Tracking has been defined as the maintenance of relative position in rank of behaviour over timeReference Kelder, Perry, Klepp and Lytle1, Reference Twisk, van Mechelen, Kemper and Post2. Only a limited number of longitudinal studies have investigated the extent of tracking of nutrient intakes from adolescence into young adulthood and the results have been inconsistent. Cusatis et al.Reference Cusatis, Chinchilli, Johnson-Rollings, Kieselhorst, Stallings and Llyod3 concluded that nutrient intake patterns did not track strongly during adolescence (between 12 and 18 years). Post et al.Reference Post, deVente, Kemper and Twisk4 investigated the extent of tracking of nutrient intakes between 13 and 33 years of age in the Amsterdam Growth and Health Longitudinal Study. Significant stability coefficients for all nutrients were found (0.28–0.52); however, over the entire study period (between ages 13 and 33 years) the authors concluded that dietary intake between adolescence and adulthood was changeableReference Post, deVente, Kemper and Twisk4. Dunn et al.Reference Dunn, Liu, Greenland, Hilner and Jacobs5 observed a poor to moderate correlation for most nutrient intakes reported by subjects aged 18–30 years over a 7-year period but concluded that, despite considerable changes in lifestyle, individuals tend to remain within broad categories of intake.
Previous work in the Northern Ireland Young Hearts cohortReference Robson, Gallagher, Livingstone, Cran, Strain and Savage6 demonstrated poor tracking of energy and nutrient intakes in subjects assessed at age 12 years and again at age 15 years. More recently, follow-up data have been collected in this cohort at age 20–25 yearsReference Gallagher, Savage, Murray, Davey Smith, Young and Robson7. Adolescence is a period of life when food and nutrient intakes can fluctuate owing to erratic eating behaviour. However, it could be speculated that towards the end of the adolescent period eating patterns may stabilise. Thus the aim of the present study was to evaluate the extent to which patterns in dietary intakes in these subjects track from age 15 years through to young adulthood (20–25 years).
Methodology
Subjects
The Young Hearts Project is an ongoing study of biological and behavioural risk factors for cardiovascular disease in a representative sample of young people from Northern Ireland. The baseline survey (YH1) was completed in 1990 on a randomly selected sample of schoolchildren from Northern Ireland (n = 1015; 251 12-year-old males, 258 12-year-old females, 252 15-year-old males and 254 15-year-old females). The original group of 12-year-olds (then aged 15 years) was followed up in 1992/93 (YH2) with complete data collected on 225 males and 230 females, representing a 90% response rate. Further details of the study design and sampling procedure used for YH1 and YH2 are reported elsewhereReference Boreham, Savage, Primrose, Cran and Strain8. Between October 1997 and October 1999, all of the original participants in the Young Hearts Project were invited to participate in the third examination phase (YH3). In this phase of the study a 48.2% response rate (251 males and 238 females) was achievedReference Gallagher, Savage, Murray, Davey Smith, Young and Robson7. Figure 1 shows the numbers of subjects participating in each sampling phase of the study. The present study is based on data obtained from participants at age 15 years and subsequently at young adulthood (YH3) (n = 245 males, n = 231 females). Ethical approval was obtained from the Medical Research Ethical Committee of The Queen's University of Belfast and written informed consent was obtained from parents or guardians (at YH1 and YH2 only) and from all participating subjects at all time points.

Fig. 1 Numbers of subjects participating in each phase of the Young Hearts (YH) Project, Northern Ireland
Anthropometry
In all phases of the study, measurements of height, weight and skinfold thicknesses were made. Subjects, wearing light indoor clothing and no shoes, were weighed to the nearest 0.1 kg using scales; height was measured to the nearest millimetre using a freestanding, portable stadiometer. Body mass index (BMI) was computed as weight (kg)/[height (m)]Reference Twisk, van Mechelen, Kemper and Post2. Skinfold thicknesses were measured in duplicate using standardised procedures to the nearest millimetre using callipers at four sites (biceps, triceps, subscapular and suprailiac) and used to estimate body fatnessReference Durnin and Rahaman9. Maturational stage was assessed using the Tanner index of pubertal development (YH1 and YH2 only)Reference Tanner10.
Dietary intakes
The diet history method with open-ended interviewReference Van Staveren, de Boer and Burema11 was used to record the usual weekly meal and snack intake of each subject at both time points, i.e. at age 15 yearsReference Strain, Robson, Livingstone, Primrose, Savage and Cran12 and in the present studyReference Gallagher, Savage, Murray, Davey Smith, Young and Robson7. Interviews were conducted in a one-to-one setting by trained fieldworkers. The number of interviews conducted by each fieldworker was approximately equal for each age–sex group. A standardised form was used to record meals and snacks, portion size and frequency of eating. Information was obtained on the usual meal and snack pattern of the subject, including place of consumption; usual foods consumed during the week and on weekends; and detailed description of these foods including methods of preparation and portion size. After the usual eating pattern had been described, a review of the information was obtained with particular emphasis on probing for foods that had not been mentioned. Amounts of foods and fluids consumed were estimated by means of photographs of known portion weights of foods supplemented with the use of common household cups, glasses and dishes. Energy and nutrient intakes were calculated using computerised databases as previously describedReference Gallagher, Savage, Murray, Davey Smith, Young and Robson7, Reference Strain, Robson, Livingstone, Primrose, Savage and Cran12. The basal metabolic rate of each subject was estimated (BMRest) using appropriate equations based on sex, height and weightReference Schofield, Schofield and James13. The same methods were used to assess anthropometric status and dietary intake at all sampling time points (i.e. at age 15 years and at young adulthood).
Statistical analyses
In an earlier paperReference Strain, Robson, Livingstone, Primrose, Savage and Cran12, the advantages of using percentiles, rather than the mean and standard deviation (SD), to describe dietary intakes were outlined. Consequently, to maintain consistency in the present study, dietary data are summarised as medians, with the 25th and 75th percentiles included as a measure of variation. These percentiles are also used to describe body composition data. The Wilcoxon matched-pairs signed-ranks test was used to test that the median of the population of paired differences of adolescent and young adulthood values is zero at a 5% significance level.
In the present study, daily energy or nutrient intake was considered to track well over time if subjects with ‘low’, ‘medium’ or ‘high’ intakes at adolescence (at age 15 years) maintained their ranking at young adulthood. This method, based on ranks, is described in detail elsewhereReference Robson, Gallagher, Livingstone, Cran, Strain and Savage6. In brief, in order to study the tracking of energy intake (EI) of males from adolescence to young adulthood, the group of males aged 15 years (n = 245) was divided into tertiles according to EI: lowest tertile (L1), middle tertile (M1) and highest tertile (H1). Similarly at young adulthood, the group was divided into three tertile classes: L2, M2 and H2. Using these two classifications, a 3 × 3 tracking matrix was constructed, the entry in a specific cell being the number of subjects belonging to the corresponding classes at adolescence and at young adulthood. The resulting matrix provides a broad picture of the relative changes in intake of the group over the period. The degree of tracking was summarised by a linear weighted kappa value (κ)Reference Altman14 calculated from the matrix; κ = 1 when the degree of tracking is perfect, i.e. when every subject maintained their tertile position at adolescence and at young adulthood. Guidelines for interpretation of the value of κ obtained have been defined as followsReference Altman14: < 0.20, poor; 0.21–0.40, fair; 0.41–0.60, moderate; 0.61–0.8, good; 0.81–1.0, very good. In addition, 95% confidence limits for κ were calculated. This procedure was undertaken for intakes of energy, macronutrients and selected micronutrients.
Results
The physical characteristics, reported EI and macronutrient intakes of the Young Hearts cohort at age 15 years (mean age 15.5 (SD 0.13) and 15.6 (SD 0.11) years for males and females, respectively) and at young adulthood (mean age 22.4 (SD 1.61) and 22.8 (SD 1.66) years for males and females, respectively) are presented in Table 1. Body weight and height of both males and females increased significantly (P < 0.001) during this period. At age 15 years, median weights of both males and females were greater than the median weights for the equivalent UK populations15 (by 3.7% and 6.7% for males and females, respectively). Similarly at young adulthood, median weights were greater by 6.1% in males and 6.8% in females than the median weights for the equivalent UK population15. BMI of males increased significantly (P < 0.001) but decreased for females over the same period (P < 0.01). Conversely, body fatness of males did not alter substantially during the study period whereas it increased in females by 7.4% in relative terms (P < 0.001).
Table 1 Physical characteristics, energy and macronutrient intakes of the Young Hearts cohort at adolescence (age 15 years) and at young adulthood

P25 – 25th percentile; P75 – 75th percentile; BMI – body mass index; EI – total energy intake; BMRest – estimated basal metabolic rate; CHO – carbohydrate.
Wilcoxon matched-pairs signed-ranks test was used to test for significant differences: **P < 0.01, ***P < 0.001 compared with group median at 15 years.
† Body fatness was estimated according to Durnin and RahamanReference Durnin and Rahaman9.
‡ BMRest predicted from height and weight using equations of Schofield et al.Reference Schofield, Schofield and James13.
§ Alcohol intakes represent the median (25th, 75th percentile) for 81 males and 60 females at age 15 years and for 209 males and 176 females at young adulthood.
Despite increases in weight, height and BMI, median EI (MJ day− 1) reported by the males decreased by 7% over time. Although weight, height and percentage body fatness increased during this period in the females, reported median EI (MJ day− 1) at young adulthood was significantly lower (10%) than at age 15 years (P < 0.001). Expressed relative to body weight (kJ kg− 1 day− 1), EI reported by males and females was significantly lower at young adulthood than at age 15 years (P < 0.001, both sexes). The ratio of total EI to predicted BMR (EI/BMRest) also decreased significantly over time in both groups (P < 0.001, both sexes) and may reflect an increase in levels of underreported EI with age within the cohort. In contrast to decreases in carbohydrate (CHO) and fat intakes, protein intakes (expressed as g day− 1 or as percentage contribution to EI, %EI) increased during the same period (both sexes, P < 0.01). At young adulthood, both the number of subjects reporting alcohol consumption and the levels of alcohol intake by alcohol consumers were significantly increased compared with those at age 15 years (P < 0.001, both sexes).
The weighted κ values for EI and macronutrients are given in Table 2. The tracking of EI and macronutrient intakes at the individual level was only poor to fair. A fair degree of tracking was found over the study period for EI (kJ kg− 1 day− 1; κ = 0.26 and 0.33 for males and females, respectively). With the exception of protein intakes in females (g day− 1; κ = 0.25, fair tracking), the tracking of macronutrients (expressed as g day− 1 or %EI) from adolescence to young adulthood was poor (κ < 0.20).
Table 2 Tracking of energy and macronutrient intakes as estimated by weighted kappa values (κ)
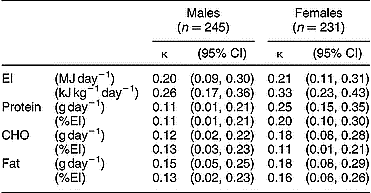
CI – confidence interval; EI – total energy intake, CHO – carbohydrate.
κ values were calculated as described in the Methodology; standard errors for κ were ± 0.05 throughout.
Table 3 presents reported intakes of selected micronutrients by the Young Hearts cohort at adolescence and at young adulthood. Changes in median micronutrient densities (mg MJ− 1 or μg MJ− 1) of the males' diets were inconsistent over time. On the other hand, the males' diets became more nutrient-dense with respect to thiamin, vitamin B6, total folate, vitamins C and D (all P < 0.001), whereas calcium (P < 0.05) and vitamin A (P < 0.001) densities fell with increasing age. No other significant changes in micronutrient densities were observed in the males' diets. The females' diets also became more nutrient-dense with respect to thiamin, vitamin B6, total folate (all P < 0.001), vitamin C (P < 0.01) and vitamin D (P < 0.05). On the other hand, calcium (mg day− 1) and vitamin A (μg day− 1) intakes did not change during this period. In contrast to the males' diets, the median intakes of riboflavin (mg MJ− 1) by females increased over time (P < 0.001). The shortfall in median iron (mg MJ− 1) intakes relative to the Estimated Average Requirement (EAR)15 reported by the females was substantial both at adolescence ( − 16.3%) and young adulthood ( − 14.7%). Moreover, median intakes of vitamin B6 (mg MJ− 1) and total folate (μg MJ− 1) reported by the females were lower than the EAR15 at age 15 years ( − 4.9% and − 18.8%, respectively). Median intakes of all other micronutrients exceeded the EAR when expressed in terms of nutrient density.
Table 3 Intakes of selected micronutrients in the diets of the Young Hearts cohort at adolescence (age 15 years) and at young adulthood
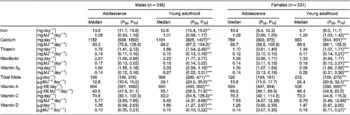
P25 – 25th percentile; P75 – 75th percentile; RE – retinol equivalents.
Wilcoxon matched-pairs signed-ranks test used to test for significant differences: *P < 0.05, **P < 0.01, ***P < 0.001 compared with median intake by group at age 15 years.
The weighted κ values obtained for the micronutrient intakes are summarised in Table 4. At the individual level, micronutrient intakes exhibited only poor to fair tracking during the study period. For intakes of vitamin A (μg RE day− 1, where RE is retinol equivalents) a fair degree of tracking was observed for both males and females (κ = 0.25 and 0.27, respectively). Intakes of calcium (mg day− 1) and riboflavin (mg day− 1) exhibited a fair degree of tracking for males, but not for females (κ = 0.25 and 0.26 vs. 0.18 and 0.20, respectively). In contrast to the poor tracking of iron (κ = 0.10) and thiamin (κ = 0.14) in the males, a fair degree of tracking was observed for intakes of these micronutrients in females (κ = 0.23 and 0.24, respectively). In terms of nutrient density, a fair degree of tracking continued to be observed for vitamin A (μg RE MJ− 1) in both males and females (κ = 0.21 and 0.24, respectively). Poor tracking was observed for males' intakes of calcium (mg MJ− 1) and folate (μg MJ− 1) (κ = 0.20 and 0.10, respectively), but in females the degree of tracking was marginally stronger (κ = 0.27 and 0.21, respectively). When adjusted for EI, intakes of iron, thiamin, riboflavin, vitamin B6, vitamin C and vitamin D exhibited only poor tracking in both sexes.
Table 4 Tracking of micronutrient intakes as estimated by weighted kappa values (κ)

CI – confidence interval; RE – retinol equivalents.
κ values were calculated as described in the Methodology; standard errors for κ were ± 0.05 throughout.
Discussion
Given the accumulating evidence of the link between nutrient intake and various chronic diseases, it seems prudent to undertake longitudinal studies to investigate the stability of nutrient intakes over time. To date, the evidence for tracking of nutrient intakes from adolescence to young adulthood has been somewhat scarce and inconsistent. Furthermore, methodological differences between dietary data collection, time period lapses between sampling periods and methods used for the calculation of tracking coefficients make it difficult to meaningfully compare different datasets.
The main criteria used for the selection of a suitable method for the collection of dietary data were that the method should be relatively quick to undertake, suitable for surveying large groups, and involve a low respondent burden (particularly apt when studying these age groups). Although the diet history used in this study is not a standardised technique, particular care was taken to ensure that the same protocol was followed at each sampling time point. As this method measures only memory and perception of usual diet, it is subjective and therefore may be particularly vulnerable to exaggeration of ‘good’ foods and underestimation of foods perceived as ‘bad’. As a result it is conceivable that nutrient intakes may have been subject to misrepresentation but this is impossible to quantify in this study. Another possible source of bias is that the diet history method is easier to undertake in those with regular eating behaviours and, given the irregularity of adolescent eating behaviours, it may be more difficult for respondents in the age groups studied to recall foods eaten. The choice of any survey method will always be a compromise between subject burden and likely inaccuracies in dietary reporting. Thus since the method chosen for the baseline study was considered the most suitable at that time, it would have been inappropriate to change it for the follow-up survey.
Dietary surveys usually report a wide range of EI with extremes of intakes that are unlikely to reflect habitual intake and such under- and overreported intakes may distort the ranking of subjectsReference Livingstone and Black16. Varying levels of under- and overreporting of intakes are apparent in the present study population. Using the Goldberg et al.Reference Goldberg, Black, Jebb, Cole, Murgatroyd and Coward17 cut-off of 1.18 for EI/BMRest, it is apparent that underreporting at the individual level increases with increasing age in the present cohort, with 2.4% of males compared with 18.2% of females at adolescence being classified as ‘underreporters’, rising to 11.0% and 29.9% respectively at young adulthood. It is thus highly likely that the increase in underreporting has attenuated the tracking of nutrient intakes within the cohort. Using a cut-off of 2.5 for EI/BMRest to define overreportersReference Strain, Robson, Livingstone, Primrose, Savage and Cran12, overall levels of overreporting decreased over time, although males continued to overreport their intakes compared with females (7.8% vs. 1.7%, respectively).
Various statistical methods are employed to assess whether dietary intakes track over time. In the present study, a method based on ranking observations was chosen to determine the extent of tracking of intakes. This was selected in preference to a method based on actual intakes because of its simplicity and its ability to show the rates of transitions between the tertile classesReference Robson, Gallagher, Livingstone, Cran, Strain and Savage6. Thus, this method provides a broad picture of the relative changes in intake from adolescence into young adulthood. A fair degree of tracking was observed in the present study for EI (adjusted for body weight) between adolescence and young adulthood in contrast to the overall poor tracking coefficients (κ < 0.20) observed for macronutrient intakes; these findings largely reflect the tracking patterns in this cohort between age 12 and 15 yearsReference Robson, Gallagher, Livingstone, Cran, Strain and Savage6. In contrast, some differences in the tracking patterns of micronutrient intakes were observed.
A recent analysis by Black and ColeReference Black and Cole18 of seven UK-based studies highlighted subject-specific bias in dietary reporting with repeated dietary assessment (with the same measurement method or with different methods). Such subject-specific bias supports the view that people who misreport dietary intakes in one survey are likely to do so (and probably to the same level of magnitude) on repeat measurement and also suggests that the extremes of intake are likely to maintain their ranking (lowest or highest tertile) within a population over time. Thus, under- and overreporting by subjects in the present study may provide one explanation for the lack of tracking of EI and energy-adjusted nutrient intakes. Furthermore, other factors (such as changes over time in body size, physical activity, attitudes, knowledge, etc.) may be important confounders when assessing the extent of tracking of intakes. Indeed, attempts to adjust for body size (e.g. EI per kg body weight) or EI (e.g. nutrient intakes as %EI) may introduce further bias and again help explain the overall poor tracking coefficients observed.
The only other comparable study of tracking in terms of the ages investigated and the dietary methods employed was the Amsterdam Growth and Health Longitudinal Study. This study has periodically assessed nutrient intake using the diet history method in a group of 73 males and 91 females (aged 13–17 years at baseline) through to adulthood (age 27 years)Reference Welten, Kemper, Post, Van Staveren and Twisk19. Post et al.Reference Post, deVente, Kemper and Twisk4 reported increases in EI between ages 13 and 21 years for males and a more stable EI pattern over time for females between ages 13 and 21 years, which is in contrast to the stable EI in males but decreasing EI in females in the present study. The former study also observed that males had significantly higher (+25%) EI than females over the whole age range (age 13–33 years) studied, which is largely comparable with findings in the present study (31–35%). When expressed relative to body weight, patterns of EI in the present study are comparable with those of Post et al.Reference Post, deVente, Kemper and Twisk4 as are patterns of protein and fat intakes. Fair to moderate tracking of calcium intakes in males and females over the same 14-year period has also been described for this cohortReference Welten, Kemper, Post, Van Staveren and Twisk19. More recently Post et al.Reference Post, deVente, Kemper and Twisk4 observed relatively low but significant stability coefficients for tracking of macro- and micronutrients between adolescence and adulthood and concluded that dietary intake between adolescence and adulthood was changeable.
Dunn et al.Reference Dunn, Liu, Greenland, Hilner and Jacobs5 concluded that despite considerable changes in lifestyle between age 18 and 30 years, individuals do tend to remain within broad categories of ranking. In contrast, in a younger cohort (12 to 18 years), Cusatis et al.Reference Cusatis, Chinchilli, Johnson-Rollings, Kieselhorst, Stallings and Llyod3 observed varying patterns of dietary intakes over time in females and concluded that nutrient intake patterns did not track strongly during this period of adolescence.
Stronger tracking coefficients for dietary intake have been reported over shorter periods and in younger age groupsReference Stein, Shea, Basch, Contento and Zybert20–Reference Zive, Berry, Sallis, Frank and Nader23. As individuals move from childhood through adolescence and on to young adulthood, it is possible that increasing autonomy and food choice is mirrored in changes to patterns of dietary intake. It is possible that, between adolescence and young adulthood, the significant lifestyle changes that these individuals experience, such as starting new careers or families, has a greater impact on dietary intakes than has been previously predicted. The substantial drift of subjects between the low, medium and high classes of EI and macronutrient intake between adolescence and young adulthood in the present study would support this case. Moreover, these data suggest that individual dietary patterns exhibited at 15 years of age are unlikely to be predictive of dietary intakes at young adulthood.
Similar analysis to that outlined in the present paper was undertaken for the subjects at age 12 years and subsequently at young adulthood. These data (not presented) suggest that tracking of intakes between these time points was even weaker, although this may also reflect the smaller size of the analysis cohort (n = 135 males, n = 119 females). In contrast to poor to fair tracking observed for dietary intakes, it is noteworthy that considerably stronger tracking coefficients were observed for anthropometric measurements (weight, BMI, body fatness) in this cohort between age 12 and 15 yearsReference Robson, Neville, Twisk, Strain, Livingstone and McGuinness24, similar to the tracking observed for these measures between age 15 and 20–25 years (data not presented). This finding has been noted in other surveysReference Power, Lake and Cole25.
The results of the present study indicate that, in a group of adolescents from Northern Ireland, dietary intakes at age 15 years did not appear to predict dietary intakes at young adulthood. That stabilising of food habits did not appear to occur during this period of life possibly reflects that this is a period within the life cycle when patterns of dietary intake are impacted by substantial lifestyle changes. However, given the extent of misreporting in many dietary surveys, it remains unclear whether the apparent lack of tracking observed in many studies is real, making it almost impossible to correlate nutrient intakes over time with the eventual development of disease.
Acknowledgements
The first two phases of the Young Hearts Project (YH1 and YH2) were supported by the Northern Ireland Chest, Heart and Stroke Association. The Project Team would also like to thank the British Heart Foundation and the Wellcome Trust for their support of YH3. The authors would like to thank the subjects who participated in the project, and all fieldworkers who collected the dietary data (Hilary Morrison, Elizabeth Archer, Siobhan Higgins, Dara Morgan, Charlotte Neville and Meabh McGuinness).







