In recent decades, excess weight and obesity have been acknowledged as some of the fastest growing non-transmissible health problems worldwide(1). An increase in the prevalence of excess weight and obesity is a particular concern because it has proven to be an important factor for numerous chronic diseases and other health disorders among adults(Reference Lew and Garfinkel2–Reference Thomsen, Ulrik and Kyvik8) as well as among children(Reference Zimmet, Alberti and Shaw9–Reference Gilliland, Berhane and Islam14). Consequently, obesity not only worsens the quality of life of affected individuals but also increases public and personal health expenditures(Reference Colditz15–Reference Seidell19). Obesity is a burdening factor in all respects; it should become a public health priority in developed and developing countries, and it is from this perspective of prevention that the relationship between childhood obesity and young adult obesity has been investigated. Several studies have shown a tendency of those who are overweight or obese as children to remain overweight or obese as adolescents and adults(Reference Whitaker, Wright and Pepe20–Reference Kvaavik, Tell and Klepp28). However, much of the research has been conducted in high-income and predominantly Western countries, while only limited data are available for middle- or low-income countries and post-socialist countries (like Slovenia), which quickly and abruptly adopted capitalist economies and consumerist lifestyles, including eating habits and physical activity patterns. Longitudinal research on these topics from rapidly changing societies might provide new insights, as well as enable comparison and consequent prediction of obesity trends among children and adults in these societies.
The present study focused on the tracking of obesity and excess weight by measuring triceps skinfold thickness (TSF) and BMI from childhood to young adulthood among Slovenian schoolchildren who entered primary school in the school years 1996–1997 and turned 18 years of age in the school years 2007–2008. Numerous studies have identified two critical periods in childhood for the development of obesity(Reference Williams, Davie and Lam27, Reference Dietz29–Reference Whitaker, Pepe and Wright34): the period of adiposity rebound between 5 years and 7 years and adolescence. According to these findings, the analysis includes the measurements at 7, 11 and 14 years to establish the excess weight and obesity outcome at 18 years. We used longitudinal data to investigate the relationship between height, weight, BMI and TSF to assess: (i) associations between the height of children and young adults; (ii) the extent to which obese or overweight children become obese or overweight young adults; and (iii) whether obese or overweight young adults were obese or overweight children.
Methods
Subjects
The 1988–1990 Slovenian cohort included 21 777 children (94·2 % of the children who started primary school in September 1996) who enrolled in the SLOFIT system(Reference Strel, Ambrožič and Kondrič35) by the written consent of their parents, who were born between January 1988 and November 1990. Information on weight and height in the last year of secondary school at an average age of 18·7 (sd 0·3) years was obtained in April 2008 for 4833 children. This analysed subsample included children whose information on body weight and body height was obtained both in the first grade of primary school and the last year of 4-year secondary school. Children with incomplete information in the 1997 (n 1624) or 2008 measurements (n 17 438) were excluded. The subsample did not include pupils who were enrolled in the 3-year vocational schools and therefore finished their schooling in 2007 or who were older than 19 years in April 2008 (n 2715). A considerable reduction of the sample in 2008 occurred because many pupils either refused to participate in the measurements after they turned 18 and no longer needed the written consent of their parents, because they could not attend the measurements due to health problems, because they refused to be weighed, or because they did not continue their schooling after primary school.
The present study presents the data from the 1997 SLOFIT measurement and the 2001, 2004 and 2008 follow-ups. The age of the participating children was calculated to the closest month on the day of the measurements every year; the average age at the 1997 measurement was 7·7 ± 0·3 years (92·8 (sd 3·7) months for males and 92·7 (sd 3·9) months for females). Mean values and standard deviations of the population sample and the subsample were very similar, with differences in mean height below 5 mm, differences in mean weight below 0·6 kg (except in 11-year-old females in whom the average weight of the subsample was 1·1 kg smaller), differences in mean TSF below 0·4 mm, and differences in mean BMI below 0·4 kg/m2 at all ages.
Measurements
Height, weight and TSF were measured by trained physical education teachers who had completed a 30-h course in anthropometric measurement during their studies. Subjects were measured each April at ages 7, 11, 14 and 18 years ± 1 year. Measurements were organised in school gyms between 08·00 and 14·00 hours. Subjects were weighed barefoot in their shorts and T-shirts to the nearest 0·5 kg with portable scales of various brands; height was measured with stadiometers of various brands to the nearest 0·5 cm; triceps skinfold was measured with Holtain-Tanner callipers to the nearest mm. All instruments were calibrated once at the beginning of measurements. Data were checked to detect coding errors. BMI was calculated at each age. The cut-off points of the International Obesity Taskforce (IOTF)(Reference Cole, Bellizzi and Flegal36) for each 0·5 year were used to define overweight and obese children. These criteria identify BMI values for each age associated with a predicted BMI of 30 kg/m2 at 18 years. Age-specific BMI (weight in kg divided by height in m2) was calculated for each individual using the nearest tabulated age method. Age-specific values are given in Table 1.
Table 1 Percentage of normal, overweight and obese children at 7, 11, 14 and 18 years

Statistics
Partial correlation coefficients, adjusted for dates of measurement, were calculated between height, weight, TSF and BMI at ages 7, 11, 14 and 18 years. To reduce skewness of the weight, TSF and BMI distributions, correlations were also performed by using Lg (weight), Lg (TSF), Lg (BMI) and Sqrt for all three variables. Differences between correlations using transformed and untransformed variables proved to be trivial (−0·26 to 0·24); therefore, only correlations of untransformed variables are presented. BMI categories at 7, 11, 14 and 18 years were cross-classified. Percentages are shown primarily to show outcomes for the obese children and to show the BMI distribution at 7 years for the different BMI groups at 18 years. All analyses were performed using SPSS 15·0 for Windows (SPSS Inc., Chicago, IL, USA).
Results
Mean (sd) height, weight, TSF and BMI for subjects 7–18 years for males and females are shown in Table 2.
Table 2 Mean height, weight, TSF and BMI at 7–18 years
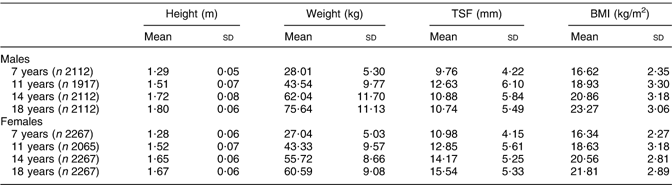
TSF, triceps skinfold thickness.
Height, weight, BMI and TSF
Correlations between height at 7 and 18 years in Table 3 tended to be strong for males (r = 0·69, P < 0·001) and females (r = 0·65, P < 0·001). As expected, correlations were greater over shorter intervals. Males had higher correlations than females at 7 and 11 years, but lower correlations at 14 years. These sex differences reflect the differences in the pubertal growth spurt, which occurs later for males(Reference Tanner, Whitehouse and Marubini37). At 14 years, the growth period of females is nearing the end and the difference between the average height of females at ages 14 and 18 years, shown in Table 2, was only 2 cm, while the difference between the average height of males in the same period was four times greater at 8 cm. The concluded growth of females at 14 years is also shown in Table 3 because the correlation between height at 14 and 18 years among females was considerably stronger (r = 0·91, P < 0·001) than among males (r = 0·72, P < 0·001). Correlations for weight were very similar to those of height (r = 0·64, P < 0·001 for males and females between 7 and 18 years). Correlations between BMI at different ages were weaker than for weight and height (r = 0·58, P < 0·001 for males and r = 0·59, P < 0·001 for females between 7 and 18 years) and the correlations among shorter periods were again slightly stronger for males than for females. The correlations indicated that BMI at 11 or 14 years explained 49–69 % of the variability in BMI at 18 years, while BMI at 7 years explained only around 34 % of the variability in BMI at 18 years for males and females. In comparison to all other measures, TSF showed the weakest correlations (Table 3) between 7 and 18 years (r = 0·39, P < 0·001 for males and r = 0·35, P < 0·001 for females). Stronger correlations were again observable among males and over shorter terms; for example, the correlations between TSF at 7 and 11 years were stronger than the correlations between TSF at 7, 14 or 18 years. The correlations indicated that only 12–25 % of the variability in TSF at 18 years was explained by TSF at 7, 11 or 14 years. Correlations of TSF with height, weight and BMI suggested that TSF correlates best with BMI and that correlations are largest at 11 years in males (r = 0·77, P < 0·001) and females (r = 0·70, P < 0·001). High correlations were also observed between TSF and weight at all ages in both sexes, while correlations of TSF with height proved to be small (r < 0·29, P < 0·001).
Table 3 Partial correlation matrix of height, weight, TSF and BMI at 7–18 years
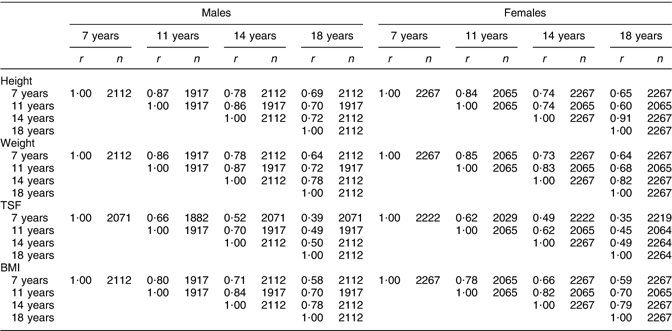
TSF, triceps skinfold thickness.
BMI outcome for obese and overweight children
The data in Table 4 show whether overweight and obese 7-years-olds became overweight 18-year-olds, giving the percentage of children who were subsequently in each BMI group according to the IOTF cut-off points at 18 years. The general trend was that the higher the BMI group, the higher was the probability of being obese at 18 years. For example, 17·3 % of 7-year-old males of normal weight were obese or overweight at 18 years compared with 53·1 % who were overweight or 77·7 % who were obese at 7 years. The chances of an obese 7-year-old becoming an obese 18-year-old increased consistently with age. For obese males at 7 years of age, the percentage of those who were obese at 18 years increased from 19·8 % at 7 years to 26·4 % at 11 years and 33·8 % at 14 years. The trends among females were similar with 17·0 % of obese 18-year-olds at 7 years, 32·7 % at 11 years and 37·1 % at 14 years.
Table 4 BMI outcome at 18 years for the normal, overweight and obese 7-, 11- and 14-year-olds
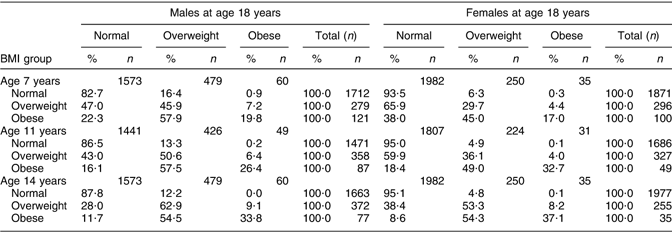
Previous BMI of obese 18-year-olds
The data in Table 5 show the percentages for BMI groups of those 18-year-olds who had been in different BMI groups in childhood and adolescence in order to assess whether obese 18-year-olds were obese as children. At every age, the majority (around 90 %) of 18-year-olds of normal weight would be identified as children of normal weight. In contrast, between 41·3 % and 57·6 % of overweight 18-year-old males, and between 53·2 % and 63·4 % of overweight or obese females would be identified as overweight children. In both sexes, there was a very high percentage of obese individuals at 18 years of age who would have been identified as overweight or obese individuals at earlier ages. The percentage grew with age in both sexes; among males, 73·3 % of obese 18-year-olds were overweight or obese at 7 years, 93·9 % at 11 years and 100 % at 14 years, while among females 85·7 % of obese 18-year-olds were obese at 7 years, 93·5 % at 14 years and 97·1 % at 14 years.
Table 5 Percentages within BMI groups at earlier ages according to BMI status at 18 years
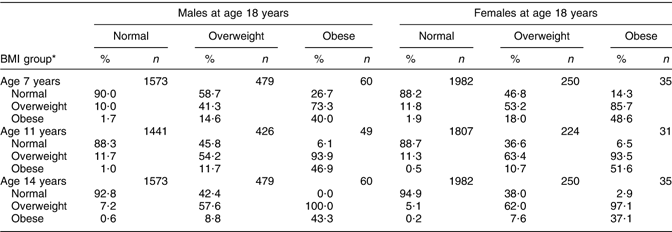
*BMI groups are not mutually exclusive, i.e. overweight includes also obesity.
Discussion
It has already been established that tracking childhood obesity beyond early adult age is a serious limitation of the literature to date(Reference Guo, Huang and Maynard24, Reference Kemper, Post and Twisk26), but the lack of studies from developing countries and the countries that experienced abrupt and major socio-political and economical changes in recent years seems to be equally limiting(Reference Williams, Davie and Lam27). Slovenia is a country that has experienced enormous socio-political and economical changes in the last 20 years, which strongly influenced the lifestyles, nutritional habits of children and youth, and their physical development(Reference Wang, Ge and Popkin38–Reference Jurak, Kovač and Strel41).
Studies in the USA and Europe that tracked obesity from childhood to adulthood generally found that about one-third of overweight and obese children remained overweight and obese as adults(Reference Guo, Huang and Maynard24, Reference Lake, Power and Cole42–Reference DiPietro, Mossberg and Stunkard46). In our cohort study, the percentage of overweight and obese 7-year-olds who became overweight or obese 18-year-olds was considerably higher (over 65 % among males and below 50 % among females). Our findings, which are more alarming, might be the outcome of the recent cohort, since most of the previous studies used cohorts from the 1950s and 1960s. The young adults included in our cohort probably lived in a very different environment as children than the ones in the older cohorts, especially regarding the abundance of fat- and sugar-rich food, availability of media and information technology and sedentary lifestyles.
In addition, we found that excess weight and obesity track slightly more consistently in females than in males, because obese and overweight females were more likely to remain in the same BMI group or move to a lower BMI group (obese to overweight and overweight to normal, respectively). The reason for this phenomenon remains unclear but it could be that body image, especially weight management, is more important to girls than to boys(Reference Serdula, Ivery and Coates47–Reference Davison and McCabe52), and they put more effort into maintaining or lowering their weight; boys, who more often moved from the lower to the higher BMI group, show much lower concern in this matter. Another possible reason that might explain this phenomenon is the use of the IOTF standard, which could misclassify some Slovenian children and adolescents because this standard was constructed on the basis of data from countries other than Slovenia.
In comparison to some other studies(Reference Power, Lake and Cole25, Reference Lake, Power and Cole42, Reference Rolland-Cachera, Deheeger and Guilloud-Bataille43), BMI in our population is well predicted from childhood. This is suggested by large correlations (above r = 0·50) between childhood and young adulthood BMI and a large proportion of obese and overweight 18-year-olds identified from childhood. According to the IOTF cut-off points for obesity, 40·0 % of Slovenian 18-year-old obese males and 48·6 % females would have been identified from their BMI at 7 years. The percentage identified increased with years, and it is notable that a large majority of the obese 18-year-olds had already been obese or overweight at 14 years (89·3 % of boys and 91·0 % of girls). The data suggest that children who today enter secondary schools obese are almost certain to remain so until the end of their schooling. Although Serdula et al.(Reference Serdula, Ivery and Coates47) estimated that less than a half of adult obesity can be attributed to childhood obesity, and our data support this finding, it should be noted that (taking into account overweight and obese BMI groups combined) over 73 % of males and over 85 % of 18-year-old obese females would have been classified as overweight or obese as early as 7 years, and the percentage identified would have increased to over 90 % with age in both sexes. In addition, the correlations of BMI at 18 years with BMI at earlier ages suggest that between one-third (at 7 years) and two-thirds (at 14 years) of the variability in BMI at 18 years was explained by BMI in childhood.
Finally, in contrast with the large correlations of height, weight and BMI between childhood and adulthood, those for TSF were only medium (r < 0·50, P < 0·001), except for the correlation of TSF at 11 and 14 years, where the correlations were large among females (r = 0·62, P < 0·001) and even larger among males (r = 0·70, P < 0·001). This finding is congruent with other findings, which suggested that precision of obesity prediction by TSF is very low up to late childhood and becomes somewhat better in adolescence, and that correlations are usually higher for males(Reference Clarke and Lauer22). This phenomenon could be attributed to the known methodological problems(Reference Must, Dallal and Dietz53). It has been reported previously that skinfold thickness measurements in obese subjects have poor reliability(Reference Forbes54), and that skin thickness and skinfold compressibility vary by age, by site and possibly also by sex(Reference Martin, Ross and Drinkwater55–Reference Clegg and Kent58). This suggests that using TSF curves for the classification of overweight and obese children should be used with all these problems in mind and that the use of BMI curves is more practical for the tracking of obesity from childhood to adulthood.
The child-to-adult relationship for obesity will continue to be of interest to researchers and policy makers, because policies to prevent childhood and adult obesity are under discussion in many countries(59). The evidence presented in the present study suggests that the prevention of adulthood obesity should focus on the identification and treatment of high-risk groups in childhood and adolescence. It is especially important to prevent and treat overweight and obesity at a young age, because children and adolescents of normal weight are very likely to retain normal weight. However, the relative risk for becoming an obese adult is greatly increased for those who are already obese or overweight as children or adolescents. From an epidemiological viewpoint, these are the critical periods when preventive measures such as nutrition and physical activity management could be applied.
Acknowledgements
Sources of funding: This study was funded by the Slovenian Ministry of Education and Sport. Conflict of interest declaration: The authors have no conflict of interest to declare. Authorship responsibilities: G.S. performed all data analysis and wrote the manuscript; J.S. wrote the protocol of the study and authored the SLOFIT system for monitoring the physical and motor development of children and youth.







