The consumption of smokeless tobacco (SLT) has been reported in 127 countries(Reference Siddiqi, Husain and Vidyasagaran1). As per the Comprehensive Smokeless Tobacco Health Education Act (CSTHEA), under the Centre for Disease Control and Prevention (CDC), SLT is defined as ‘any finely cut, ground, powdered, or leaf tobacco that is intended to be placed in the oral cavity’ (CDC). SLT comes as either snuff or chewing tobacco. More than 85 % of the SLT-related burden is present in South and South-East Asia, followed by Africa(Reference Siddiqi, Husain and Vidyasagaran1). When compared to smoking, SLT use was found to be higher among women in many low- and middle-income countries mainly due to its social acceptability and the notion of it being less harmful than smoking(Reference Sansone, Raute and Fong2). About 2·5 million disability-adjusted life years and 90 791 lives were lost globally due to oral, pharyngeal and oesophageal cancers owing to SLT use(Reference Siddiqi, Husain and Vidyasagaran1). Though studies have also shown adverse health impacts of SLT including cardiovascular and reproductive morbidities amongst both men and women(Reference Hergens, Alfredsson and Bolinder3), so far no attempts have been made to understand the linkages with food insecurity and nutrition. This study examined the available global shreds of evidence that links SLT use with public health nutrition and food insecurity and its possible implications on malnutrition, poor birth outcomes and metabolic disorders.
There have been a plethora of evidence-based researches that study the impact of smoking on diet, dietary antioxidants, body weight and metabolic disorders(Reference Zhang, Wang and Li4–Reference Cosnes and S Karger10). Some previous studies have also shown linkages between smoking and malnutrition in developing countries. For instance, a survey amongst the low- and middle-income households in rural Indonesia reported that households comprising one smoker spent less on food budget (68 %) as compared to the households with non-smokers (75 %)(Reference Block and Webb11). Habitual smoking by parents not only hampers the quality but also the quantity of the food consumed by the poorest households, which further leads to significant fall in the nutritional status of children in these households(Reference Block and Webb11).
The available literature is deficient in studies that links SLT and nutrition despite its well-established linkages with various cancers(Reference Sinha, Suliankatchi and Gupta12) and non-communicable diseases(Reference Thakur, Garg and Narain13). Particularly lacking is an understanding of SLT control as an important development issue and its linkages to the food and nutrition discourse. There was thus a clear and significant information gap in the existing literature on SLT use and its implication on nutritional outcomes based on the global evidence.
Methods
Protocol and guidelines
We followed the guidelines of Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) checklist for the elaboration of this systematic review(Reference Shamseer, Moher and Clarke14). The systematic review entails evidence on articulated questions that use systematic and explicit methods to identify, select and critically assess relevant primary research, and to extract and analyse data from the studies included in the review (Systematic Reviews: CRD’s guidance for undertaking reviews in health care, 2009)(Reference Tacconelli15). This review examines the highest level of evidence by combining the relevant data from the existing literature to derive informed decisions. The review has constructed themes based on the reported evidence and synthesised qualitative summary tables. The systematic review protocol has been registered with PROSPERO International’s prospective register of systematic reviews (registration number: CRD42021253694). The review was formulated to address mainly two research questions: (1) What is the impact of SLT on nutritional outcomes which includes body weight, malnutrition, anaemia and birth outcomes? and (2) What is the impact of SLT on metabolic disorders?
The preliminary conceptual framework that is used has given information on the development and segregation of many domains that had been impacted by SLT. This was seen after triangulating the findings of the thirty-four review papers. In generating the framework, a panel of experts panel considered themes and concepts identified under each of the categories from the evidence systematic review, post which, the authors structured and reorganised concepts under main health, functioning and conceptually related domains. This framework provided the basis and insights to comprehend the impact of SLT on health and functioning and conceptually related domains(Reference Afolalu, Spies and Bacso16).
Database and search strategy
The total number (n 1880) of research papers were extracted from an electronic advance search of PubMed and Scopus published from January 2000 to December 2020 for review. Search criteria included MeSH terms with ‘smokeless tobacco’ and ‘underweight’, ‘anemia’, ‘BMI’, ‘diet’, ‘food’, ‘food security’, ‘fruit’, ‘hunger’, ‘malnutrition’, ‘obesity’, ‘vegetables’, ‘hunger’, ‘nutrition’, ‘fruits’ and ‘vegetables’.
Inclusion and exclusion criteria
Research articles were then selected after discerning relevant literature pertaining to the underlying objective. Organisation of research articles was done on seeking any sort of relationship between SLT and nutritional outcomes like BMI, malnutrition, anaemia, poor birth outcomes and metabolic disorders (n 355). The search strategy is shown in Fig. 1. A total number of thirty-four papers were finally used in the systematic review (Tables 1 and 2).

Fig. 1 The PRISMA flowchart for selection of epidemiological studies. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analysis; SLT, smokeless tobacco
Table 1 Summary of the selected studies used in systematic review (high-income countries)
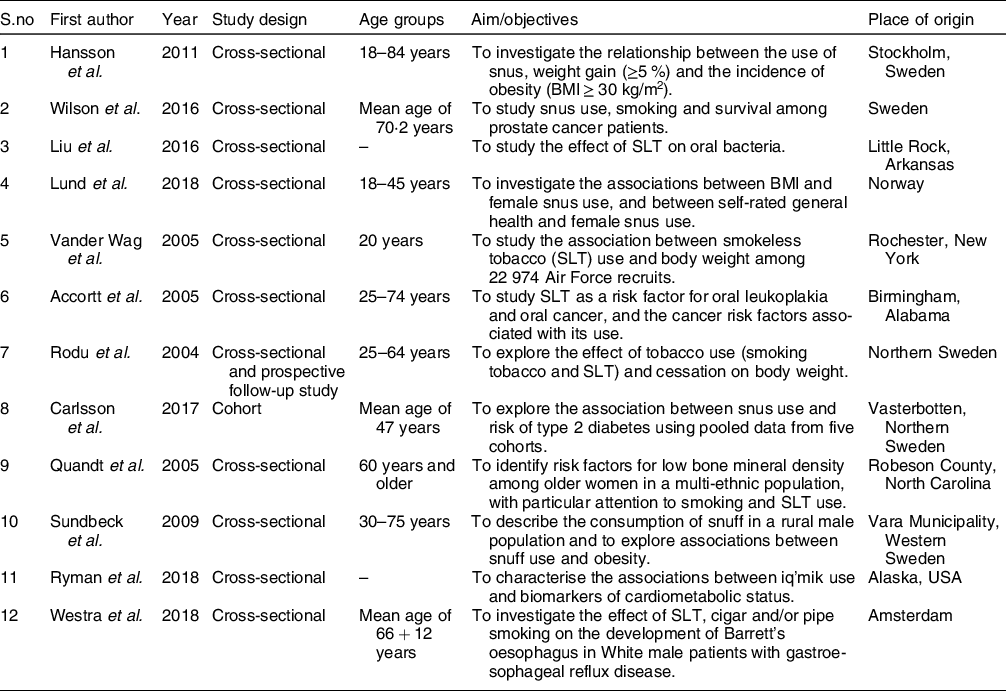
Table 2 Summary of the selected studies used in systematic review (low-income and middle-income countries)

SLT, smokeless tobacco.
Following was the inclusion criteria: (i) primary research papers published from 2000 to 2020 in English language and (ii) exposure variable: SLT. Studies that encompassed smoking were considered only if SLT had contributed to the outcome in combination with smoking; (iii) outcome variable: nutrition-related factors, that is, BMI, body weight, malnutrition, anaemia, poor birth outcomes and metabolic disorders; and (iv) study design: cross-sectional, cohort and review papers.
Following was the exclusion criteria: (i) studies that solely focused on smoking conducted on mice were excluded; and (ii) extracts from Cochrane articles, case reports and letters were also excluded.
Results
Characteristics of the included studies
The selected studies comprised cross-sectional (thirty-one) and cohort (three) studies. Half of the studies were from the South-East Asia region (seventeen), out of which eleven were from India followed by Bangladesh (two), Pakistan (two) and Nepal (one). Six studies were included from Scandinavian countries which were based on the impact of snus use. Five studies were from the USA and three were from African countries. These studies represented age diversity with the inclusion of primary school children, teenagers and adults (13–60 years of age). Studies described the general population with different demographic and socio-economic conditions.
Smokeless tobacco, food expenditure and food insecurity
The cause of food insecurity is not merely access and consumption but adequate absorption of the nutrients in adequate quantity and quality(Reference Headey17). For studying the association between tobacco and household food insecurity, a study from Nepal reported a higher household food insecurity among households where men either smoked or consumed SLT as compared to those households where none of the members either smoked or consumed SLT(Reference Sreeramareddy and Ramakrishnareddy18). In the USA, a study based on National Health and Nutrition Examination Survey (NHANES) from 1999 to 2014 showed that not only smoking but also the usage of alternative tobacco products including SLT has been significantly associated with household food insecurity(Reference Mayer, Gueorguieva and Ma19).
A household survey on tobacco and food expenditure patterns in Bangladesh reported that the tobacco expenditure was quite high in households with dual-tobacco users (smoking and smokeless) as compared to expenses incurred on cooking oil and other fat items(Reference Virk-Baker, Husain and Parascandola20). Further comparisons were made on the basis of food energy gain on a daily basis if the money was spent to purchase cereals among the smoking-only, smokeless-only and dual-tobacco user households. A potential gain of daily food energy found was 857, 437 and 1512 kcals, respectively(Reference Virk-Baker, Husain and Parascandola20). Another study from Mumbai, India, found higher daily expenditure on gutka (a form of SLT) as compared to food items among street children aged 6 to 8 years(Reference Shoba, Efroymson and FitzGerald21). While choosing tobacco over food, the study noted that these children were not only limiting their options to a healthy diet but also suffering from its ill effects, that is, cough, sore throat, weakness and breathing problems(Reference Shoba, Efroymson and FitzGerald21).
Smokeless tobacco and BMI
There is adequate evidence of nicotine cessation leading to weight gain among smokers due to the regulation of appetite(Reference Jo, Talmage and Role22), but there is hardly any literature on the proven linkage between the interference of SLT use and appetite or dietary outcomes. However, the reviewed literature demonstrated evidence of change in the weight and thus BMI with SLT use. For instance, a study from the USA observed that users consuming SLT for more than 30 d were found obese. Likewise, the chances of being categorised as overweight was higher for daily (OR = 1·29, 95 % CI (1·07, 1·54)), occasional (OR = 1·50, 95 % CI (1·17, 1·93)), former (OR = 1·33, 95 % CI (1·05, 1·67)) and experimental (OR = 1·13, 95 % CI (1·02, 1·24)) SLT users relative to non-SLT users(Reference Vander Weg, Klesges and DeBon23). There have also been evidence of increased weight (≥5 %) (OR = 1·31, 95 % CI (1·04, 1·65)) and incidence of obesity (BMI ≥ 30 kg/m2) (OR = 1·93, 95 % CI (1·13, 3·30)) after adjustment of other factors in males with snus use in the USA(Reference Hansson, Galanti and Magnusson24). Weight gain has been reported among the former smokers who substituted smoking with snuff(Reference Sundbeck, Grahn and Lönngren25).
Among women, SLT use was linked with lower BMI in northern rural Ghana(Reference Nonterah, Debpuur and Agongo26,Reference Blankson and Hall27) . In a cross-sectional population-based sample among female snus users in Sweden, daily snus use was found to be associated with lower chances of being overweight and higher chances of being underweight and worse general health(Reference Lund, Kvaavik and Nygard28). Tobacco consumed in different forms also has an impact on weight. A population-based cohort study in Mumbai, India, found that men and women using different types of SLT had BMI less than 16·0 and had approximately twice the risk of death as compared to the non-users(Reference Pednekar, Gupta and Hebert29).
Smokeless tobacco and malnutrition
Based on BMI scores, a higher prevalence of malnutrition co-existing with poor oral health was reported amongst the primary school children consuming SLT in Pakistan(Reference Mustufa, Jamali and Sameen30). Similarly, a higher prevalence of undernutrition was found among those tribal women who were SLT users in Wayanad, India(Reference Mohandas, Amritesh and Lais31). Low consumption of fruits and vegetables (90 % below recommended levels) has been found among the SLT users in Bangladesh(Reference Razzaque, Nahar and Mustafa32). While examining the relationship between smoking and diet, it has also been also reported that tobacco users should be counselled to increase their intake of fruits, vegetables and high-fibre grains as they substantially have low intakes of foods related to cancer risk(Reference Subar and Harlan33). Increased undernourishment, dental problems and high blood pressure were also seen among the rural women consuming SLT in Burkrina Faso(Reference Diendere, Zeba and Nikiema34).
SLT is also being consumed due to a common misbelief of promoting oral health and benefits to teeth. Tobacco containing dentifrices in the form of toothpaste, tooth powder roasted and powdered tobacco, etc., are widely available in the markets. Some people in the north-eastern parts of India also believe that tobacco water (tuibur) protects from the bites of insects and possesses antiseptic and anti-snake venom properties(Reference Sinha, Gupta and Pednekar35). The use of such aqueous tobacco extracts has shown to affect the growth of some oral bacterial species that influence the healthy ecological balance of oral bacteria in humans. The growth and viability of these species were found to be affected in a concentration-dependent manner. The growth of these bacterial strains thus varies due to incongruence between nutrients and toxic compounds in SLT products(Reference Liu, Jin and Pan36). It is well understood that anthropometric data, BMI and skinfold thickness are significantly related to bone mineral density. The bone mineral density is a reflection of the nutritional status of women, and osteoporosis is higher among the malnourished(Reference Violeta and Violeta37). While the decrease in bone mineral density is evident with ageing, the decline was greater for women who were SLT users as compared to those who never had SLT in North Carolina(Reference Quandt, Spangler and Case38).
Alteration in taste has also been reported with the use of SLT which could also be one of the reasons for malnutrition. Another study has reported a significantly higher intake of cereals (β = 152·46, P < 0·0001 sugar (β = 8·16, P < 0·0001) and lower consumption of dairy and milk products (β = -17·11, P < 0·01) and oil/fat (β = -10·30, P < 0·01) in households consuming any form of tobacco (smoke, smokeless or dual form)(Reference Virk-Baker, Husain and Parascandola20). SLT has also been associated with low BMI and low haemoglobin levels in Fe deficiency anaemia among women(Reference Pednekar, Gupta and Shukla39,Reference Subramoney and Gupta40) . Fe deficiency anaemia is not just limited to women SLT users but also men SLT users(Reference Geldsetzer, Didzun and De Neve41).
Smokeless tobacco and poor birth outcomes
Initiation of SLT usage can occur during pregnancy to relieve symptoms associated with high physiological stress, that is, nausea, vomiting and constipation. The pooled prevalence of SLT use among pregnant women was highest in the South-East Asia region (2·6 %) and lowest in Europe (0·1 %). SLT use during pregnancy also increases the likelihood of stillbirth in low-income countries(Reference Gupta and Subramoney42). SLT use during pregnancy is also associated with low haemoglobin levels (10·00 g/dl or less) among the population-based cohort of 918 women in Mumbai(Reference Subramoney and Gupta40). Mean haemoglobin (g %) was found significantly lesser (t = -15·24, P = 0·000) among pregnant users of Mishri (a form of SLT) (10·4 ± 0·90) compared to non-users (11·6 ± 1·05)(Reference Pratinidhi, Ganganahalli and Kakade43). In another prospective cohort of 1217 women in Mumbai, India, SLT use has been linked to a decrease in gestational age as well as the significant decrease in birth weight(Reference Gupta and Sreevidya44).Similarly, 81 % of pregnant women users that were SLT users delivered babies with birth weight of less than 2·5 kg. Complications like oligohydramnios and fetal distress were found to be significantly more among the SLT users(Reference Pratinidhi, Ganganahalli and Kakade43). Moreover, the cotinine levels among users were found to be negatively correlated with anthropometric measurements of newborn babies(Reference Ganganahalli, Pratinidhi and Patil45). In a study conducted in Papua New Guinea, initiating betel nut chewing and low BMI during pregnancy have resulted in significant birth weight reduction(Reference Senn, Baiwog and Winmai46). The primary reasons listed for chewing betel nuts were to prevent morning sickness, halitosis and addiction(Reference Senn, Baiwog and Winmai46).
Smokeless tobacco and metabolic disorders
A higher odds of developing Barrett’s oesophagus was found when a cigar or pipe was used in combination with SLT(Reference Westra, Lutzke and Mostafavi47). Snus used in the Scandinavian countries has been linked with the high risk of type 2 diabetes(Reference Carlsson, Andersson and Araghi48). Similarly, Chimo (SLT) increased the odds of type 2 diabetes associated with low-fat mass in an Andean Venezuelan population by 77 %, representing a significant public health problem(Reference Gonzalez-Rivas, Nieto-Martínez and García Santiago49). Despite the abundant consumption of marine-based food and physical activity which are considered to be cardioprotective, the use of Yup’ik (a form of SLT) may increase cardiometabolic risk amongst the Alaska Native people(Reference Ryman, Boyer and Hopkins50). Evidence of snuff use and its association with CVD is conflicted. Swedish moist snus has not been proven to be associated with the heightened risk of fatal myocardial infarction(Reference Hergens, Alfredsson and Bolinder3). However, a significant and positive association was found between metabolic syndrome and areca nut chewers with SLT additives(Reference Shafique, Zafar and Ahmed51). A population-based longitudinal study in Sweden reported a positive independent association between metabolic syndrome and snus use. High doses of snus not only increases the risk of metabolic syndrome and hypertension but also has an effect on obesity and hypertriglyceridemia(Reference Norberg, Stenlund and Lindahl52). Besides physical inactivity, non-vegetarian diet, high intake of fat, family history and SLT consumption were also found to be some of the predisposing factors for the development of gallstone disease in North India(Reference Dhamnetiya, Goel and Dhiman53).
Discussion
To the best of our knowledge, this is the first systematic review to ascertain the linkage between SLT consumption and public health nutrition. Our findings suggest that SLT consumption is linked with various dimensions of public health nutrition (Fig. 2). Several public health and nutritional outcomes are mentioned in Table 3. These include nutritional, oral health, poor birth, behavioural and metabolic outcomes. SLT consumption is widespread in more than half of the countries globally. Synthesised results show that the use of SLT products leads to increased household food insecurity. Further, the household’s expenditure on tobacco outweighs the expenditure made on oil and fat. Increased intake of cereals and sugar and lower consumption of milk and fat products were seen in households consuming SLT or any other form of tobacco(Reference Virk-Baker, Husain and Parascandola20). This may not only lead to decreased total energy intake but also compromise on the concept of a healthy eating plate or healthy diet. Reduction in perceived intensity of salt, sour and bitter taste leading to an aberration in the dietary pattern has been documented among SLT users(Reference Mela54). In addition to this, greater alcohol intake and lower consumption of carbohydrates, confections and sweets, fruits and grains were also seen among the SLT users(Reference Mela54). Therefore, alteration in taste, poor oral health and low consumption of fruits and vegetables associated with SLT use can be responsible for malnutrition. The differences in the food consumption pattern among the SLT and non-SLT users highlight the urgent need to address SLT usage as one of the underlying causes of food insecurity, malnutrition and poor nutritional outcomes. Hence, the inclusion of SLT cessation is crucial in the context of nutritional interventions.

Fig. 2 Diagrammatic representation of the association between smokeless tobacco use and public health nutrition; SLT, smokeless tobacco
Table 3 Public health and nutrition-related outcomes identified from the evidence systematic review

Malnutrition and its relation to SLT use have been reported among South-East Asian countries. Women who used any form of SLT had lower BMI(<16) and had twice the risk of death when compared to non-SLT users(Reference Pednekar, Gupta and Hebert29). Anaemia due to SLT use is not just limited to women, but it concurs in men as well(Reference Geldsetzer, Didzun and De Neve41). Studies from both low middle income countries and high income countries support that different SLT products have an insignificant impact on fetal growth and increased risk of preterm delivery(Reference England, Kim and Tomar55). Low birth weight is another associated consequence of the consumption of SLT among pregnant women(Reference Subramoney and Gupta40,Reference Gupta and Sreevidya44,Reference Senn, Baiwog and Winmai46) . In order to reduce exposure to SLT before and during pregnancy, there is a need to develop culturally appropriate behavioural interventions in low-income countries(Reference Yakoob, Menezes and Soomro56). Attention should be given to reduce SLT use as a component of routine antenatal care in order to minimise the adverse perinatal outcome(Reference Pratinidhi, Ganganahalli and Kakade43).
The review also confirms linkage of SLT use to loss of weight(Reference Lund, Kvaavik and Nygard28) and also to weight gain(Reference Hansson, Galanti and Magnusson24). Evidence of weight gain (≥5 %) and incidence of obesity (BMI ≥ 30 kg/m2) in males with snus use has been reported in the USA. However, BMI as a measure of body composition has limitations due to the exclusion of body fat or muscle mass(Reference Hansson, Galanti and Magnusson24). One of the studies focused on the relationship between snus use and obesity depending on three different parameters (BMI ≥ 30, waist-hip-ratio ≥1·0 and waist circumference >102 centimetres). It was found that only waist-to-hip ratio is positively associated with snus use(Reference Wallenfeldt, Hulthe and Bokemark57). The possible explanation of the co-occurrence of obesity and high prevalence of SLT in rural areas was due to effective nicotine delivery system(Reference Green, Silveira and Kimmel58), body image, physical activity and diet(Reference Green, Silveira and Kimmel58). Moreover, no information was gathered on eating habits including the consumption of fruits and vegetables and frequency of eating(Reference Greenwood and Stanford59). Therefore, weight gain or loss remains an important question to investigate in future studies. Metabolic disorders, including type 2 diabetes, increased cardiometabolic risk, myocardial infarction, gallstone disease and Barrett’s oesophagus, are the other comorbidities that occur with the consumption of SLT(Reference Hergens, Alfredsson and Bolinder3,Reference Westra, Lutzke and Mostafavi47,Reference Carlsson, Andersson and Araghi48,Reference Ryman, Boyer and Hopkins50,Reference Dhamnetiya, Goel and Dhiman53) . Several studies in the past have also anticipated the metabolic effects of SLT use due to the sustained exposure to nicotine on the central nervous system as in the case of cigarette smoking(Reference Bolinder, Noren and Wahren60,Reference Eliasson, Asplund and Evrin61) .
SLT usage also affects the diverse and composite human oral microbiome. The growth of some bacterial species is affected by SLT usage. The SLT aqueous extracts enhance the growth rate of some bacterial species which interferes with the opportunistic pathogens. These interactions potentially cause oral diseases like gingival and periodontal inflammation and dental caries. It is crucial to identify the toxicological effects of SLT products on the oral microbiota and the genotoxicity of their metabolites produced by oral microbiota(Reference Liu, Jin and Pan36). Further studies are also warranted to identify the impact of various brands of SLT on oral bacterial species which could hamper the oral bacterial physiology and metabolism(Reference Sun, Jin and Beger62). The decrease in the bone mineral density with ageing was more prominent among elderly women who consumed SLT, which distinctly shows the influence of SLT on their nutritional status. With the available evidences(Reference Razzaque, Nahar and Mustafa32,Reference Subar and Harlan33) of low consumption of fruits and vegetables among SLT users, there is a need to do further research on the relationship between SLT and diet.
Conclusion
To conclude, this study highlights the linkages between SLT usage and poor nutritional outcomes. These interplaying factors are crucial and need to be understood well for planning comprehensive interventions. Tobacco control research should be conducted in convergence with nutrition research for overall health benefits(Reference Pednekar, Gupta and Shukla39). The review suggests the integration of public health nutrition programmes and tobacco cessation efforts. It also provides a clear picture of how SLT usage is linked to poor nutritional outcomes. Attention is needed to find appropriate mechanisms for the inclusion of SLT cessation in nutrition-sensitive programmes at the community level. Hence, both the issues should be seen together to arrive at a comprehensive solution in order to attain public health goals.
One of the limitations of this review is that PubMed and Scopus were the only search engines used which may exclude relevant papers not retrievable through it. We adhered to include articles from PubMed and Scopus, because these are proven sources of highly regarded scientific journals. The included studies were heterogeneous in nature due to the differences in the study outcomes, quality and designs. Results obtained should be carefully interpreted as the studies involved were not uniformly distributed across the countries.
Acknowledgements
Acknowledgements: I am very thankful to all the staff members of the Division of Preventive Oncology and Population Health. I would also like to thank the administrative staff for providing the paraphernalia to carry out this work. Financial support: This work was carried out under ASTRA (University of York, UK) and funded by the National Institute for Health Research (NIHR), using UK government aid to support global health research (program reference 17/63/76/ Global Health Research Groups). The views expressed are those of the author(s) and not necessarily those of the NIHR or the UK Department of Health and Social Care. Authorship: P.K.S. and S.S. conceptualised the study. P.K.S. and S.S. finalised the selected papers and wrote the first draft of the manuscript. S.S. and S.S. contributed to the interpretation of the results. P.K.S., S.K. and L.S. conducted critical revisions and contributed to writing the final manuscript. Ethics of human subject participation: Not applicable.
Conflicts of interest:
The authors have no conflicts of interest to declare.
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S1368980022001331








