Although breakfast is widely promoted as essential for the nutritional well-being of young people, breakfast skipping is relatively common among adolescents in developed countries( Reference Rampersaud, Pereira and Girard 1 , Reference Alexy, Wicher and Kersting 2 ). The breakfast meal and the frequency with which it is consumed, together with genetic and environmental factors, may influence appetite, dietary intake and food choices. These factors may have important implications for body weight regulation and to prevent diseases( Reference Giovannini, Agostoni and Shamir 3 ). There is some evidence that breakfast skipping behaviour is related to higher BMI in adolescents( Reference Rampersaud, Pereira and Girard 1 , Reference Diethelm, Huybrechts and Moreno 4 – Reference Cho, Dietrich and Brown 6 ). In addition, regular breakfast consumption has been associated with a healthier cardiovascular profile in European adolescents( Reference Hallström, Labayen and Ruiz 5 ).
Breakfast has been documented to make an essential contribution to nutrient intakes( Reference Rampersaud, Pereira and Girard 1 , Reference Rampersaud 7 , Reference Miller, Forgac and Cline 8 ), due to its contribution of approximately 20 % to total daily energy intake and content of specific nutrients such as vitamins( Reference Wyon, Abrahamsson and Järtelius 9 ). Adolescents who consume breakfast are more likely to meet recommended intakes of Ca, Fe, Mg, Zn, Cu, folate, vitamins A, D, B6, B1, C and E, and total energy than those who skip breakfast( Reference Rampersaud, Pereira and Girard 1 ). Likewise, breakfast consumption may reduce the risk of chronic diseases due to its potential impact on overall diet quality( Reference Matthys, De Henauw and Bellemans 10 – Reference Viteri and Gonzalez 13 ). Consequently, breakfast is considered an important key component of a healthy diet contributing to adequate growth and adolescent development.
On the other hand, previous reports from the HELENA (Healthy Lifestyle in Europe by Nutrition in Adolescence) Study have stated that deficient blood vitamin concentrations, at least at the subclinical level, are prevalent in European adolescents: plasma folate (PF; 15 %), vitamin D (25-hydroxyvitamin D (25(OH)D); 27 %), vitamin B6 (pyridoxal phosphate (PLP); 5 %), β-carotene (25 %) and vitamin E (5 %)( Reference González-Gross, Valtueña and Breidenassel 14 – Reference Breidenassel, Valtuena and Gonzalez-Gross 16 ). Likewise, some vitamin deficiencies could be associated with fatness and other diseases( Reference Kremer, Campbell and Reinhardt 17 ). In addition, in Europe, there is a lack of comparable data on vitamin intakes and blood concentrations( Reference Al-Tahan, González-Gross and Pietrzik 18 ). Therefore, a balanced breakfast could be a good strategy to avoid these blood vitamin deficiencies. However, there are no studies analysing the relationship of blood vitamin levels with breakfast patterns. Thus, the aim of the present study was to examine the association between vitamin intakes, blood vitamin concentrations and different patterns of breakfast consumption in European adolescents participating in the HELENA Study.
Participants and methods
Study design, recruitment and participants
The HELENA Study was a multicentre cross-sectional study aiming at obtaining reliable and comparable data from a random sample of 3000 European adolescents, aged between 12·5 and 17·5 years, from ten different cities, on a broad range of nutrition and health-related parameters( Reference De Henauw, Gottrand and De Bourdeaudhuij 19 , Reference Moreno, De Henauw and Gonzalez-Gross 20 ). Selection of cities was based on two criteria: regional distribution and presence of an active research group assuring sufficient expertise and resources to successfully perform epidemiological studies. Within the study, Stockholm (Sweden), Athens and Heraklion (Greece), Rome (Italy), Zaragoza (Spain), Pécs (Hungary), Ghent (Belgium), Lille (France), Dortmund (Germany) and Vienna (Austria) were included. On a regional basis, diversity of the sample with respect to cultural and socio-economic aspects was achieved by selecting a random proportional distribution of all schools taking into account the site of the school (district/zone of the city) and the type of school (public or private). One partner centrally performed the school and class random selection procedure for all study centres, including the subset of classes for blood sampling. In case a selected school refused to participate, a school with comparable characteristics from a list of substitutes was chosen. The number of adolescents to be studied was estimated at 3000 using variance of BMI. BMI has the greatest dispersion in the study population with regard to the hypotheses under consideration. The sample size was calculated with a confidence level of 95 % and with ± 0·3 error in the parameter BMI. Error of 0·3 was chosen as a worst-case scenario for precision level as described by the HELENA Study( Reference De Henauw, Gottrand and De Bourdeaudhuij 19 , Reference Moreno, De Henauw and Gonzalez-Gross 20 ). One-third of the classes were randomly selected for blood collection, resulting in a total of 1089 blood samples for the subsequent clinical biochemistry assays. After validating data on breakfast assessment and blood samples, 1058 (52·8 % females) adolescents were included in the present study.
Exclusion criteria were limited to adolescents who were not able to speak the local language, those who were participating in another clinical trial at the same time, those aged <12·5 or >17·5 years and adolescents having suffered from infection one week before the visit. Exclusions from the study were done a posteriori, without the knowledge of the affected individuals. All procedures involving human participants were approved by the ethics committee of each city involved and have therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. Informed written consent was obtained from adolescents and parents or guardians. A complete description of ethical issues and good clinical practice within the HELENA Study is given elsewhere( Reference Beghin, Castera and Manios 21 ).
Breakfast consumption patterns assessment
The ‘Food Choices and Preferences’ questionnaire was developed based on forty-four focus groups which explored attitudes and issues of concern among adolescents regarding food choices, preferences, healthy eating and lifestyle( Reference Hallstrom, Vereecken and Ruiz 22 ). This questionnaire has been used in previous research( Reference Hallström, Labayen and Ruiz 5 ). Adolescents reported their breakfast habits by responding to the following statement: ‘I often skip breakfast’. There were seven possible answers ranging as ‘strongly disagree’ (=1), ‘moderately disagree’ (=2), ‘slightly disagree’ (=3), ‘neither agree nor disagree’ (=4), ‘slightly agree’ (=5), ‘moderately agree’ (=6) and ‘strongly agree’ (=7). Adolescents were categorized into three groups: (i) consumers (answered ‘1’ or ‘2’); (ii) occasional consumers (answered ‘3’, ‘4’ or ‘5’); and (iii) skippers (answered ‘6’ or ‘7’).
Supplement use
Information on vitamin supplement use was obtained via the clinical recall of the adolescents (case report form). Adolescents were asked about taking any micronutrient supplement and were classified into two groups: (i) supplement users; and (ii) non-supplement users.
Socio-economic status
The complete description of the self-reported socio-economic questionnaire is provided elsewhere( Reference Iliescu, Beghin and Maes 23 ). The Family Affluence Scale (FAS) is based on the concept of material conditions in the family as the basis for the selection of items. Currie et al. chose a set of items which reflected family expenditure and consumption that were relevant to family circumstances( Reference Currie, Elton and Todd 24 ). FAS was used in the present study as an index of socio-economic status (SES)( Reference Currie, Molcho and Boyce 25 ) and included four questions answered by the adolescent: (i) ‘Do you have your own bedroom?’ (‘no’ (=0); ‘yes, one’ (=1)); (ii) ‘How many cars are there in your family?’ (‘no’ (=0); ‘yes, one’ (=1); ‘yes, two’ (=2); ‘yes, more than two’ (=3)); (iii) ‘How many PCs [personal computers] are there in your home?’ (‘none’ (=0); ‘one’ (=1); ‘two’ (=2); ‘more than two’ (=3)); and (iv) ‘Do you have Internet access at home?’ (‘no’ (=0); ‘yes, one’ (=1)). We defined low, medium and high SES based on the final score obtained from the four questions. We gave a numerical value to each possible answer in the four questions and then we summed the final score from all the questions, which ranged from 0 to 8. Finally, we grouped these scores into three levels: low (from 0 to 2), medium (from 3 to 5) and high (from 6 to 8).
Family structure
We obtained information about family structure through the aforementioned questionnaire. Family structure was defined as ‘traditional family’ when the adolescent was living at home with two parents (parents and/or step-parents) or ‘single/shared-care’ when the adolescent was living in a single-parent family or had ‘shared care’ between parents. Those living in other family structures (e.g. in a foster home or with grandparents) were categorized into the ‘single/shared-care’ family structure( Reference Hallström, Labayen and Ruiz 5 ).
Dietary intake assessment
Dietary consumption was assessed using the self-administered, computerized 24 h recall (24HR), HELENA Dietary Assessment Tool (DIAT), which is based on the Young Adolescents’ Nutrition Assessment software validated in European adolescents( Reference Vereecken, Covents and Sichert-Hellert 26 ) (r s=0·86–0·91) for all nutrient and energy intakes. The adolescents completed the 24HR twice (within 2 weeks) during school time; both times, trained staff including a dietitian were present. The HELENA-DIAT used special techniques to support and enhance respondents’ memory, which allowed a more detailed description and quantification of the foods consumed. The European Food Consumption Survey Method project indicated the repeated 24HR as the most suitable method to obtain population means and distributions( Reference Brussaard, Lowik and Steingrimsdottir 27 ). To calculate energy and nutrient intakes, data from HELENA-DIAT were linked to the German Food Code and Nutrient Database (BLS (Bundeslebensmittelschlüssel), version II.3.1, 2005)( Reference Dehne, Klemm and Henseler 28 ). The use of the German Food Code and Nutrient Database has been evaluated and was considered equally valid as using the local food composition databases from each individual country( Reference Julian-Almarcegui, Bel-Serrat and Kersting 29 ). The Multiple Source Method was used to estimate the habitual dietary intake of nutrients and foods( Reference Harttig, Haubrock and Knüppel 30 ). This statistical modelling technique takes into account within-person variability. Likewise, this technique calculates habitual intakes taking into account age, sex and study centre. Participants from Heraklion and Pécs were excluded from these analyses as no nutrient intake information was calculated for these two cities due to logistical problems (insufficient local staff available). Therefore, eight out of the ten study centres were included in the 24HR analyses, resulting in a sample size decrease. Moreover, under-reporters were excluded from all analyses. The BMR was calculated from age-specific FAO/WHO/United Nations University equations( 31 ). Under-reporting was considered when the ratio of energy intake to estimated BMR was <0·96, as proposed by Black( Reference Black 32 ).
Medical examination
The day prior to the study day, participants were asked to abstain from eating and drinking after 20.00 hours. On the study day, a medical doctor visited the school classes and interviewed all participants for medical history and acute diseases. A blood sampling questionnaire was used to assess fasting status, acute infections, allergies, smoking, vitamin and mineral supplements, and medication. Maturity was assessed by means of Tanner stage( Reference Tanner and Whitehouse 33 ).
Specimen collection and biochemical analyses
A specific handling, transport and traceability system for biological samples was developed for the HELENA Study as described by González-Gross et al.( Reference Gonzalez-Gross, Breidenassel and Gómez-Martínez 34 ). Blood samples were obtained at the same time as dietary intake assessment. Fasting blood samples were collected by venepuncture at school between 08.00 and 10.00 hours in the morning. PF, cobalamin (CBL) and red-blood-cell (RBC) folate were measured by competitive immunoassay (Immulite 2000; DPC Biermann GmbH, Bad Nauheim, Germany; CV for PF=5·4 %, RBC folate=10·7 %, CBL=5·0 %)( Reference Ueland, Refsum and Stabler 35 ). PLP was measured by HPLC (Varian Deutschland GmbH, Darmstadt, Germany; CV=1 %) with the modified method of Kimura et al.( Reference Kimura, Kanehira and Yokoi 36 ). Serum holo-transcobalamin (a marker of vitamin B12 status; holo-TC) was measured by microparticle enzyme immunoassay (Active B12; Axis-Shield Ltd, Dundee, UK; CV=5·1 %) with the use of an AxSYM analyser (Abbott Diagnostics, Abbott Park, IL, USA)( Reference Brady, Wilson and McGregor 37 ).
Plasma 25(OH)D was analysed using an ELISA kit (OCTEIA 25(OH)D; IDS Immunodiagnostic Systems Deutschland GmbH, Frankfurt am Main, Germany) and measured with a Sunrisee photometer (TECAN, Männedorf, Germany). The CV for the method was less than 1 %( Reference González-Gross, Valtueña and Breidenassel 14 ). Plasma vitamin C, retinol, α-tocopherol and β-carotene were analysed using reversed-phase HPLC (Sykam GmbH, Gilching, Germany) with UV detection (UV-Vis 205; Merck, Darmstadt, Germany). The CV of the method was 2·9 % for retinol, α-tocopherol and β-carotene, and 1·7 % for vitamin C( Reference Breidenassel, Valtuena and Gonzalez-Gross 16 ).
Body composition measurements
The anthropometric methods used within the HELENA Cross-Sectional Study were described by Nagy et al.( Reference Nagy, Vicente-Rodriguez and Manios 38 ). Briefly, body weight was measured in kilograms using a standard beam balance scale (type SECA 861, UK; precision 100 g, range 0–150 kg). Height was measured in centimetres using a precision stadiometer (type SECA 225, UK; precision 0·2 cm, range 70–200 cm). BMI was calculated as weight in kilograms divided by the square of height in metres (kg/m2). Adolescents were classified according to the international BMI cut-off values as non-overweight or overweight/obese( Reference Cole, Bellizzi and Flegal 39 ).
Statistical analyses
Associations between sex and BMI status (non-overweight and overweight/obese), SES (low, medium and high), family structure (traditional family and single/shared-care) and breakfast consumption categories (consumer, occasional consumer and skipper) were assessed by the χ 2 test. Mean vitamin intakes and serum vitamin levels were compared across breakfast consumption categories using one-way ANCOVA with breakfast consumption category as the fixed factor, vitamin intakes or blood vitamin concentrations as the dependent variables and age, BMI, supplement use, centre, sex, SES and family structure as covariates( Reference Hallstrom, Vereecken and Ruiz 22 ). The analyses were stratified by sex. On the other hand, the associations between breakfast pattern categories and different statuses of vitamin concentrations (sufficiency, insufficiency or deficiency) were analysed by the χ 2 test. Likewise, adolescents were grouped in relationship with their vitamin blood status according to accepted reference values and as previously published by Moreno et al.( Reference Moreno, Gottrand and Huybrechts 40 ). All analyses were performed using the statistical software package IBM SPSS Statistics for Windows Version 22.0 and the level of significance was set at 5 %.
Results
Table 1 presents anthropometric and body composition data, blood vitamin status and vitamin intake data; and also displays BMI status, SES, family structure and distribution of breakfast consumption patterns in the study sample. There were significant differences between sexes in serum PLP, CBL, vitamin C, β-carotene, α-tocopherol, retinol and tHcy (P<0·05). In addition, there were differences between breakfast consumers compared with occasional consumers and breakfast skippers according to sex (P<0·001).
Table 1 Mean anthropometric indices, mean blood vitamin concentrations, BMI status, socio-economic status, sociodemographic status and breakfast consumption patterns of the study sample by sex; adolescents (n 1058) aged 12·5–17·5 years from ten European cities, HELENA (Healthy Lifestyle in Europe by Nutrition in Adolescence) Study

Significant P values are shown in bold font.
Table 2 presents vitamin intake data according to different breakfast patterns, by sex, after controlling for centre, age, BMI, supplement use, SES and family structure. Higher vitamin D (cholecalciferol) and total folate intakes were observed in adolescent breakfast consumers compared with breakfast skippers (P<0·05). Likewise, female breakfast consumers had higher intakes of vitamin B6 (pyridoxine) and vitamin E (tocopherol equivalents) than skippers (P<0·05).
Table 2 Mean energy and vitamin intakes of the study sample by breakfast consumption pattern and sex; adolescents (n 1058) aged 12·5–17·5 years from ten European cities, HELENA (Healthy Lifestyle in Europe by Nutrition in Adolescence) Study

All analyses were adjusted for centre, age, BMI, supplement use, family structure and socio-economic status (covariables).
P values from one-way ANCOVA indicate statistical significance among different breakfast patterns; significant P values are shown in bold font.
Significant differences among breakfast consumption groups (P<0·05) by Bonferroni post hoc test: *v. consumers; †v. occasional consumers.
Table 3 presents differences in blood vitamin concentrations across breakfast consumption categories, stratified by sex, after controlling for centre, age, BMI, supplement use, SES and family structure. Male adolescents who regularly consumed breakfast presented higher blood concentrations of 25(OH)D, CBL and vitamin C than breakfast skippers. Higher BMI was also observed in male breakfast skippers compared with occasional consumers and consumers (P<0·05). Female breakfast consumers had higher blood concentrations of 25(OH)D, holo-TC and vitamin C compared with skippers (P<0·05). Moreover, BMI and tHcy were lower in female breakfast consumers in contrast to skippers (P<0·05).
Table 3 Mean age, BMI and blood vitamin concentrations of the study sample by breakfast consumption pattern and sex; adolescents (n 1058) aged 12·5–17·5 years from ten European cities, HELENA (Healthy Lifestyle in Europe by Nutrition in Adolescence) Study

All analyses were adjusted for centre, age, BMI, supplement use, family structure and socio-economic status (covariables).
P values from one-way ANCOA indicate statistical significance among different breakfast patterns; significant P values are shown in bold font.
Significant differences among breakfast consumption groups (P<0·05) by Bonferroni post hoc test: *v. consumers; †v. occasional consumers.
Table 4 presents the frequency and percentage of different blood vitamin concentration statuses by breakfast consumption pattern in the study sample. Adolescent breakfast skippers presented higher 25(OH)D, CBL and PF deficiency prevalence compared with habitual breakfast consumers.
Table 4 Frequency and percentage of different blood vitamin concentration statuses by breakfast consumption pattern in the study sample; adolescents (n 1058) aged 12·5–17·5 years from ten European cities, HELENA (Healthy Lifestyle in Europe by Nutrition in Adolescence) Study
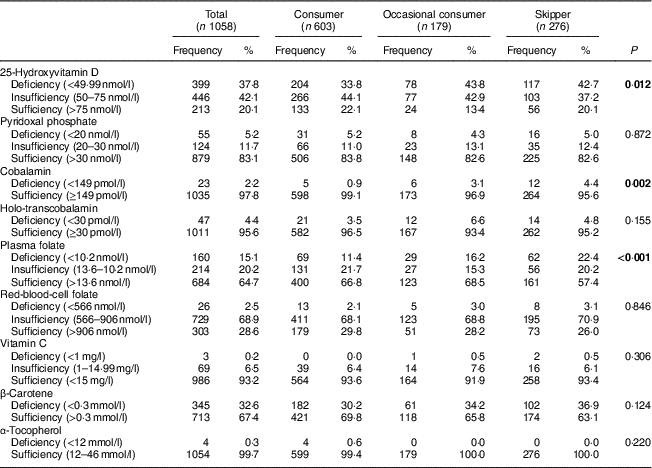
Reference values used to analyse different blood vitamin concentration statuses in adolescents aged 12·5–17·49 years were from the HELENA Study( Reference Moreno, Gottrand and Huybrechts 40 ).
Significant P values are shown in bold font.
Discussion
To the best of our knowledge, the current study is the first to analyse the associations among vitamin intakes, blood vitamin concentrations and different patterns of breakfast consumption in European adolescents, taking into account several potential influential factors such as centre, age, sex, BMI, supplement use, family structure and SES. The main results showed that regular breakfast consumption is associated with a healthier profile for only some blood vitamins in European adolescents and for tHcy only in females.
In agreement with our data, other articles have shown that only half of European adolescents are breakfast consumers, while 25 % of males and 33 % of females are breakfast skippers( Reference Moreno, Gottrand and Huybrechts 40 , Reference Hallstrom, Vereecken and Labayen 41 ), similar to other areas in the world( Reference Nicklas, O’Neil and Myers 42 ). Age influences breakfast consumption, with the older adolescents being more likely to skip breakfast (4–8 years: 2 %; 9–13 years: 9 %; 14–18 years: 18 %)( Reference Alexy, Wicher and Kersting 2 , Reference Barr, DiFrancesco and Fulgoni 43 ). Another interesting fact is that although habitual breakfast consumers have higher energy intake, those skipping breakfast are more likely to be obese( Reference Rampersaud, Pereira and Girard 1 ). In agreement, our data showed that male and female breakfast skippers had higher BMI compared with consumers.
Breakfast consumption has been consistently associated with a more favourable nutrient intake profile and improved diet quality (i.e. favourable nutrient and energy intakes) in children and adolescents( Reference Barr, DiFrancesco and Fulgoni 43 ), as we have observed in the present study. Thus, skipping breakfast has been proposed to indirectly influence BMI by overcompensating intake later in the day, consuming more snacks and fewer meals( Reference Affenito 44 ). Further, including low-fat dairy foods, wholegrain breads and cereals, and citrus fruits, other fruits and juices for breakfast could possibly have a positive influence on body mass( Reference Utter, Scragg and Mhurchu 45 ).
Moreover, in order to assess an individual’s nutritional status, vitamin, food and nutrient intake data should be complemented with biochemical data( Reference Moreno, Gonzalez-Gross and Kersting 46 ), owing to the fact that micronutrient intake data are not always associated with blood concentrations( Reference Brady, Lamb and Sokol 47 ). Our results showed an association of regular breakfast consumption with greater vitamin D (cholecalciferol), vitamin B9 (total folate) and vitamin B6 (pyridoxine) intakes and blood vitamin C, vitamin D (25(OH)D), vitamin B12 (CBL, holo-TC), folate (PF, RBC folate) and tHcy concentrations, after controlling for centre, age, BMI, supplement use, SES and family structure. However, both vitamin D and folate intakes were considerably below average requirements for adolescents (e.g. as estimated for the USA and Canada in the Dietary Reference Intakes). Specifically, the average requirements for vitamin D and folate for adolescents are ~10 µg/d and 320 µg/d, respectively, whereas mean intakes were only 2–3 µg/d (vitamin D) and <250 µg/d (folate), even among breakfast consumers( 48 , 49 ). Thus, the intakes of breakfast consumers cannot be considered to be optimal. Nevertheless, this association between breakfast eating, higher vitamin intakes and higher concentrations of some vitamins and tHcy in females may be due to some specific foods consumed at breakfast, rather than eating breakfast per se ( Reference Cho, Dietrich and Brown 6 ). In addition, intakes of other foods in the remaining meals consumed during the rest of the day could explain the association.
Breakfast is an ideal opportunity for adolescents to begin the day by eating bread, other cereals, dairy products and fruits, which have been associated with better dietary intakes of the aforementioned micronutrients( Reference Utter, Scragg and Mhurchu 45 ). Likewise, some of these breakfast products, especially packaged foods, are fortified with vitamins and minerals that also can contribute to achieving recommended intakes of these micronutrients. Based on our results, vitamin D concentrations are highly and positively affected by breakfast in both sexes. Furthermore, vitamin D has a major role in bone mass development. Adolescence is a critical life stage for bone mineral accrual: peak bone mass is acquired at approximately 18 years of age( Reference Vicente-Rodríguez, Ezquerra and Mesana 50 ) and 50 % of adult total bone mass is achieved during this period of life( Reference Peters, Verly and Marchioni 51 ). Thus, 25(OH)D deficiency at these early ages could be considered a risk factor for bone health (i.e. osteoporosis), but also other diseases including diabetes, cancer, CVD and metabolic syndrome( Reference González-Gross, Valtueña and Breidenassel 14 , Reference Parker, Hashmi and Dutton 52 ). Moreover, 25(OH)D is identified as one of the vitamins most highly at risk, with almost 80 % of adolescents below optimal levels( Reference González-Gross, Valtueña and Breidenassel 14 – Reference Breidenassel, Valtuena and Gonzalez-Gross 16 , Reference Moreno, Gottrand and Huybrechts 40 ). Dairy products at breakfast could be an important target for improving vitamin D concentrations, especially if these dairy products are fortified( Reference Peters, Verly and Marchioni 51 ). Likewise, the other very important vitamin D source is sunlight exposure. In our study we took into account the latitude of the adolescent’s residence, with study centre being added as a confounding factor. However, other confounders regarding vitamin D such as physical activity were not accounted for.
Furthermore, our results illustrate that adolescents consuming breakfast habitually had higher concentration levels for some B-vitamin markers. CBL was higher only in male breakfast consumers and holo-TC was higher only in female breakfast consumers compared with breakfast skippers. On the other hand, tHcy was lower in female breakfast consumers than in skippers. There is increasing evidence of sub-deficient PF, PLP and CBL status in several population groups, including children and adolescents. Concretely, in a previous study, subclinical deficiency of PF and PLP in about 20 % of European adolescents was reported( Reference González-Gross, Benser and Breidenassel 15 ). Results from the present study also showed higher frequency of CBL and PF deficiency in breakfast skippers than in habitual breakfast consumers. Vitamin B6, vitamin B12 and folic acid contribute to healthy growth and development, thus deficiency during adolescence is related to irreversible neurological damage and several diseases, such as CVD and cancer( Reference Al-Tahan, González-Gross and Pietrzik 18 ). In addition, low blood CBL concentrations have been associated with suppressed osteoblast activity( Reference Carmel, Lau and Baylink 53 ) and low concentrations of PF, PLP and CBL have also been associated with stimulated osteoclast activity( Reference Carmel, Lau and Baylink 53 ). Moreover, these vitamins are determinants of tHcy, which may also have an independent effect on different diseases( Reference Gjesdal, Vollset and Ueland 54 – Reference Murakami, Iemitsu and Sanada 57 ). Likewise, several studies have shown an association between increased total tHcy concentration and the risk of osteoporotic fracture( Reference Gjesdal, Vollset and Ueland 54 ). Better tHcy profile and higher blood B-vitamin concentrations have been reported in young people aged 4–18 years( Reference Gibson 58 ) and adults who consume breakfast cereals( Reference Tucker, Olson and Bakun 59 ).
Moreover, retinol, α-tocopherol, vitamin C and β-carotene are considered antioxidant nutrients and are widely found in plant or plant-derived foods( Reference Dybkowska, Waszkiewicz-Robak and Piekot 60 ). It is well recognized that dietary antioxidants play an active role in affording protection against chronic diseases like cardiovascular and cerebrovascular disease, cancer and diabetes in adulthood( Reference Breidenassel, Valtuena and Gonzalez-Gross 16 , Reference Nothlings, Schulze and Weikert 61 ). Retinol and vitamin C deficiency are not common; however, β-carotene and α-tocopherol show significant deficiency prevalence in adolescents( Reference Moreno, Gottrand and Huybrechts 40 ). In our study, blood vitamin C concentration was associated with breakfast habits. Breakfast consumers presented higher vitamin C concentrations compared with the breakfast skippers. However, this made no difference to the prevalence of deficiency or sufficiency in both groups. It should be noted that the association between vitamin C intake and plasma concentration in the general population shows that in order to achieve protective plasma concentrations, it is necessary to increase the intake of vitamin C up to the RDA levels( Reference Krajcovicova-Kudlackova, Babinska and Valachovicova 62 ). Daily breakfast consumption containing fruit seems to be an ideal strategy to achieve this goal. Further to the above, β-carotene concentrations below the normal range have been identified in European adolescents (25 %)( Reference Moreno, Gottrand and Huybrechts 40 ).
The large sample size and the standardized methodology are notable strengths of the present study. On the other hand, the use of the statement ‘I often skip breakfast’ for the assessment and categorization of breakfast consumption habits of adolescents could be a possible limitation of our study. The term ‘breakfast consumers’ in the literature includes a variety of definitions, such as consuming breakfast every day, every weekday, on the dietary survey day, or usual or habitual consumption( Reference Rampersaud, Pereira and Girard 1 , Reference Dialektakou and Vranas 63 ), which makes comparisons difficult. In addition, there is no consensus regarding the definition of breakfast consumption. A study conducted by Dialektakou and Vranas found that the percentage of breakfast skippers varied greatly according to how breakfast was categorized( Reference Dialektakou and Vranas 63 ). An important limitation is that we are comparing total vitamin concentrations from only one meal per day (i.e. breakfast; requirements for the vitamins could be completed with other meals during the day). In addition, we are analysing consuming breakfast or not, instead of the quality of the breakfast. Furthermore, because of the cross-sectional nature of the study design, no conclusion can be drawn about the directionality and causality of the associations observed between breakfast consumption and serum vitamin concentrations.
Conclusions
Regular breakfast consumption is associated with higher intakes of some vitamins, higher blood concentrations of some vitamins and lower blood concentration of tHcy in European adolescents. Particularly, skipping breakfast is associated with lower blood vitamin D (25(OH)D) and CBL concentrations in males and with lower vitamin D (25(OH)D) and holo-TC, and higher tHcy, concentrations in females; and with low intakes of vitamin D (cholecalciferol) and vitamin B9 (total folate) in both sexes, and with low intakes of vitamin B6 (pyridoxine) and vitamin E (tocopherol equivalents) in females. Consuming breakfast regularly could be an indicator for a healthier dietary pattern overall.
Acknowledgements
Acknowledgements: The authors thank all of the adolescents who took part in the HELENA Study. They also wish to thank Rosa Maria Torres, Ulrike Albers, Petra Pickert, Christel Bierschbach, Adelheid Schuch and Anke Berchtold for their contribution to laboratory work. Financial support: The HELENA Study was conducted with financial support of the European Community Sixth RTD Framework Programme (Contract FOOD-CT-2005-007034). Additional support was provided by the Spanish Ministry of Education (grant numbers EX-2007-1124, EX-2008-0641, AP2006-02464, AGL2007-29784-E/ALI and AP-2005-3827); Universidad Politécnica de Madrid (grant number CH/018/2008); Swedish Council for Working Life and Social Research; the ALPHA study, a European Union-funded study, in the framework of the Public Health Programme (reference 2006120); the Spanish Ministry of Health: Maternal, Child Health and Development Network (grant number RD08/0072); and Instituto de Salud Carlos III (ISCIII) (CIBER: CB12/03/30038 Pathophysiology of Nutrition and Obesity), Spain. The content of this article reflects only the authors’ views, and the European Community is not liable for any use that may be made of the information contained therein. Conflict of interest: The authors declare no conflict of interest. Authorship: The writing group takes sole responsibility for the content of this article. J.M.-A., J.V. and M.G.-G. wrote the manuscript and performed the statistical analysis; J.M.-A., J.V., M.C.-G, F.G., C.B., M.F., Y.M., S.D.H., K.W., A.K., P.S., M.K. and LA.M. contributed to the interpretation and discussion of the results and critically revised the drafted manuscript. Ethics of human subject participation: All procedures involving human participants were performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments, and approved by the ethics committee of each city involved. Informed written consent was obtained from adolescents and parents or guardians.
HELENA Study Group. Coordinator: Luis A. Moreno. Core Group members: Luis A. Moreno, Fréderic Gottrand, Stefaan De Henauw, Marcela González-Gross, Chantal Gilbert. Steering Committee: Anthony Kafatos (President), Luis A. Moreno, Christian Libersa, Stefaan De Henauw, Sara Castelló, Fréderic Gottrand, Mathilde Kersting, Michael Sjöstrom, Dénes Molnár, Marcela González-Gross, Jean Dallongeville, Chantal Gilbert, Gunnar Hall, Lea Maes, Luca Scalfi. Project Manager: Pilar Meléndez. Universidad de Zaragoza (Spain): Luis A. Moreno, Jesús Fleta, José A. Casajús, Gerardo Rodríguez, Concepción Tomás, María I. Mesana, Germán Vicente-Rodríguez, Adoración Villarroya, Carlos M. Gil, Ignacio Ara, Juan Revenga, Carmen Lachen, Juan Fernández Alvira, Gloria Bueno, Aurora Lázaro, Olga Bueno, Juan F. León, Jesús Mª Garagorri, Manuel Bueno, Idoia Labayen, Iris Iglesia, Silvia Bel, Luis A. Gracia Marco, Theodora Mouratidou. Consejo Superior de Investigaciones Científicas (Spain): Ascensión Marcos, Julia Wärnberg, Esther Nova, Sonia Gómez, Ligia Esperanza Díaz, Javier Romeo, Ana Veses, Belén Zapatera, Tamara Pozo, David Martínez. Université de Lille 2 (France): Laurent Beghin, Christian Libersa, Frédéric Gottrand, Catalina Iliescu, Juliana Von Berlepsch. Research Institute of Child Nutrition Dortmund, Rheinische Friedrich-Wilhelms-Universität Bonn (Germany): Mathilde Kersting, Wolfgang Sichert-Hellert, Ellen Koeppen. Pécsi Tudományegyetem (University of Pécs) (Hungary): Dénes Molnar, Eva Erhardt, Katalin Csernus, Katalin Török, Szilvia Bokor, Mrs Angster, Enikö Nagy, Orsolya Kovács, Judit Répasi. University of Crete School of Medicine (Greece): Anthony Kafatos, Caroline Codrington, María Plada, Angeliki Papadaki, Katerina Sarri, Anna Viskadourou, Christos Hatzis, Michael Kiriakakis, George Tsibinos, Constantine Vardavas, Manolis Sbokos, Eva Protoyeraki, Maria Fasoulaki. Institut für Ernährungs- und Lebensmittelwissenschaften – Ernährungphysiologie, Rheinische Friedrich Wilhelms Universität (Germany): Peter Stehle, Klaus Pietrzik, Marcela González-Gross, Christina Breidenassel, Andre Spinneker, Jasmin Al-Tahan, Miriam Segoviano, Anke Berchtold, Christine Bierschbach, Erika Blatzheim, Adelheid Schuch, Petra Pickert. University of Granada (Spain): Manuel J. Castillo, Ángel Gutiérrez, Francisco B. Ortega, Jonatan R. Ruiz, Enrique G. Artero, Vanesa España, David Jiménez-Pavón, Palma Chillón, Cristóbal Sánchez-Muñoz, Magdalena Cuenca-García. Agricultural Research Council–Food and Nutrition Research Centre (CRA-NUT) (Italy): Davide Arcella, Elena Azzini, Emma Barrison, Noemi Bevilacqua, Pasquale Buonocore, Giovina Catasta, Laura Censi, Donatella Ciarapica, Paola D’Acapito, Marika Ferrari, Myriam Galfo, Cinzia Le Donne, Catherine Leclercq, Giuseppe Maiani, Beatrice Mauro, Lorenza Mistura, Antonella Pasquali, Raffaela Piccinelli, Angela Polito, Romana Roccaldo, Raffaella Spada, Stefania Sette, Maria Zaccaria. University of Napoli ‘Federico II’ Department of Food Science (Italy): Luca Scalfi, Paola Vitaglione, Concetta Montagnese. Ghent University (Belgium): Ilse De Bourdeaudhuij, Stefaan De Henauw, Tineke De Vriendt, Lea Maes, Christophe Matthys, Carine Vereecken, Mieke de Maeyer, Charlene Ottevaere, Inge Huybrechts. Medical University of Vienna (Austria): Kurt Widhalm, Katharina Phillipp, Sabine Dietrich, Birgit Kubelka, Marion Boriss-Riedl. Harokopio University (Greece): Yannis Manios, Eva Grammatikaki, Zoi Bouloubasi, Tina Louisa Cook, Sofia Eleutheriou, Orsalia Consta, George Moschonis, Ioanna Katsaroli, George Kraniou, Stalo Papoutsou, Despoina Keke, Ioanna Petraki, Elena Bellou, Sofia Tanagra, Kostalenia Kallianoti, Dionysia Argyropoulou, Katerina Kondaki, Stamatoula Tsikrika, Christos Karaiskos. Institut Pasteur de Lille (France): Jean Dallongeville, Aline Meirhaeghe. Karolinska Institutet (Sweden): Michael Sjöstrom, Jonatan R. Ruiz, Francisco B. Ortega, María Hagströmer, Anita Hurtig Wennlöf, Lena Hallström, Emma Patterson, Lydia Kwak, Julia Wärnberg, Nico Rizzo. Asociación de Investigación de la Industria Agroalimentaria (Spain): Jackie Sánchez-Molero, Sara Castelló, Elena Picó, Maite Navarro, Blanca Viadel, José Enrique Carreres, Gema Merino, Rosa Sanjuán, María Lorente, María José Sánchez. Campden BRI (UK): Chantal Gilbert, Sarah Thomas, Elaine Allchurch, Peter Burgess. SIK – Institutet foer Livsmedel och Bioteknik (Sweden): Gunnar Hall, Annika Astrom, Anna Sverkén, Agneta Broberg. Meurice Recherche & Development asbl (Belgium): Annick Masson, Claire Lehoux, Pascal Brabant, Philippe Pate, Laurence Fontaine. Campden & Chorleywood Food Development Institute (Hungary): Andras Sebok, Tunde Kuti, Adrienn Hegyi. Productos Aditivos SA (Spain): Cristina Maldonado, Ana Llorente. Cárnicas Serrano SL (Spain): Emilio García. Cederroth International AB (Sweden): Holger von Fircks, Marianne Lilja Hallberg, Maria Messerer. Lantmännen Food R&D (Sweden): Mats Larsson, Helena Fredriksson, Viola Adamsson, Ingmar Börjesson. European Food Information Council (Belgium): Laura Fernández, Laura Smillie, Josephine Wills. Universidad Politécnica de Madrid (Spain): Marcela González-Gross, Jara Valtueña, Raquel Pedrero, Gonzalo Palacios, Agustín Meléndez, Pedro J. Benito, David Cañada, Juan Mielgo-Ayuso, Alejandro Urzanqui, Juan Carlos Ortiz, Francisco Fuentes, Juan José Gómez Lorente, David Jiménez-Pavón, Ulrike Albers, Rosa María Torres, Paloma Navarro.







