Micronutrient deficiencies are a major public health problem, especially in low-income countries, mostly affecting children and women of reproductive age( Reference Black, Victora and Walker 1 ). Micronutrient deficiencies are associated with increased prevalence of infectious diseases and hampered cognitive development in childhood. By impairing growth and school achievement in children and affecting reproductive functions and fetal development in women( Reference Black, Victora and Walker 1 ), they accentuate the intergenerational cycle of malnutrition( Reference Delisle 2 ).
The accuracy of micronutrient status assessment can be hampered by factors affecting biomarkers independently from status. Inflammation, for example, is known to affect biomarkers for Fe status (plasma ferritin (FER) concentration) and vitamin A status (plasma retinol and retinol-binding protein (RBP) concentrations) even in apparently healthy individuals( Reference Thurnham, Mburu and Mwaniki 3 ). A complex chain reaction involving immunoregulatory cytokines follows injury or infection and contributes to a redistribution of nutrients to different body compartments. This redistribution results in a withholding of essential nutrients to infectious agents (‘nutritional immunity’) as well as benefits repair processes( Reference Thurnham 4 ). However, this redistribution also affects the concentrations of biomarkers for micronutrient status in the circulation, while overall micronutrient status remains the same, unless an infection is prolonged or severe. At the same time, it appears that micronutrient deficiency can affect the acute-phase response (APR)( Reference Thurnham 4 ). Therefore, more precision is needed about the biological response to inflammation of biomarkers for micronutrient status. For biomarkers of vitamin A status (plasma retinol and RBP concentrations)( Reference Thurnham, McCabe and Northrop-Clewes 5 – Reference Rosales, Ritter and Zolfaghari 7 ), correction factors based on a meta-analysis have been proposed to adjust values in subjects during different stages of inflammation( Reference Thurnham, McCabe and Northrop-Clewes 5 ). A similar approach has been used for FER concentrations( 8 ). Fe deficiency (ID) is indicated by depleted Fe stores, corresponding to low plasma FER concentrations, and/or tissue Fe deficiency, indicated by elevated soluble transferrin receptor (TfR) concentrations( Reference Cook, Flowers and Skikne 9 ). The impact of inflammation on TfR concentrations is less clear, with some studies suggesting that TfR is not or less sensitive to inflammation than FER( Reference Beguin 10 ). Other studies, however, showed a significant impact of the APR on TfR values( Reference Phiri, Calis and Siyasiya 11 , Reference Punnonen, Irjala and Rajamäki 12 ). The effect of the APR on plasma Zn concentration remains unclear too( Reference Brown 13 , Reference Brown, Rivera and Bhutta 14 ).
The objective of the present study was therefore to investigate the impact of subclinical inflammation on the assessment of micronutrient deficiencies in three different populations and settings (Senegalese schoolchildren, Cambodian schoolchildren, Cambodian women of reproductive age) by investigating the impact of having elevated acute-phase proteins (APP), namely C-reactive protein (CRP) and α1-acid glycoprotein (AGP), on biomarkers for Fe, vitamin A and Zn status.
Participants and methods
Study area, design, population surveyed and ethics
Three data sets were available for the current study as follows.
Schoolchildren from Senegal
The study was a representative cross-sectional survey conducted in 2010 in children from primary state schools of Dakar, Senegal( Reference Fiorentino, Bastard and Sembène 15 ). A two-stage cluster sampling method was chosen, with schools considered primary sampling units. Within thirty randomly selected schools, and without criteria for age and gender, twenty children were randomized as final sampling units in each school, resulting in a sample of 594 apparently healthy schoolchildren.
Schoolchildren from Cambodia
Data were collected as part of a randomized placebo-controlled trial investigating the impact of multi-micronutrient-fortified rice on the health and development of Cambodian schoolchildren: the FORISCA (FOrtified RIce for School meals in CAmbodia) UltraRice®+NutriRice® study. Baseline data collection was conducted in November 2012 in twenty primary schools from Kampong Speu Province in Cambodia( Reference Perignon, Fiorentino and Kuong 16 ). The schools were randomly selected from primary schools participating in school meal or take-home ration programmes of the UN World Food Programme. Children attending the selected schools were eligible to be part of the study if they were between 6 and 16 years of age, had written informed consent from parents/caregivers and did not have any mental or severe physical handicap. In each school, 132 children were randomly selected after stratification by sex and grade; hence 2640 children in total. One hundred and sixty-nine children were not recruited because they were absent on the day of data collection or refused to participate. Thus, a total of 2471 apparently healthy schoolchildren, aged 6–16 years, participated in the blood sample collection.
Women of reproductive age from Cambodia
The last data set was provided by a serological survey for antibodies for tetanus, rubella and measles conducted in women of reproductive age in Cambodia in 2012. Six hundred and eleven enumeration areas were selected from the 28 764 enumeration areas in the 2008 Cambodia General Population Census by probability proportional to size( Reference Scobie, Mao and Buth 17 ). In each enumeration area, twenty-two households were randomly selected. All eligible women in selected households were invited to participate after providing written information about the survey and obtaining consent from women. In total, 2117 apparently healthy women participated in the blood sample collection. We used residual serum from this survey to determine FER, TfR, RBP, CRP and AGP concentrations.
Blood collection and laboratory analyses
Venous blood samples (4–5 ml) were collected by experienced phlebotomists using sterile single-use material: into a vacutainer with heparin (Heparin Venosafe®; Terumo, Japan) in Senegal and in Cambodian women, and into a vacutainer with no anticoagulant (Vacuette®; Greiner Bio One, Austria) in Cambodian children, all trace-element-free. Blood samples were stored immediately in the field in an icebox containing ice-packs and transported to the laboratory within a maximum of 5 h after the first sample withdrawal. Plasma/serum samples were separated by centrifugation, aliquoted and stored at −20°C. Centrifugation resulted in serum samples in Cambodian children and in plasma samples in Cambodian women and Senegalese children. However, all samples are referred to as ‘plasma’ herein for simplicity.
For plasma Zn measurement, samples collected from Senegalese schoolchildren were sent on dry ice to the Nutripass laboratory of the Institut de Recherche pour le Développement (IRD, Montpellier, France); and samples collected from Cambodian schoolchildren to the National Institute of Nutrition (NIN, Hanoi, Vietnam). Zn status was not measured in Cambodian women of reproductive age. In both laboratories, plasma Zn was measured by flame atomic absorption spectrophotometry, using trace-element-free procedures and certified controls.
Samples from the three studies were sent on dry ice to the VitMin Laboratory (Willstaett, Germany) for determination of RBP, CRP, FER, TfR and AGP concentrations. RBP, FER, TfR, CRP and AGP were measured by a sandwich ELISA technique( Reference Erhardt, Estes and Pfeiffer 18 ).
Inflammation was defined as elevated CRP (>5 mg/l) and/or elevated AGP (>1 g/l), allowing differentiation between incubation phase (high CRP and normal AGP), early convalescence phase (both high AGP and CRP) and late convalescence phase (high AGP and normal CRP)( Reference Thurnham, McCabe and Haldar 19 ).
Tissue Fe deficiency was defined as TfR>8·3 mg/l( Reference Erhardt, Estes and Pfeiffer 18 ) and depleted Fe stores was defined as FER<15 µg/l( 20 ). ID was defined as depleted Fe stores (low FER) and/or tissue Fe deficiency (high TfR).
Body Fe was calculated according to the formula of Cook et al.( Reference Cook, Flowers and Skikne 9 ): body Fe (mg/kg)=−[log (TfR/FER)−2·8229]/0·1207. Body Fe deficiency (BID) was defined as body Fe<0 mg/kg( 8 ).
A third way to evaluate ID as ‘high TfR/log FER index’, called ID-TFI herein, was defined using a cut-off of 7·06 (=8·3/log(15))( Reference Cook, Flowers and Skikne 9 ).
Vitamin A status was determined by RBP concentration, which reflects plasma retinol concentration. RBP occurs in a 1:1:1 complex with retinol and transthyretin, which is assumed to be less affected by inflammation than retinol concentrations( Reference de Pee and Dary 21 ). Vitamin A deficiency (VAD) was defined as RBP <0·7 µmol/l( Reference Engle-Stone, Haskell and Ndjebayi 22 ). Marginal VAD was defined as RBP value ≥ 0·7 µmol/l and <1·05 µmol/l, as it was initially defined in adults( Reference West 23 , Reference Tanumihardjo 24 ). Zn deficiency was defined as plasma Zn<9·9 µmol/l, 10·1 µmol/l or 10·7 µmol/l for children aged <10 years, girls aged >10 years and boys aged >10 years, respectively( Reference Gibson 25 ). Children were considered severely Zn deficient when plasma Zn was <7·7 µmol/l( Reference Brown, Rivera and Bhutta 14 ).
Statistical analysis
Data entry, including quality checks and validation by double entry of questionnaires, was performed with EpiData version 3.1 (EpiData, Odense, Denmark). Data management and analyses were performed using the statistical software package IBM SPSS Statistics version 20.0. Significance was defined as P<0·05. The distributions of biomarker concentrations were checked for normality using normality plots and Kolmogorov–Smirnoff tests. Because distributions of AGP, CRP, TfR and FER were skewed, they were log-transformed before statistical analysis. Spearman’s rank correlation coefficients were determined to assess relationships between APP and micronutrient status biomarkers. For biomarkers that were correlated to APP concentrations, we calculated geometric mean values in each subgroup with inflammation (incubation, early convalescence, late convalescence, high AGP or high CRP) and in each reference group (no inflammation, normal AGP or normal CRP). We then calculated the ratio of the geometric mean value in the subgroup with inflammation to the mean value in the corresponding reference group without inflammation( Reference Thurnham, McCabe and Northrop-Clewes 5 , Reference Thurnham, McCabe and Haldar 19 , Reference Righetti, Glinz and Adiossan 26 , Reference Grant, Suchdev and Flores-Ayala 27 ). The correction factor (CF) was calculated as 1/ratio. Prevalence of poor micronutrient status was calculated: (i) without correction; (ii) only in the subjects with no inflammation; (iii) using biomarker concentrations adjusted with CF calculated in the present study; (iv) using FER and RBP concentrations adjusted with CF recommended by Thurnham and co-workers( Reference Thurnham, McCabe and Northrop-Clewes 5 , Reference Thurnham, McCabe and Haldar 19 ); and (v) using 30 µg/l as low FER cut-off in children with inflammation( 8 ). Corrected prevalences were compared with the uncorrected prevalences using McNemar’s χ 2 test.
Results
Biochemical characteristics of the participants from the three different samples are presented in Table 1. Although similar in gender proportions (half of children were female) and age (approximately 10 years), the populations of schoolchildren from Cambodia and Senegal differed in inflammatory and micronutrient status. Prevalence of inflammation was less than 15 % in Senegalese children and Cambodian women but was 40 % in Cambodian children, most of them being in the late convalescence phase (high AGP and normal CRP). ID was prevalent, being present in one in three children in the Senegalese study population, half of the children and in one in seven women in the Cambodian study populations. In Cambodian children, ID was mostly related to a high TfR (only 1 % had low FER); while in Senegalese children, respectively one-third and one-fifth had a high TfR and a low FER. In Cambodian women, the proportion of high TfR was similar to the proportion of low FER (8−10 %). Prevalence of VAD was less than 5 % in all samples. Marginal vitamin A status was present in more than 40 % of Senegalese children, while less than 15 % of the Cambodian subjects had a marginal vitamin A status. Zn deficiency affected one-quarter of the children in Senegal and more than 90 % of the Cambodian children.
Table 1 Demographic and biochemical characteristics of participants: schoolchildren from Senegal, schoolchildren from Cambodia and women of reproductive age (WRA) from Cambodia

CRP, C-reactive protein; AGP, α1-acid glycoprotein; FER, ferritin; TfR, soluble transferrin receptor; ID, Fe deficiency; BID, body Fe deficiency; ID-TFI, Fe deficiency defined by high TfR/log FER index; RBP, retinol-binding protein; VAD, vitamin A deficiency.
Data are presented as mean and standard deviation or as percentage.
† Zn<9·9 µmol/l, 10·1 µmol/l or 10·7 µmol/l, respectively, in children aged <10 years, girls aged >10 years and boys aged >10 years.
‡ Zn<7·7 µmol/l.
Correlations between APP and biomarkers of Fe, vitamin A and Zn status are presented in Table 2. CRP and AGP were highly positively correlated in all samples. FER was highly positively correlated with both CRP and AGP in all samples. TfR was positively correlated with AGP in all samples, and with CRP in the Cambodian schoolchildren. Contradictory results were found between indicators based on the TfR/FER ratio (body Fe and TfR/log FER index) and APP among the different groups: TfR/log FER index was negatively correlated with CRP in both Senegalese children and Cambodian women, while this index was positively correlated with AGP in Cambodian children. Similarly, correlations between body Fe and AGP were positive in Senegalese children and Cambodian women, but negative in Cambodian children.
Table 2 Spearman correlation coefficients (ρ) between inflammatory biomarkers and micronutrient status variables in Senegalese schoolchildren, Cambodian schoolchildren and Cambodian women of reproductive age (WRA)
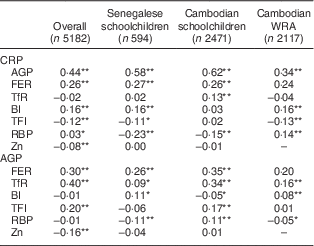
CRP, C-reactive protein; AGP, α1-acid glycoprotein; FER, ferritin; TfR, soluble transferrin receptor; BI, body Fe; TFI, TfR/FER index; RBP, retinol-binding protein.
*P<0·05; **P<0·001.
In most subjects, RBP was negatively correlated with CRP and AGP. In Cambodian schoolchildren, however, RBP was negatively associated with CRP but positively associated with AGP. No correlation between Zn and CRP or AGP was found in subgroups but in all subjects combined, Zn was negatively associated with CRP and AGP. For biomarkers that were significantly correlated with CRP and/or AGP (FER, TfR, RBP and Zn), CF were calculated for groups according to inflammatory status (Tables 3–6).
Table 3 Ferritin (FER) concentrations, ratios with 95 % confidence intervals and correction factors (CF) by inflammatory status in Senegalese schoolchildren, Cambodian schoolchildren and Cambodian women of reproductive age (WRA)
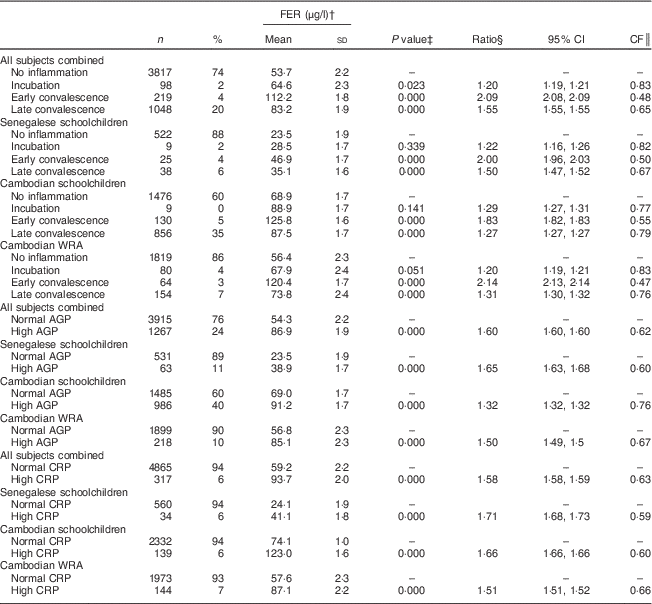
AGP, α1-acid glycoprotein; CRP, C-reactive protein.
† Geometric means and sd.
‡ ANOVA on log-transformed FER means of positive group v. control group.
§ Ratio of back-transformed FER concentrations of positive group v. control group.
║ CF=1/ratio.
Table 4 Soluble transferrin receptor (TfR) concentrations, ratios with 95 % confidence intervals and correction factors (CF) by inflammatory status in Senegalese schoolchildren, Cambodian schoolchildren and Cambodian women of reproductive age (WRA)
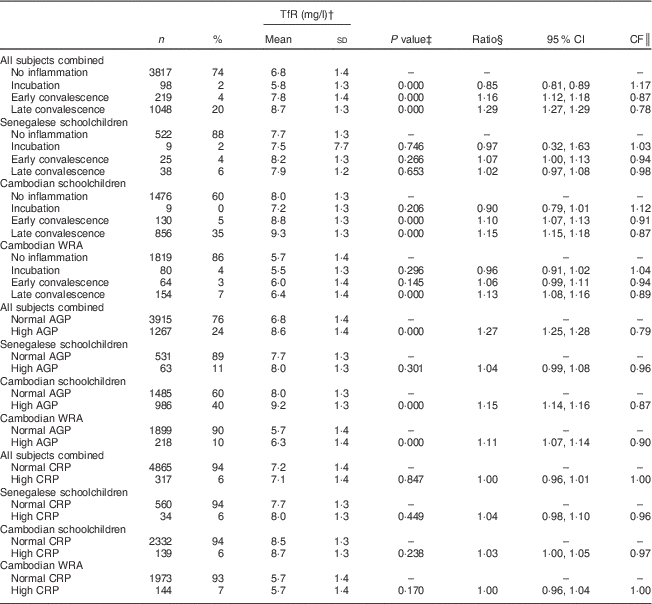
AGP, α1-acid glycoprotein; CRP, C-reactive protein.
† Geometric means and sd.
‡ ANOVA on log-transformed TfR means of positive group v. control group.
§ Ratio of back-transformed TfR concentrations of positive group v. control group.
║ CF=1/ratio.
Table 5 Retinol-binding protein (RBP) concentrations, ratios with 95 % confidence intervals and correction factors (CF) in Senegalese schoolchildren, Cambodian schoolchildren and Cambodian women of reproductive age (WRA)
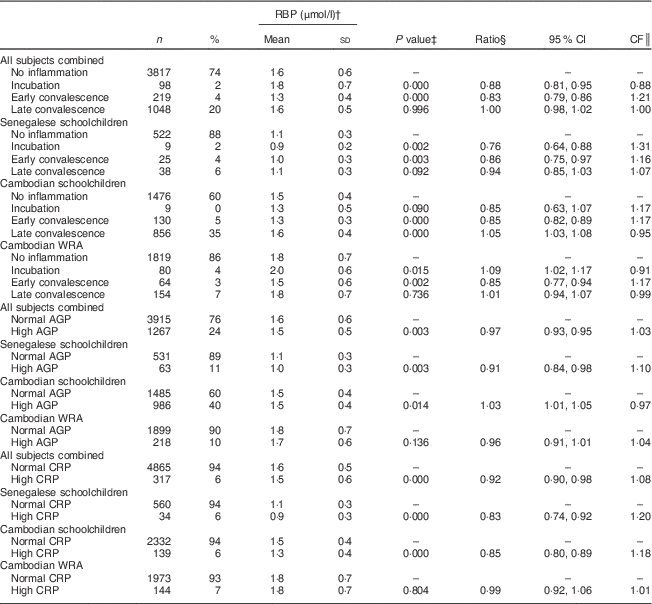
AGP, α1-acid glycoprotein; CRP, C-reactive protein.
† Geometric means and sd.
‡ ANOVA on log-transformed RBP means of positive group v. control group.
§ Ratio of back-transformed RBP concentrations of positive group v. control group.
║ CF=1/ratio.
Table 6 Zinc concentrations, ratios with 95 % confidence intervals and correction factors (CF) in Senegalese schoolchildren, Cambodian schoolchildren and Cambodian women of reproductive age (WRA)

AGP, α1-acid glycoprotein; CRP, C-reactive protein.
† Geometric means and sd.
‡ ANOVA on log-transformed Zn means of positive group v. control group.
§ Ratio of back-transformed Zn concentrations of positive group v. control group.
║ CF=1/ratio.
For indicators of Fe status, the effect of inflammation was stronger on FER than on TfR with all CF being lower than 0·8. Mean FER was significantly higher in participants in incubation, early convalescence and late convalescence phase, or with elevated AGP or CRP in all subjects (Table 3). Elevated CRP had no influence on TfR concentrations (Table 4), whereas elevated AGP was associated with higher TfR concentrations. TfR concentrations were significantly lower in all subjects combined during the early incubation phase, and higher in later stages of inflammation. The maximum inflammation effect on TfR concentrations in the later stages of inflammation was less than 25 % (lowest CF being 0·78).
In children, but not in women, RBP concentrations were significantly lower when CRP was elevated (Table 5). During incubation, mean RBP concentrations were lower in children but higher in women. Concentrations were lower to the same extent in all populations during early convalescence.
In all children combined, Zn concentrations tended to be lower in early convalescence and were significantly lower during late convalescence and with elevated AGP (Table 6). These effects were not significant in the two subgroups of Senegalese children and Cambodian children.
The effect of correcting micronutrient biomarkers for inflammation on the estimated prevalence of deficiency is shown in Table 7. Uncorrected prevalence of high TfR was higher than the corrected prevalence in all samples combined, as well as in subgroups of Cambodian children and women (Table 7). Uncorrected prevalence of Fe stores deficiency was lower than the corrected prevalence, especially in Senegalese children and Cambodian women. The difference between the corrected and uncorrected prevalence of ID was lower than 2 % in Senegalese children and Cambodian women, but about 8 % in Cambodian children. BID and ID-TFI were less sensitive to inflammation. Prevalence of VAD was below 4 % in all samples and difference between adjusted and unadjusted prevalence was below 1 %.
Table 7 Effect of correcting ferritin (FER), soluble transferrin receptor (TfR) and retinol-binding protein (RBP) concentrations on the prevalence of low iron status and low vitamin A status in Senegalese schoolchildren, Cambodian schoolchildren and Cambodian women of reproductive age (WRA)
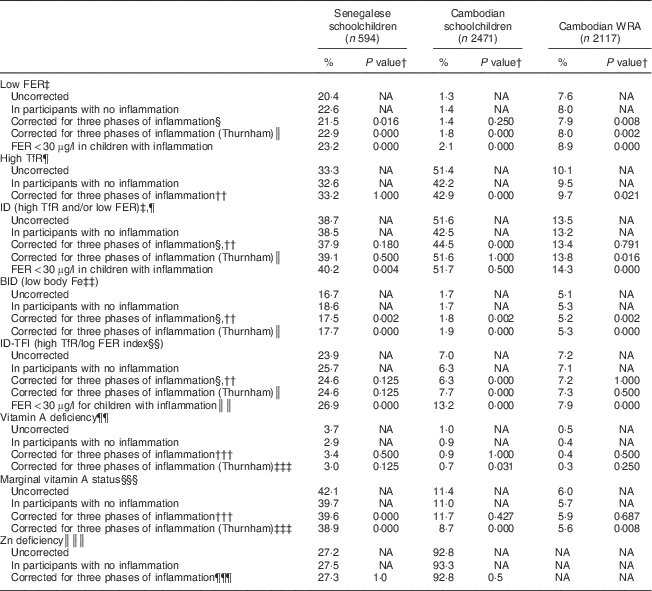
ID, Fe deficiency; BID, body Fe deficiency; ID-TFI, Fe deficiency defined by high TfR/log FER index; NA, not applicable; CF, correction factor.
† McNemar’s χ 2 test of proportion to compare uncorrected prevalence and corrected prevalence.
‡ FER<15 µg/l for children and WRA, except if notified.
§ Using CF in Table 3.
║ Thurnham’s CF for incubation, early convalescence and late convalescence: 0·64, 0·39 and 0·65 in children; 0·73, 0·58 and 0·85 in women.
¶ TfR>8·3 mg/l.
†† Using CF in Table 4.
‡‡ Body Fe<0 mg/kg.
§§ TfR/logFER>7·05 (corresponding to 8·3/log15).
║║ TfR/logFER>5·3 (corresponding to 8·3/log30).
¶¶ RBP<0·7 µmol/l.
††† Using CF in Table 5.
‡‡‡ Thurnham’s CF for incubation, early convalescence and late convalescence: 0·87, 0·76 and 0·89.
§§§ RBP<1·05 µmol/l.
║║║ Zn<9·9 µmol/l, 10·1 µmol/l or 10·7 µmol/l, respectively, in children aged <10 years, girls aged >10 years and boys aged >10 years.
¶¶¶ Using CF in Table 6.
Discussion
Inflammation is known to affect indicators of Fe status (FER concentration) and vitamin A status (retinol and RBP concentrations), an effect we confirmed in the present study. Other indicators such as plasma Zn and TfR concentrations are thought to be affected as well, but these indicators have been less studied. In animal models, the fall in plasma Zn concentration occurs before clinical symptoms arise (hence just after infection) and returns to normal quickly( Reference Brown 13 ). A rapid fall in plasma Zn following infection was also observed in human subjects( Reference Beisel 28 ). However, in our cohorts of schoolchildren in Cambodia and Senegal, inflammation affected plasma concentrations of Zn only slightly. The prevalence of Zn deficiency after adjustment was similar to the prevalence among children without inflammation. Duncan et al. ( Reference Duncan, Talwar and McMillan 29 ) reported Zn concentrations of Scottish hospital patients to be significantly negatively correlated to CRP (ρ=−0·16). However, in these hospital patients, the degree of inflammation was much more severe than in our normal populations with the causes of inflammation differing, and therefore the subjects in the Scottish study cannot be classified as having ‘subclinical inflammation’. Indeed, in the study of Duncan et al., Zn concentrations were not significantly lower in patients with CRP concentrations <20 g/l, a situation similar to ours. Therefore, our findings suggest that for determining Zn status in populations with a low prevalence of subclinical infection, taking the APR into account will not lead to a significant improvement in the estimate of the prevalence of Zn deficiency. This is consistent with the conclusions of an earlier review indicating no effect of inflammation on Zn status in children( Reference Thurnham, Mburu and Mwaniki 3 ).
To our knowledge, no correction factors for adjusting TfR concentration for inflammation have been suggested. TfR was positively correlated with AGP, as well as with CRP in Cambodian schoolchildren. However, TfR concentrations were higher only in groups in early and late convalescence phases. This is consistent with studies suggesting that FER reacts faster to the APR than TfR( Reference Fiorentino, Bastard and Sembène 15 , Reference Perignon, Fiorentino and Kuong 30 ). It is possible that TfR responds only indirectly to the APR, as a regulating response during convalescence. During the first phase of inflammation FER responds directly, regulated through higher hepcidin concentrations reducing circulating Fe and Fe absorption( Reference Wessling-Resnick 31 ). Then when the convalescence starts, the shortage of tissue Fe indicated by elevated TfR may result from the compensation of reduced circulating Fe. However, significantly lower TfR concentrations were observed in all subjects combined during the incubation phase, similar to reports by Righetti et al. ( Reference Righetti, Glinz and Adiossan 26 ). Although TfR has been long considered more reliable than FER to indicate poor Fe status of populations when inflammation is prevalent( Reference Bui, Stein and DiGirolamo 32 ), TfR was significantly higher during inflammation phases in Cambodian women and children, as also found in other studies( Reference Black 33 ). In our cohorts, TfR concentrations were higher during inflammation leading to an overestimation of ID when using TfR, which is again in line with results from Righetti et al. ( Reference Righetti, Glinz and Adiossan 26 ). In the cohort of Cambodian children, who had a high prevalence of inflammation, the prevalence of high TfR (indicative of tissue Fe deficiency) was almost 10 % higher, as compared with children without inflammation (51 v. 42 %). Similar effects on TfR were reported for Ivorian infants who also had a high prevalence of inflammation( Reference Thurnham, McCabe and Northrop-Clewes 5 – Reference Rosales, Ritter and Zolfaghari 7 ). However, when inflammation was not very prevalent, such as in the Cambodian women and Senegalese children, adjusting the prevalence of tissue Fe deficiency for inflammation did not modify the estimated prevalence of tissue Fe deficiency in these populations.
Use of TfR/log FER rather than TfR/FER( Reference Thurnham 4 ) to predict ID has been recommended, especially in areas with endemic infection( Reference Rosales, Ritter and Zolfaghari 7 ). Instead of calculating CF to apply to TfR/log FER index as has been done by Righetti et al. ( Reference Righetti, Glinz and Adiossan 26 ), we used corrected values of FER and TfR to calculate TfR/log FER index or body Fe, with prevalence of ID defined by TfR/log FER index>7·06 or by a negative value for body Fe. Depending on the biomarker for Fe status, that is whether ID was indicated primarily by low FER concentration (depleted Fe stores) or by elevated TfR concentration (tissue Fe deficiency), inflammation resulted in either over- or underestimation of the prevalence of ID defined by high TfR/log FER index (ID-TFI) or negative body Fe (BID). Overall, the difference of corrected prevalence between uncorrected prevalence and the reference prevalence in children without inflammation was low (<2 %), suggesting that indicators based on both TfR and FER were least sensitive to inflammation, compared with others. Thus, only when CRP and AGP cannot be measured do our findings suggest that indicators based on both TfR and FER, such as TfR/log FER index, may be used to assess Fe status and ID prevalence of populations with a high prevalence of inflammation. However, assessment of Fe store levels and tissue Fe levels would still be affected by inflammation.
The adjusted prevalence of ID using our CF for both FER and TfR was very close to that obtained using Thurnham’s CF for FER in Cambodian women and Senegalese children, suggesting that in these cohorts adjusting FER concentrations for inflammation is more important than adjusting TfR for inflammation to obtain a more precise estimate of ID. However, using Thurnham’s CF for FER only in the Cambodian children, without adjusting for TfR, led to overestimation of ID defined by high TfR and/or low FER, because in this sample FER concentrations were high, making TfR the most important factor for indicating ID.
Not adjusting FER concentrations for inflammation can lead to underestimation of the prevalence of low Fe stores, especially in populations with endemic inflammation. Thurnham’s CF were shown to be accurate to adjust FER status( Reference Cichon, Ritz and Fabiansen 34 ). Also in the present study, the prevalence of depleted Fe stores in the three samples adjusted with either Thurnham’s CF or ours was similar to the prevalence of depleted Fe stores in subgroups with no inflammation. The small effect of adjusting the prevalence of depleted Fe stores for inflammation in the three populations in our study is readily explained either by the low prevalence of inflammation in Cambodian women and Senegalese children or by the low prevalence of low Fe stores in Cambodian children and women. Hence, inflammation only had a large impact on the estimate of the prevalence of low Fe stores when both the prevalence of inflammation and the prevalence of low Fe stores were high. Overall, using 30 µg/l as a cut-off for FER in children with inflammation as suggested by WHO( 35 ) performed less efficiently than adjustment for inflammation by use of APP for accurate prevalence of ID.
In children from Senegal and Cambodia, RBP was lower in the incubation and early convalescence phases, which is consistent with other studies( Reference Thurnham, McCabe and Northrop-Clewes 5 – Reference Rosales, Ritter and Zolfaghari 7 ). Indeed, retinol is known to fall rapidly, within 48 h of the onset of inflammation( Reference Goodman 36 ). During late convalescence, RBP was still lower in Senegalese children but higher in Cambodian children compared with participants with no inflammation; the latter has been reported earlier, in a small sample of Zambian schoolchildren( Reference Bresnahan, Chileshe and Tanumihardjo 37 ). A meta-analysis using the same CRP and AGP cut-offs as in the present study showed that the APR-induced decrease of retinol lasted during the late convalescence phase( Reference Thurnham, McCabe and Northrop-Clewes 5 ). However, this meta-analysis was conducted in populations with a poor vitamin A status (median retinol<1·05 µmol/l). Retinol (and hence RBP) concentrations are expected to rebound during convalescence( Reference Brown 13 ). We believe that baseline vitamin A status might explain the difference between Senegalese and Cambodian children. Forty per cent of the Senegalese children had marginal vitamin A status, whereas vitamin A status in Cambodian children was adequate. Therefore, the reaction of vitamin A biomarkers to the different phases of inflammation may differ according to the initial vitamin A status, suggesting that baseline vitamin A status should be taken into account when correcting for inflammation. The interaction between inflammation and initial vitamin A status and its effect on the assessment of vitamin A status should be further investigated. More data on populations with a relatively good vitamin A status are needed to confirm this. But if found correct, it could affect the way to correct vitamin A status for inflammation. In Senegalese children, the CF proposed by Thurnham (and calculated using populations with poor vitamin A status) resulted in a prevalence of VAD very close to the prevalence in children without inflammation. In Cambodian subjects, however, the CF proposed by Thurnham led to a significant but slight underestimation of the prevalence of VAD compared with adjusting by using the CF calculated in the present study, presumably because vitamin A concentrations rebounded earlier in this population.
Prevalence of inflammation is considered low under 15 % and to have only a modest impact on the assessment of micronutrient deficiencies( Reference Beisel 38 , Reference Mitra, Alvarez and Guay-Woodford 39 ). However, consensus about cut-offs to define what should be considered an elevated APP is needed. Indeed, the sensitivity of the <5 mg/l cut-off commonly used for CRP has recently been questioned( Reference Dillon, Htet and Chiwile 40 ). Several studies used a cut-off different from 5 mg/l for CRP and/or 1 g/l for AGP, or a series of cut-offs to define categories of inflammation( Reference Beisel 38 ). Some studies showed similar patterns of impact of the APR on micronutrient biomarkers using either 5 or 10 mg/l for the CRP cut-off( Reference Hotz, Chileshe and Siamusantu 41 ), while one study indicated that the best CRP cut-off could vary from 5, 10 to 20 mg/l depending on the measured biomarker( Reference Wieringa, Dijkhuizen and West 42 ). Moreover, different cut-offs for different ages may be needed( Reference Thurnham, McCabe and Northrop-Clewes 5 ). Finally, the definition of ‘apparently healthy individuals’ might have differed between researchers involved in the different studies. Hence, an international recommendation about APP cut-offs to categorize inflammation is needed as well as a meta-analysis on the impact of inflammation on a broad range of micronutrient biomarkers.
Our study had several limitations. First, the Fe status of populations was heterogeneous with Cambodian children having adequate Fe stores but a high proportion of high TfR, while ID in Cambodian women and in Senegalese children was both due to low Fe stores and Fe tissue deficiency. However, this gave us the opportunity to examine the impact of inflammation on micronutrient status in different population profiles and to bring out original observations compared with previous research. Second, the prevalence of inflammation in Senegalese children and Cambodian women was low, reducing the power of the study to investigate the impact of inflammation. Third, the sample size of Senegalese children was small, hence more data should be gathered in African populations on the impact of inflammation on indicators of micronutrient status.
Conclusion
Inflammation affected the biomarkers of vitamin A and Fe status, but had little impact on the biomarker for Zn status in the populations studied. This suggests that ideally FER, TfR and RBP should be adjusted for inflammation measured by both CRP and AGP to differentiate the different phases of inflammation, especially in areas with a high infection pressure. We also proposed a correcting factor for TfR that has to be confirmed, ideally through a large meta-analysis or pooled analyses such as those carried out on retinol and FER( Reference Thurnham, McCabe and Northrop-Clewes 5 , Reference Thurnham, McCabe and Haldar 19 ). Divergent responses of RBP to incubation and convalescence phases were observed according to the studied populations, presumably depending on the vitamin A status of the population. More research should be conducted on APP cut-offs to define the different phases of inflammation and the sensitivity of micronutrient biomarkers, especially TfR and RBP, to inflammation in relation to the underlying micronutrient status.
Acknowledgements
Acknowledgements: The authors thank all the children and their families from Senegal and Cambodia, and the women from Cambodia, who participated into the study. They would like to thank Richard de Groot for his help with statistical analysis. Financial support: The study on Cambodian children was funded by the US Department of Agriculture/Foreign Agricultural Service (USDA/FAS), the UN World Food Programme (WFP)–DSM consortium and the Institut de Recherche pour le Développement (IRD). The USDA/FAS and the WFP–DSM consortium had no role in the design, data analysis or writing of this article. The study on Cambodian women was funded by the Centers for Disease Control and Prevention (CDC). The CDC had no role in the design, data analysis or writing of this article. The study in Senegal was funded by Danone. Danone had no role in the design, data analysis or writing of this article. Conflict of interest: None. Authorship: M.F., F.T.W. and J.B. formulated the research questions. M.F., F.T.W., M.P., K.K. and C.C. designed and carried out the study in Cambodian children, and M.F. and J.B. the study in Senegalese children. M.F. and J.B. designed and M.F. carried out the data analysis. M.F., F.T.W. and J.B. and wrote the article that was reviewed and agreed by all co-authors. Ethics of human subject participation: The study was conducted according to the guidelines laid down in the Declaration of Helsinki. All procedures involving human subjects were approved by the National Health Research of Senegal (for the study in Senegal); by the National Ethics Committee for Health Research (NECHR) of the Ministry of Health, Phnom Penh, Cambodia (for the study in Cambodian children and in Cambodian women); by the Ministry of Education, Youth and Sports, Phnom Penh, Cambodia and the Research Ethical Committee (REC) of PATH, Seattle, WA, USA (for the study in Cambodian children); and by the CDC, Atlanta, GA, USA (for the study in Cambodian women). Written informed consent was obtained from all women enrolled in the study and from all parents of children enrolled in the study.










