53 ). Moreover, certain correlation coefficients between dietary and plasma TAC in the present study were comparable to the results of Rautiainen et al. ( Reference Rautiainen, Serafini and Morgenstern 54 ). However, whether plasma TAC is a good reference to validate FFQ-based TAC estimates is still inconclusive( Reference Rautiainen, Serafini and Morgenstern 54 , Reference Pellegrini, Salvatore and Valtuena 55 ). Low bioavailability of flavonoids and proanthocyanidins might interfere the correlation( Reference Manach, Williamson and Morand 56 ). Plasma TAC was found to increase immediately after a high-antioxidant diet but decrease to normal level after a few hours( Reference Cao, Russell and Lischner 57 ). Besides, plasma TAC may be affected by plasma protein, uric acid and antioxidant enzymes rather than antioxidant nutrients and their metabolites( Reference Ghiselli, Serafini and Natella 58 ). As a result, plasma TAC may not be used as a surrogate measurement of dietary TAC( Reference Pellegrini, Salvatore and Valtuena 55 ). Furthermore, the diet–plasma correlations of the FFQ and 30 d FR demonstrated comparable patterns, such as the significant associations between plasma and dietary carotenoids (except for lycopene). These results were indicative of a relatively strong association between the two dietary assessment tools.
There are several strengths of the present study. The FFQ captures intake of a wide range of vitamins and flavonoids with antioxidant properties with one administration, and is also useful to estimate the integrated TAC parameter. The food list derived from dietary TAC for commonly consumed food items covered the dietary sources richest in specific antioxidants, regularly consumed and most influencing antioxidant variations. Besides, involvement of supplements increased the ability of the FFQ for evaluating overall antioxidant status. Furthermore, a 30 d FR served as a reference for the FFQ for 1-month diet estimates.
Our study also had limitations. Although there was reasonable validity of the FFQ, the fact that it was validated in a small sample of American college students in Connecticut does not imply it will perform equally well in the other US populations. Cross-validation studies of the FFQ in external populations are important before its application. Additionally, a period of 1 month was used in the newly developed FFQ, considering the most accurate reference method selected (i.e. 30 d FR) to capture high variations of antioxidant intake. However, the short period may serve as a major drawback and restrict its application in epidemiological studies. As a result, whether it could be extended to adequately assess long-term habitual antioxidant intake needs further investigation. Moreover, whether the participation fatigue caused by the consecutive 30 d FR would affect the reliability of this reference may also need justification.
Conclusions
Associations between dietary estimates from the FFQ, the 30 d FR and plasma antioxidant concentrations were in accordance with those reported by previous validation studies. The brief FFQ generally performed as well as the 30 d FR in estimating a comprehensive dietary antioxidant profile during a short time. This FFQ may be used in epidemiological or clinical studies to capture short-term antioxidant intakes or to simply document variations of antioxidant intakes in intervention trials.
Acknowledgements
Sources of funding: The present study was supported by University of Connecticut USDA Hatch Grant No. CONS00846. Conflicts of interest: All authors have no conflict of interest to declare. Authors’ contribution: O.K.C developed the concept. O.K.C. and M.Y. prepared the preliminary data, developed the FFQ and designed the validation study. M.Y., Y.W. and C.G.D. acquired the data. S.G.L. analysed the plasma biomarkers. M.Y. conducted the statistical analysis and drafted the manuscript. S.I.K., M.L.F, E.C. and W.O.S provided technical support and advice as members of the project steering group. All authors were involved in the data interpretation and manuscript preparation. Acknowledgements: The authors would like to thank all of the college students who participated in the study.






