Food insecurity exists when an individual is unable to access or does not have enough money to buy an adequate amount of safe and nutritious food that meets their dietary needs and food preferences for an active and healthy life(1). An estimated 821 million people around the globe were affected by food insecurity in 2017, with a disproportionate burden in sub-Saharan Africa (SSA)(2,3) . The prevalence of severe food insecurity, specifically defined by global public health authorities as those who ran out of food and could not eat for a whole day or more in the past year, was only 1·4 % in North America and Europe but 29·8 % in Africa(2). Except for North America and Europe, the burden of food insecurity has progressively increased in all other regions of the world between 2014 and 2017(2–5). In SSA, the prevalence of severe food insecurity increased from 25·0 % in 2014 to 33·8 % in 2017, compared to a much smaller increase from 11·2 % to 12·4 % in Northern Africa over the same period(2,3) . A 2018 UN report revealed that 153 million people representing approximately 26 % of those over 15 years in SSA were affected by severe food insecurity between 2014 and 2015(6).
Poverty, underdeveloped and underperforming agricultural sectors have been identified as underlying causes of food insecurity in developing countries, particularly those in SSA(7,Reference Alabi8) . Agricultural output in SSA is greatly impacted by climate change, a high burden of HIV, social conflicts and poor governance(Reference Alabi8). Previous studies have also highlighted sociodemographic characteristics of households and health status as predictors of food insecurity(Reference Choi, Fram and Frongillo9–Reference Mohammed and Dlamini12). In the USA, low income, unmet medical needs, poor health and limited participation in food assistance programs were associated with food insecurity(Reference Choi, Fram and Frongillo9). In SSA, food insecurity has been more commonly observed in households with lower income, lower education, poor health status and larger family size/number of dependents(Reference Mustapha, Kamaruddin and Dewi11,Reference Mohammed and Dlamini12) .
SSA carries a disproportionate burden of HIV infection, accounting for two-thirds of the global burden of the disease(13). A two-way relationship between food insecurity and HIV has been reported. On the one hand, HIV-related morbidity and mortality have been observed to negatively impact the socio-economic status of households and take a toll on the agricultural labour force, thereby predisposing households to food insecurity(Reference Mustapha, Kamaruddin and Dewi11). Among farming households in rural Nigeria, a decline in food production efficiency and utilised farm area, as well as in financial contributions to households were observed among people living with HIV (PLWH)(Reference Mohammed and Dlamini12). On the other hand, food insecurity could potentially increase the risk of HIV transmission(13–Reference Oyekale and Adeoti15), by triggering risk behaviours such as transactional sex(Reference Rollins16,Reference Young, Wheeler, McCoy and Weiser17) . Food insecurity has also been identified as a barrier to HIV care and may be associated with poor treatment outcomes(Reference Young, Wheeler, McCoy and Weiser17–Reference Anema, Vogenthaler, Frongillo, Kadiyala and Weiser19).
To date, studies that assessed food insecurity in SSA have tended to be small, limited to single countries and rarely involved a mixed population of people living with and without HIV(Reference Mustapha, Kamaruddin and Dewi11,Reference Mohammed and Dlamini12,Reference Cheng, Kamano and Kirui20–Reference Masa, Chowa and Nyirenda25) . Most studies that explored the relationship between HIV and food insecurity were either conducted in the era of limited access to antiretroviral therapy (ART) or had a preponderance of PLWH with advanced disease(14,Reference Rollins16,Reference Anema, Vogenthaler, Frongillo, Kadiyala and Weiser19,Reference Weiser, Tsai and Gupta26–Reference Tsai, Bangsberg and Emenyonu28) .
A large, multinational evaluation of the prevalence and predictors of food insecurity could inform interventions to combat the growing epidemic of food insecurity in SSA. Moreover, in light of the ongoing COVID-19 pandemic, which has disrupted socio-economic activities and food security in SSA(Reference Amewu, Asante, Pauw and Thurlow29,Reference Dear, Duff and Esber30) , there is a critical need to identify at-risk groups for interventions to improve food security in the region. We determined the prevalence and identified predictors of food insecurity in people living with and without HIV in four African countries.
Methods
Study design and participants
The ongoing African Cohort Study (AFRICOS) is a longitudinal observational study that enrols PLWH and a smaller group of adults without HIV at twelve President’s Emergency Plan for AIDS Relief-supported clinical care sites in Uganda, Kenya, Tanzania and Nigeria as previously described(Reference Ake, Polyak and Crowell31). The vast majority of PLWH in our sample were invited to the study based on random selection from existing clinic lists (stratified by gender and ART status) or new enrolees to the clinic, while a minority (less than 5 %) are recruited from other HIV studies. Participants without HIV were recruited from individuals who screened negative for HIV at the counselling and testing units of the clinics. A few participants without HIV were serodiscordant partners of enrolled PLWH. All the President’s Emergency Plan for AIDS Relief clinics where AFRICOS is conducted serve the general population. Recruitment/enrolment is ongoing for up to a maximum of 4200 participants (3500 PLWH and 700 people without HIV). Individuals were eligible if they were aged ≥18 years and consented to data and specimen collection. An additional inclusion criterion for PLWH was the ongoing receipt of HIV care at the enrolling clinic. We excluded individuals who were pregnant at enrolment.
At enrolment and every 6 months thereafter, participants underwent medical history taking, physical examination and laboratory assessments, including HIV screening and confirmatory tests. Participants also completed a broad demographic and socio-behavioral questionnaire. Data from enrolment visits that occurred between 23 January 2013 and 1 March 2020 were included in these cross-sectional analyses. All participants provided written informed consent prior to enrolment.
Data collection and definitions
Demographic variables were obtained from participants by self-report, including age, sex, marital status, education, employment status, primary occupation, total number of people in the household and total number of dependents. For PLWH, clinical/laboratory data on WHO clinical staging, ART status, self-reported ART adherence, CD4 count and viral load (VL) were also collected. Although information on the presence of opportunistic infection was obtained in PLWH, it was not reported as a separate variable since it is a component of WHO clinical staging. PLWH were considered suppressed if they had a VL <1000 copies/ml.
The following distinct questions were initially used to assess three food insecurity metrics: (i) ‘Have you had enough food to eat over the past 12 months?’ (ii)‘On average, how many meals do you have in a day?’ and (iii) ‘Of these meals, how many have been cut or reduced in size because there is not enough food or money for food?’ Possible responses to the first question were either ‘yes’ or ‘no’, and were coded as binary in the models. Responses to the last two questions were collected as a discrete number of meals. For analyses, the number of meals per day variable was categorised as <3, 3 or >3, while the number of meals cut or reduced in size per day variable was categorised as none or ≥1. Using a combined index, food insecurity was defined as a report of not having enough food to eat over the past 12 months or having less than three meals per day on average, while food security was defined as a report of having enough food to eat over the past 12 months and having three or more meals per day on average. The metric assessing the number of meals reduced in size per day because there is not enough food or money for food was excluded from the combined food insecurity index due to the disproportionately low number of participants with available data for this variable, as this question was added to the subject questionnaire in late 2017.
Statistical analyses
Descriptive statistics (χ 2 tests for categorical variables and Wilcoxon rank-sum tests for continuous variables) were used to describe differences between participants classified as food insecure using the combined food insecurity index and those classified as food secure. Generalised linear models with a Poisson distribution and robust error variances were used to estimate unadjusted and adjusted prevalence ratios (aPR) and 95 % confidence interval (CI) for associations between pre-specified factors of interest and not having enough food to eat in the past 12 months and the combined food insecurity index. The PR is a measure of association quantifying the relationship between a predictor variable and a dichotomous outcome of interest in a cross-sectional analysis, especially suitable for outcomes with a high prevalence. Poisson regression models for count outcomes were used to estimate unadjusted and adjusted rate ratios for associations between pre-specified factors and the number of meals per day.
Independent variables were selected for inclusion in the model based on a review of existing literature, including HIV status, age, sex, marital status, education, employment status, primary occupation, farming status, household size and number of dependents. To potentially account for country-specific and seasonal/climatic variabilities, we also adjusted for site and year of enrolment. To evaluate HIV-specific factors, a subgroup analysis was performed among PLWH. A p-value <0·05 was considered statistically significant. All analyses were performed using SAS 9·3 (SAS, Cary, NC) and Stata 15·0 (StataCorp, College Station, TX).
Results
Characteristics of the study population
As of 1 March 2020, a total of 3551 participants were enrolled in the study, including 2937 PLWH and 614 participants without HIV. Of these, 3496 participants comprising 2884 PLWH and 612 people without HIV had complete data for analyses. Among all participants, the median age was 37·8 years (interquartile range 30·5–45·8 years). The majority of participants were female (n 2029, 58·0 %) and 2070 (59·2 %) were married. A total of 1390 (39·8 %) reported being employed at the time of enrolment, including 356 (10·2 %) who reported that their current primary occupation was farming. The median household size was 5 (interquartile range 3–6), and the median number of dependents was 2 (interquartile range 2–3) (Table 1).
Table 1 Characteristics of the study population by food insecurity status
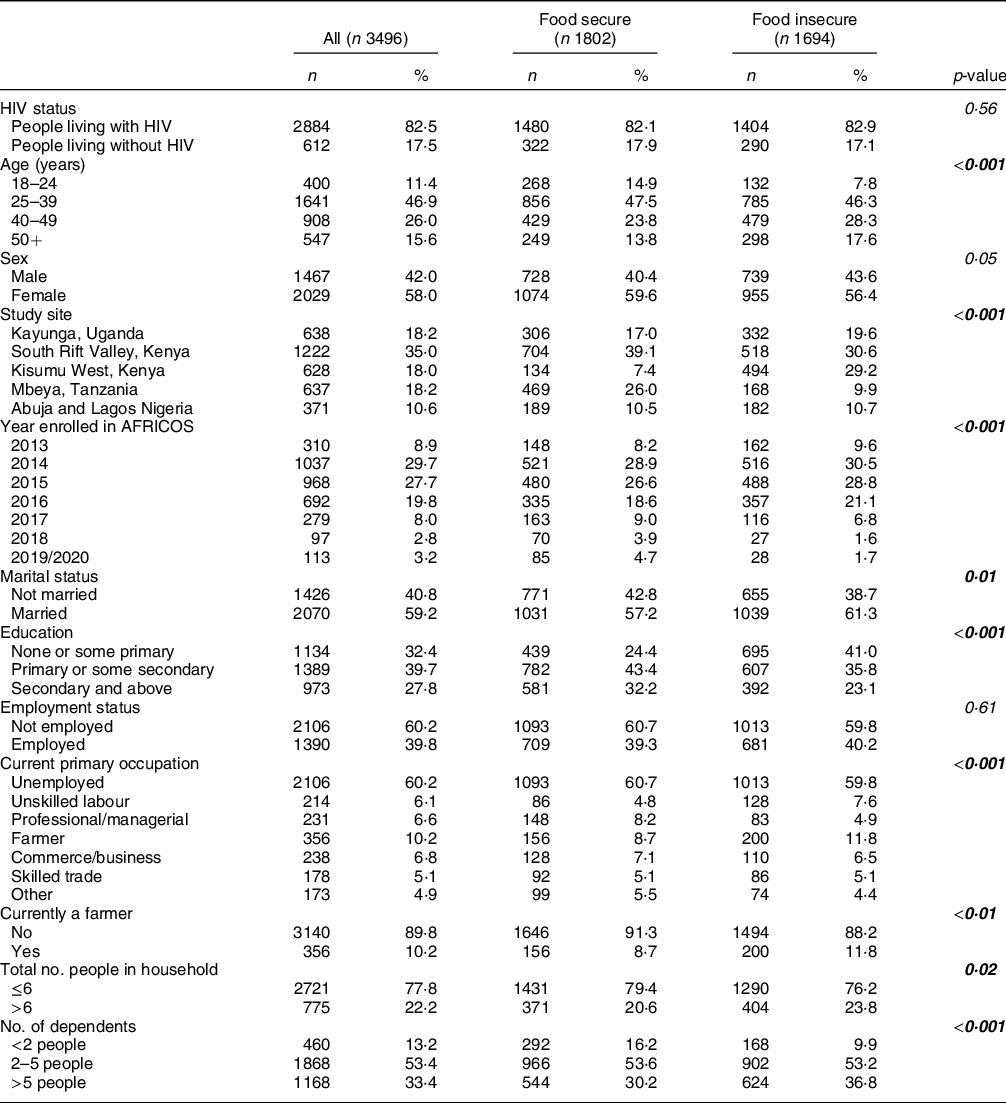
Data are presented as n (column %). Pearson’s χ 2 and Wilcoxon rank-sum tests were used to describe differences between participants classified as food insecure and those classified as food secure, with food insecurity defined as a report of not having enough food to eat over the past 12 months or having less than three meals per day on average. Statistically significant p-values are presented in bold. AFRICOS, African Cohort Study.
The median age among PLWH was 38·3 years (interquartile range 31·1–46·1 years). Among PLWH, 889 (30·8 %) were not on ART at enrolment, while 1691 (58·6 %) were on ART with a VL < 1000 copies/ml and 304 (10·5 %) were on ART with a VL ≥ 1000 copies/ml. Only 564 (19·6 %) had a CD4 count below 200 cells/mm3. WHO clinical stages III and IV disease were documented in 998 (34·6 %) and 183 (6·3 %) PLWH, respectively (Table 2).
Table 2 Characteristics of the study population living with HIV by food insecurity status
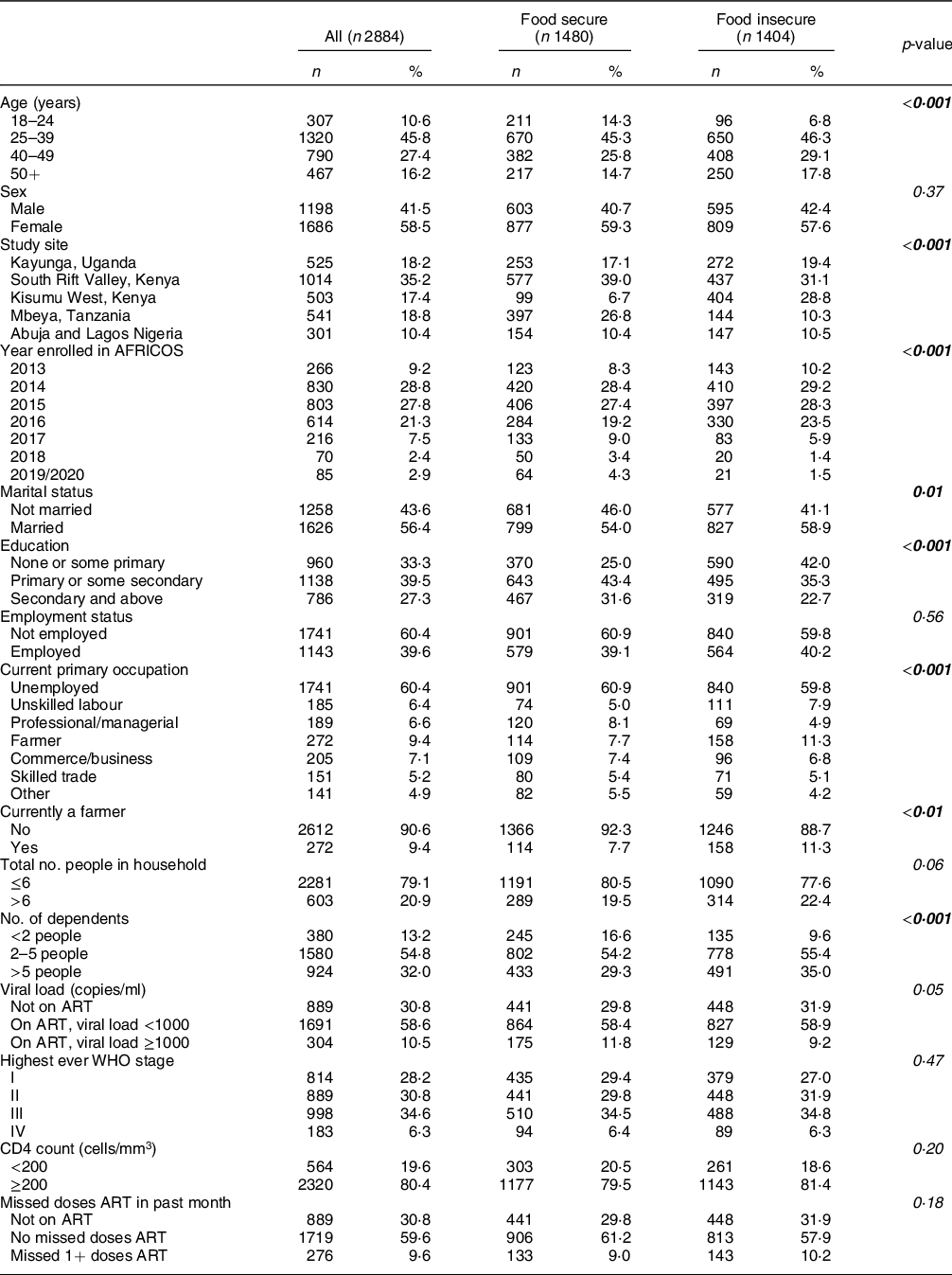
Data are presented as n (column %). Pearson’s χ 2 and Wilcoxon rank-sum tests were used to describe differences between participants living with HIV classified as food insecure and those classified as food secure, with food insecurity defined as a report of not having enough food to eat over the past 12 months or having less than three meals per day on average. Statistically significant p-values are presented in bold. ART, antiretroviral therapy.
Prevalence of food insecurity
Of 3496 participants, 1204 (34·4 %) reported not having enough food to eat in the past 12 months, while receiving <3 meals/d on average was documented in 1004 (28·7 %) participants. Reduction in number or size of ≥1 meals because there was not enough food or money for food was observed in 68 (22·8 %) out of 298 participants with available data.
Statistically significant inter-site differences were observed in the proportion of individuals who reported not having enough food to eat over 12 months, with a greater proportion of participants in Kisumu West, Kenya (n 473,75·3 %) and South Rift Valley, Kenya (n 405, 33·1 %) affected than in other sites (P < 0·001, Fig. 1A). Similarly, the average number of meals received per day varied significantly by site, with a greater proportion of participants in Uganda (n 300, 47·0 %) and Nigeria (n 145, 39·1 %) reporting <3 meals/d on average as compared to the other sites (P < 0·001, Fig. 1B). There was no significant inter-site difference in the proportion of individuals who reported a cut or reduction in the size of meals (P = 0·18, Fig. 1C).
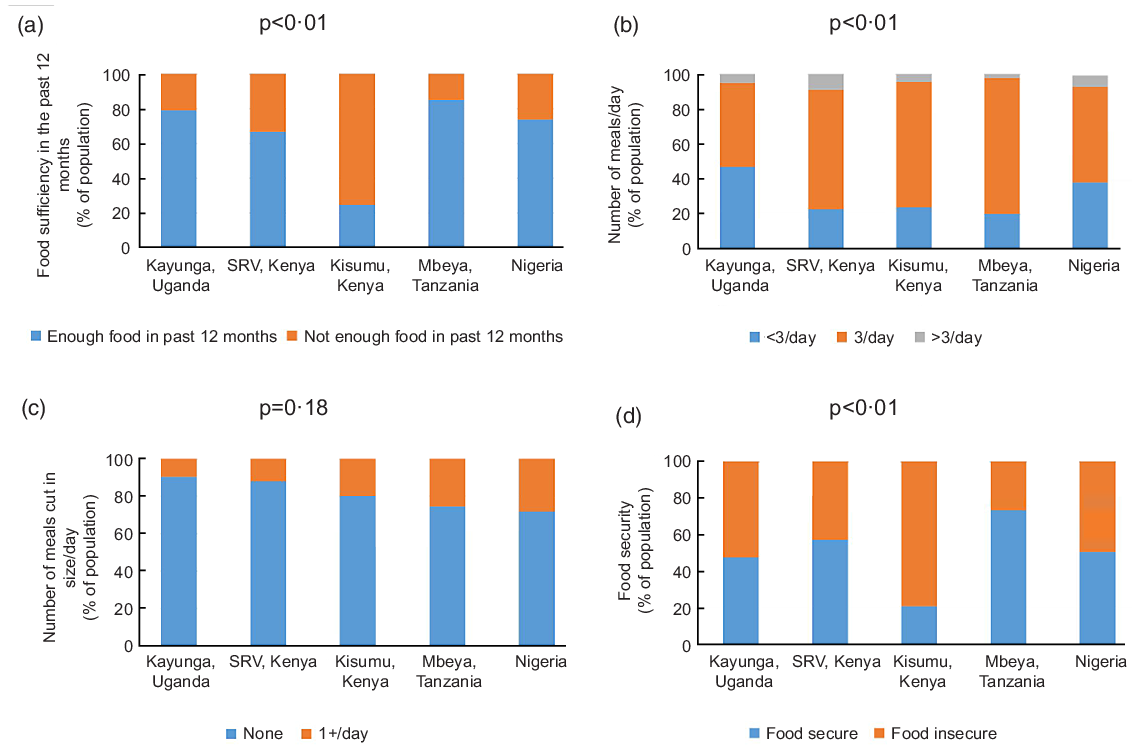
Fig. 1 Food security indicators stratified by site in the African Cohort Study
Based on the combined index, the prevalence of food insecurity was 48·5 % (n 1694), with no statistically significant difference between participants with and without HIV (48·7 % v. 47·4 %, P = 0·56). A statistically significant difference was observed in the prevalence of food insecurity by site, with the highest prevalence in Kisumu West, Kenya (n 494, 78·7 %), followed by Uganda (n 332, 52·0 %) and the lowest prevalence in Tanzania (n 168, 26·4 %), (P < 0·001, Fig. 1D).
Predictors of not having enough food over the past 12 months
Compared to participants 18–24 years old, not having enough food to eat in the past 12 months was more common among those 25–39 years old (aPR 1·35, 95 % CI 1·10, 1·64), 40–49 years old (aPR 1·58, 95 % CI 1·28, 1·94) and 50+ years old (aPR 1·33, 95 % CI 1·07, 1·65; Table 3). As compared to having less than two dependents, having 2–5 dependents (aPR 1·27, 95 % CI 1·06, 1·52) or more than five dependents (aPR 1·41, 95 % CI 1·16, 1·71) was associated with not having enough food to eat in the past 12 months. Residing in South Rift Valley, Kenya (aPR 1·78, 95 % CI 1·44, 2·21), Kisumu West, Kenya (aPR 3·99, 95 % CI 3·22, 4·94) or Nigeria (aPR 1·78, 95 % CI 1·37, 2·32) as compared to residing in Uganda, was independently associated with not having enough food to eat in the past 12 months. As compared to those with none or some primary education, having a primary or some secondary level education (aPR 0·80, 95 % CI 0·73, 0·88) or a secondary level education or above (aPR 0·65, 95 % CI 0·57, 0·74) was protective against not having enough food to eat in the past 12 months after adjusting for potential confounders. Living with HIV was not significantly associated with not having enough food to eat over the past 12 months (aPR 1·01, 95 % CI 0·90, 1·14).
Table 3 Unadjusted and adjusted analyses of factors associated with not having enough food to eat in the past 12 months among all participants
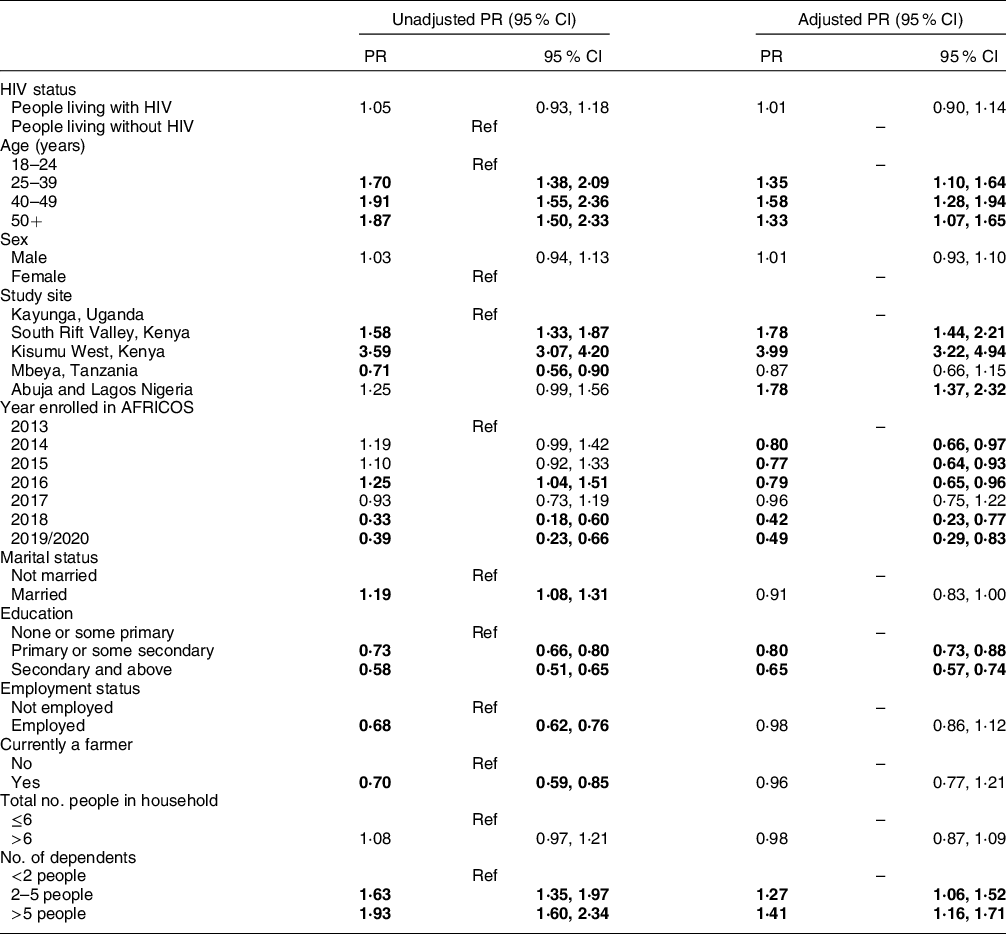
Generalised linear models with a Poisson distribution and robust error variances were used to estimate unadjusted and adjusted prevalence ratios (aPR) and 95 % CI for associations between pre-specified factors of interest and not having enough food to eat in the past 12 months among all participants. Statistically significant association is in bold. AFRICOS, African Cohort Study.
In the adjusted model for only PLWH, compared to participants 18–24 years old, not having enough food to eat in the past 12 months was more common among those 25–39 years old (aPR 1·40, 95 % CI 1·11, 1·78) and 40–49 years old (aPR 1·52, 95 % CI 1·19, 1·95; Supplementary Table 1). Similar to the model for all participants, living in Kenya (South Rift Valley or Kisumu West) or Nigeria, and having more dependents independently predicted not having enough food to eat over the past 12 months while higher education and being enrolled between 2014 and 2016 compared to 2013 were protective. WHO clinical stage, ART status and CD4 count were not found to be significantly associated with not having enough food to eat in the past 12 months after adjusting for other factors.
Predictors of average number of meals per day
Among all participants, after adjusting for potential confounders, compared to participants 18–24 years old, those who were 40–49 years (aRR 0·97, 95 % CI 0·94, 1·00) and 50+ years (aRR 0·95, 95 % CI 0·92, 0·98), male (aRR 0·98, 95 % CI 0·97, 1·00) and employed (aRR 0·97, 95 % CI 0·95, 0·99) had a decreased rate of meals per day (Table 4). Compared to participants from Uganda, participants living in South Rift Valley, Kenya (aRR 1·10, 95 % CI 1·06, 1·14), Kisumu West, Kenya (aRR 1·07, 95 % CI 1·03, 1·11) or Tanzania (aRR 1·07, 95 % CI 1·03, 1·11), and those with a primary or some secondary level education (aRR 1·04, 95 % CI 1·02, 1·06) or a secondary level education or above (aRR 1·06, 95 % CI 1·03, 1·08) as compared to those with none or some primary education had a higher rate of meals per day. There was no significant association between living with HIV and number of meals received per day (aRR 1·01, 95 % CI 0·99, 1·03).
Table 4 Unadjusted and adjusted analyses of factors associated with number of meals per day among all participants
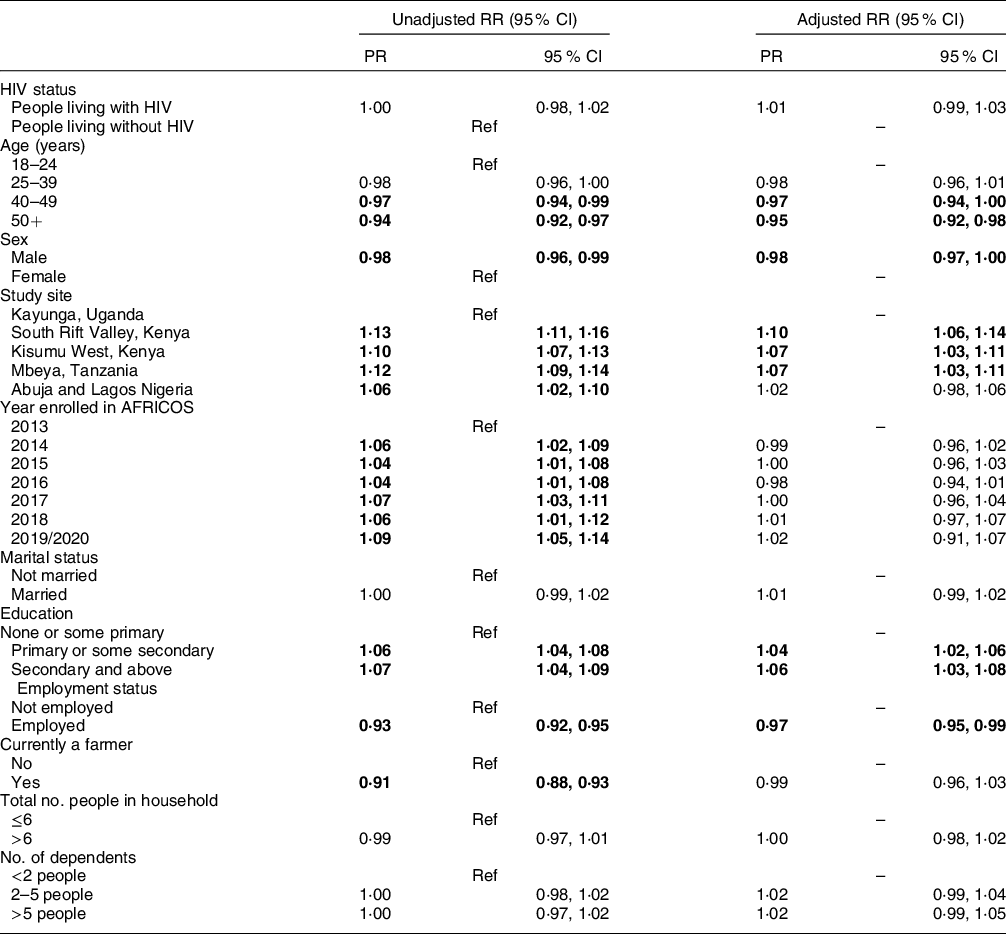
Poisson regression models were used to estimate unadjusted and adjusted rate ratios for associations between pre-specified factors and the number of meals per day among all participants. Statistically significant association in bold. AFRICOS, African Cohort Study.
Similarly, PLWH who were older, male and employed had a reduced rate of meals per day, while living in Kenya (South Rift Valley or Kisumu West) or Tanzania compared to Uganda and higher education was associated with an increased rate of meals per day (Supplementary Table 2). ART-experienced PLWH had higher rates of meals per day as compared to ART-naïve participants, irrespective of VL (on ART/VL < 1000 copies/ml aRR 1·04, 95 % CI 1·02, 1·06 and on ART/VL ≥ 1000 copies/ml aRR 1·03, 95 % CI 1·00, 1·07).
Predictors of food insecurity based on the combined index
After adjusting for potential confounding factors, compared to participants 18–24 years old, food insecurity as assessed using the combined index, was more common among those 25–39 years (aPR 1·26, 95 % CI 1·08, 1·46), 40–49 years (aPR 1·41, 95 % CI 1·20, 1·65) and 50+ years (aPR 1·35, 95 % CI 1·15, 1·59; Table 5). As compared to having less than two dependents, having 2–5 dependents (aPR 1·14, 95 % CI 1·01, 1·30) or more than five dependents (aPR 1·17, 95 % CI 1·02, 1·35) was independently associated with food insecurity. Residing in Kisumu West, Kenya (aPR 1·63, 95 % CI 1·42, 1·87) or Nigeria (aPR 1·20, 95 % CI 1·01, 1·41) as compared to residing in Uganda, was independently associated with food insecurity while residing in Tanzania (aPR 0·60, 95 % CI 0·50, 0·72) was protective against food insecurity. As compared to those with none or some primary education, having a primary or some secondary level education (aPR 0·82, 95 % CI 0·76, 0·88) or a secondary level education or above (aPR 0·73, 95 % CI 0·66, 0·81) was protective against food insecurity. Living with HIV was not a predictor of food insecurity as assessed by the combined index (aPR 1·01, 95 % CI 0·92, 1·10).
Table 5 Unadjusted and adjusted analyses of factors associated with food insecurity among all participants
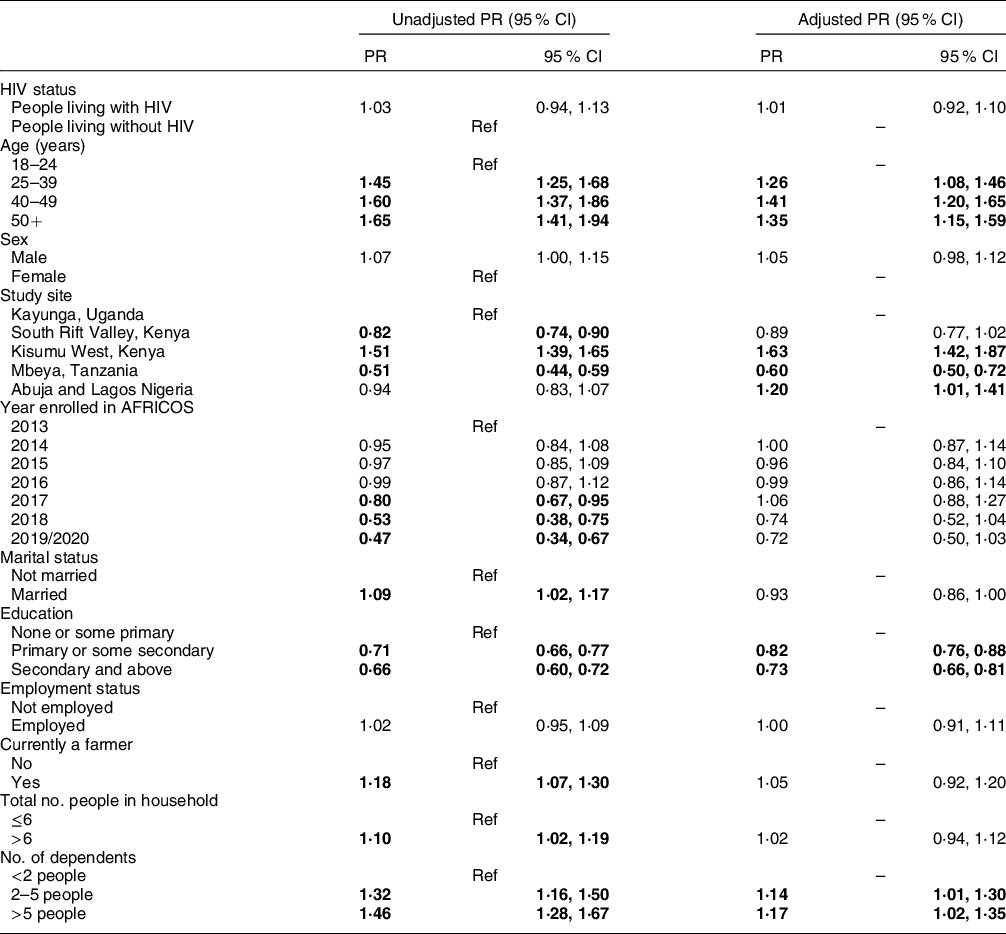
Generalised linear models with a Poisson distribution and robust error variances were used to estimate unadjusted and adjusted prevalence ratios (aPR) and 95 % CI for associations between pre-specified factors of interest and food insecurity, defined as a report of not having enough food to eat over the past 12 months or having less than three meals per day on average, among all participants. Statistically significant association is indicated in bold. AFRICOS, African Cohort Study.
After adjusting for potential confounding factors, among PLWH, compared to participants 18–24 years old, food insecurity was more common among those 25–39 years old (aPR 1·31, 95 % CI 1·09, 1·57), 40–49 years (aPR 1·40, 95 % CI 1·16, 1·69) and 50+ years (aPR 1·39, 95 % CI 1·14, 1·69; Supplementary Table 3). As compared to having less than two dependents, having 2–5 dependents (aPR 1·19, 95 % CI 1·03, 1·37) or more than five dependents (aPR 1·21, 95 % CI 1·03, 1·41) was independently associated with food insecurity among PLWH. Residing in Kisumu West, Kenya (aPR 1·83, 95 % CI 1·56, 2·15) or Nigeria (aPR 1·30, 95 % CI 1·08, 1·57) as compared to residing in Uganda, was independently associated with food insecurity while residing in Tanzania (aPR 0·65, 95 % CI 0·53, 0·80) was protective. Compared to those with none or some primary education, having a primary or some secondary level education (aPR 0·81, 95 % CI 0·75, 0·88) or a secondary level education or above (aPR 0·73, 95 % CI 0·66, 0·82) was protective against food insecurity. ART-experienced PLWH were significantly more likely to be food secure irrespective of VL compared to ART-naïve PLWH (on ART/VL < 1000 copies/ml v. ART naïve, aPR 0·90, 95 % CI 0·82, 0·99; on ART/VL ≥ 1000 copies/ml v. ART naïve, aPR 0·86, 95 % CI 0·74, 0·99). WHO clinical stage and CD4 count were not significantly associated with food insecurity in the multivariable analysis.
Discussion
Between one-fifth and one-third of participants in our study recorded at least one of three food insecurity metrics with significant inter-site differences. As defined by the combined index, nearly half of the participants reported food insecurity, underscoring a large unmet need for strategies to ensure adequate food supply to people with and without HIV in SSA. This finding is generally consistent with prior reports of high but variable prevalence of food insecurity in SSA ranging from 19·5 to 100 %(Reference Mustapha, Kamaruddin and Dewi11,Reference Hong, Fanelli and Jonas18,Reference Cheng, Kamano and Kirui20–Reference Tsai, Bangsberg and Emenyonu28,Reference Orewa and Iyangbe32–Reference Perkins, Nyakato and Kakuhikire38) . In Western Kenya, 32 % of patients attending diabetes clinics were found to be food insecure(Reference Cheng, Kamano and Kirui20), while studies from other parts of Kenya found much higher rates of food insecurity of 85 % in urban slum residents following post-election crises(Reference Kimani-Murage, Schofield and Wekesah37) and 100 % in a very small population of PLWH(Reference Nagata, Magerenge and Young24). Available studies from Uganda have also demonstrated high levels of food insecurity of 75·4–93 % in the general population(Reference Yikii, Turyahabwe and Bashaasha23,Reference Perkins, Nyakato and Kakuhikire38) and among predominantly ART-naïve PLWH(Reference Weiser, Tsai and Gupta26,Reference Tsai, Bangsberg and Emenyonu28) . Among ART-naïve PLWH in Tanzania, 52·2 % were found to be food insecure(Reference Semali, Edwin and Mboera27). The prevalence of food insecurity in various studies conducted in largely rural and low-income Nigerian households ranged from 40·8 to 70 %(Reference Mustapha, Kamaruddin and Dewi11,Reference Oyebanjo, Ambali and Akerele21,Reference Orewa and Iyangbe32,Reference Akerele, Momoh and Aromolaran33,Reference Sanusi, Badejo and Yusuf35) . In Southern Ethiopia, a study conducted in predominantly stable, ART-experienced PLWH found a relatively lower prevalence of food insecurity of 19·5 %(Reference Belijo and Mensa36). In addition to the differences in the methodologies used to assess food security, characteristics of the study population, sample size, environmental and sociopolitical issues may account for the disparity in these studies. Several tools are available for the measurement of food insecurity(Reference Pérez-Escamilla and Segall-Corrêa39). Most of the previous studies in SSA used the Household Food Insecurity Access Scale, an experience-based tool with a 30-day recall(Reference Nagata, Magerenge and Young24,Reference Weiser, Tsai and Gupta26,Reference Tsai, Bangsberg and Emenyonu28,Reference Kimani-Murage, Schofield and Wekesah37,Reference Oluma, Abadiga and Mosisa40) . Some studies in SSA have used the food security index(Reference Mustapha, Kamaruddin and Dewi11,Reference Akerele, Momoh and Aromolaran33) or different questionnaire-based tools(Reference Cheng, Kamano and Kirui20,Reference Napier, Oldewage-Theron and Makhaye22) , while others used three or single-question food security indicators over a 12-month recall similar to our methods(Reference Semali, Edwin and Mboera27,Reference Alaimo, Olson and Frongillo41) . It has been shown that all the methods have advantages and inherent measurement errors, and no single tool can account for all the dimensions of food security(Reference Pérez-Escamilla and Segall-Corrêa39).
In this study, similar socio-demographic factors consistently predicted food insecurity whether assessed by singular metrics or the combined index. Also, the socio-demographic predictors of food insecurity were similar for all participants and the subgroup of PLWH, who constituted the majority of the study population. The predictors of food insecurity among all participants, as defined by the combined index, are largely consistent with findings of prior studies conducted in SSA that identified low educational status(Reference Mustapha, Kamaruddin and Dewi11,Reference Oyebanjo, Ambali and Akerele21,Reference Nagata, Magerenge and Young24,Reference Akerele, Momoh and Aromolaran33–Reference Sanusi, Badejo and Yusuf35,Reference Oluma, Abadiga and Mosisa40) , age(Reference Mustapha, Kamaruddin and Dewi11,Reference Oyebanjo, Ambali and Akerele21,Reference Nagata, Magerenge and Young24) and a high number of dependents(Reference Akerele, Momoh and Aromolaran33) as predictors of food insecurity. A high level of education is likely to enable people to access better job opportunities, attract higher income and make more appropriate decisions regarding food production and/or consumption. Older age may be associated with a decline in physical strength leading to reduced farming activities or limited ability to engage in relatively better-paying jobs, thereby predisposing to food insecurity(Reference Tauer42,Reference Babatunde, Omotesho and Sholotan43) . We also observed that the geographical location of participants impacted food security, whether assessed by single metrics or the combined index. Compared to participants from Uganda, living in Kisumu West, Kenya was independently predictive of food insecurity in our cohort. Few studies have investigated the impact of geographical location on food insecurity. In tandem with our report, a previous study in Brazil observed that residing in the North and Northeastern regions of the country was predictive of food insecurity(Reference Santos, Silveira and Longo-Silva44). Among all participants, married persons were significantly more likely to be food secure. This observation was the only socio-demographic predictor of food insecurity seen in the mixed population but not in the PLWH subgroup in the adjusted model. In Western Ethiopia, being single predicted food insecurity in PLWH(Reference Oluma, Abadiga and Mosisa40). It is possible that the care and support provided by a spouse could positively impact food access and the availability of prepared meals at home.
Other factors commonly associated with food insecurity in previous studies are low household income, large family size, unemployment, occupation, temporal changes/year and limited involvement in farming(Reference Mustapha, Kamaruddin and Dewi11,Reference Oyebanjo, Ambali and Akerele21,Reference Akerele, Momoh and Aromolaran33–Reference Sanusi, Badejo and Yusuf35,Reference Oluma, Abadiga and Mosisa40) . Contrary to some previous studies, employment status, being a farmer and size of household were not independent predictors of food insecurity defined by the combined index in our cohort. Nevertheless, we made an interesting observation that being employed rather predicted having a lower number of meals per day on average among all participants. It is understandable that being at work may lead to missing meals on some days. However, this cannot be stretched too far as employment status neither impacted report of not having enough food to eat over the past 12 months nor food insecurity assessed by the combined index. Although the year of enrolment did not emerge as an independent predictor of food insecurity in our cohort, it proved to be a significant factor associated with the report of not having enough food to eat over the past 12 months in all participants and in the subgroup of PLWH. The impact of temporal changes on food insecurity has been previously documented(Reference Santos, Silveira and Longo-Silva44). Multiple factors may be responsible such as the impact of seasonal/climatic variations on agricultural activities and the role of socio-political crises on food access. Due to the variability of occupation and income/national currencies in our population, we did not specifically examine the relationship between food insecurity and various occupations or household income.
Although a household size of more than six and surprisingly being a farmer were significantly associated with food insecurity in unadjusted models, these associations were not statistically significant after adjusting for potential confounders. While we did not find any significant association between sex and food insecurity as defined by the combined index, we observed that being male was significantly associated with fewer meals per day among all participants and in the subgroup of PLWH. The association of male sex with fewer meals per day may be because males are more likely to be employed and may thus need to miss a meal due to being at work. Although being male was significantly associated with food insecurity compared to female sex among PLWH in a previous study in Western Ethiopia(Reference Oluma, Abadiga and Mosisa40), another study in Southern Ethiopia identified being female as a predictor of food insecurity in PLWH(Reference Belijo and Mensa36). This disparity was observed even though both studies assessed food insecurity using the same tool. A systematic review of the effect of sex on food security among PLWH receiving ART showed higher odds of food insecurity among female PLWH than their male counterparts, especially in low and middle-income countries where women lack control over resources and household food allocation decision-making(Reference Boneya, Ahmed and Yalew45).
There is a paucity of studies comparing the prevalence of food insecurity between PLWH and those without HIV. Surprisingly, this study revealed that PLWH were not disproportionately affected by food insecurity both in the combined index and for the single metrics, contrary to the traditional view that HIV infection predisposes to food insecurity(14,Reference Oyekale and Adeoti15) . A possible reason for our observation is that the PLWH in our study were engaged in care and the majority were on suppressive ART, hence were unlikely to be at substantial risk of decreased food production efficiency and higher out-of-pocket expenditure on healthcare. Moreover, this study predominantly spans an era of improved access to HIV care and treatment during which a relatively smaller proportion of PLWH presented with advanced disease, compared to studies conducted predominantly in the era of limited access to care with a substantial proportion of PLWH manifesting advanced disease(Reference Weiser, Tsai and Gupta26–Reference Tsai, Bangsberg and Emenyonu28).
Nevertheless, we conducted more focussed analyses in the subgroup of PLWH to better understand the predictors of food insecurity in the population, beyond socio-demographic factors which themselves are similar between PLWH and the mixed population in this study. In terms of HIV care/outcome variables, we observed that ART-experienced PLWH were significantly more likely to be food secure irrespective of their viral suppression status compared to ART-naïve PLWH. However, advanced HIV disease was not significantly associated with food insecurity. Previous studies have demonstrated an inverse relationship between food insecurity and key HIV care/outcome variables(Reference Young, Wheeler, McCoy and Weiser17–Reference Anema, Vogenthaler, Frongillo, Kadiyala and Weiser19). An inverse relationship between food insecurity and ART access has been documented in SSA(Reference Young, Wheeler, McCoy and Weiser17). For example, food insecurity was shown to be a barrier to ART in PLWH yet to initiate treatment and a key contributor to incomplete adherence among individuals on ART(Reference Young, Wheeler, McCoy and Weiser17). Studies conducted in the USA have reported a significant association between food insecurity and poor virologic response in ART-experienced PLWH after controlling for potential confounders(Reference Weiser, Frongillo, Ragland, Hogg, Riley and Bangsberg46,Reference Wang, McGinnis and Fiellin47) . Improved food security seen in the ART-experienced PLWH in this study is not surprising considering that the attendant improved health and well-being is likely to have downstream consequences on psychosocial and economic factors known to improve food access. Sustained improvement of access to ART could potentially be exploited as a strategy for improving food security in SSA, especially among PLWH.
Contrary to our observation, advanced HIV disease as evidenced by WHO clinical stage III/IV disease or low CD4 count predicted food insecurity in Southern Ethiopia and North America(Reference Belijo and Mensa36,Reference Normen, Chan and Braitstein48,Reference Weiser, Bangsberg, Kegeles, Ragland, Kushel and Frongillo49) . Some previous studies have demonstrated heavy household expenditure on healthcare in PLWH with advanced disease thereby predisposing to poverty and invariably food insecurity(Reference Gregson, Mushati and Nyamukapa50,Reference Ngalula, Urassa and Mwaluko51) . In another study in Western Ethiopia, the relationship between food insecurity and WHO clinical stage was inconsistent on multivariate analysis(Reference Oluma, Abadiga and Mosisa40). Beyond any potential association with advanced disease, the two-way relationship between HIV-related mortality and food insecurity highlights the critical need to address food security challenges in PLWH(Reference Anema, Vogenthaler, Frongillo, Kadiyala and Weiser19,Reference Twine and Hunter52) . On a broader perspective, the negative impact of food insecurity on health outcomes has been documented for several diseases other than HIV(Reference Kushel, Gupta and Gee53,Reference Vozoris and Tarasuk54) . Integrating food security interventions into HIV care/treatment programs appears to be a pragmatic step towards improving the health of PLWH. However, as recommended in a previous study, policies and interventions aimed at enhancing food security in SSA should target vulnerable groups broadly, rather than solely targeting those directly affected by HIV(Reference Twine and Hunter52).
The limitations of these analyses are acknowledged. Our measurement of food insecurity was not based on one of the more commonly used validated tools such as Household Food Insecurity Access Scale and the food security index, which makes direct comparison with a number of prior studies challenging. As applicable to several food insecurity survey tools, our method is subjective and vulnerable to recall social desirability bias. Also, caution should be exercised when generalising our findings for two major reasons. First, the study participants were enrolled during clinic visits so it may represent a disproportionate sample of vulnerable individuals at increased risk of food insecurity. Secondly, our study population comprised predominantly PLWH and a smaller proportion of adults without HIV, which is not the typical picture in the general population. The study participants were enrolled over a period of 7 years during which diverse seasonal and socio-political factors could have impacted food production and access in Africa thereby constituting a potential limitation to these analyses. Despite these limitations, the strengths of this study are worthy of note. We examined food insecurity among a large cohort in four African countries, highlighting significant inter-country differences in food insecurity and invariably filling some gaps in previous studies. We were able to control for multiple potential confounders, including socio-demographic variables and year of enrolment, some of which were not adjusted for in some previous studies. The potential impact of seasonal/climatic variations on food insecurity was partly controlled by the inclusion of the year of enrolment in the models. While the impact of our study limitations on the high prevalence of food insecurity and the lack of association with HIV is worthy of consideration, the fact that the majority of previous studies in SSA have either reported similarly high or much higher burden of food insecurity in the general population, and in PLWH lays credence to our observations. These analyses have also provided findings that argue for further studies on the relationship between HIV outcomes and food insecurity in the era of improved access to care and treatment.
In conclusion, we found a high prevalence of food insecurity in four African countries, with significant inter-country variability. Surprisingly, the prevalence of food insecurity among PLWH was similar to that of people without HIV. This study revealed that age, education, number of dependents, country of residence and marital status were independent predictors of food insecurity assessed using a combined index. The sociodemographic predictors of food insecurity were similar between the mixed population and the subgroup of PLWH. Irrespective of VL suppression status, ART-experienced PLWH were significantly more likely to be food secure compared to ART-naïve populations. On the other hand, no significant association was demonstrated between advanced HIV disease and food insecurity. The high prevalence of food insecurity in SSA is concerning, especially in the setting of COVID-19-induced socio-economic crises and reduction in food security. Moreover, a high level of food insecurity remains a threat to the attainment of Sustainable Development Goals in Africa. Increased access to education for the entire population and providing social support for older populations could potentially improve food security in SSA. Given the strong extended family system in Africa, in addition to intensified family planning sensitisation, the economic empowerment of eligible family members will reduce the pressure on household heads with a potentially favourable downstream impact on food security. Improved access to ART is a pragmatic strategy for promoting food security in PLWH. African governments should prioritise agricultural policies that support food production, distribution and access. We recommend further prospective cohort studies to investigate the relationship between HIV outcomes and food insecurity.
Acknowledgments
We thank the study participants, local implementing partners and hospital leadership at Kayunga District Hospital, Kericho District Hospital, AC Litein Mission Hospital, Kapkatet District Hospital, Tenwek Mission Hospital, Kapsabet District Hospital, Nandi Hills District Hospital, Kisumu West District Hospital, Mbeya Zonal Referral Hospital, Mbeya Regional Referral Hospital, Defence Headquarters Medical Center and the 68th Nigerian Army Reference Hospital.
We would also like to thank the AFRICOS Study Group – from the US Military HIV Research Program Headquarters team: Danielle Bartolanzo, Alexus Reynolds, Katherine Song, Mark Milazzo, Leilani Francisco, Shauna Mankiewicz, Steven Schech, Alexandra Golway, Badryah Omar, Tsedal Mebrahtu, Elizabeth Lee, Kimberly Bohince, Ajay Parikh, Jaclyn Hern, Emma Duff, Kara Lombardi, Michelle Imbach and Leigh Anne Eller; from the AFRICOS Uganda team: Michael Semwogerere, Prossy Naluyima, Godfrey Zziwa, Allan Tindikahwa, Hilda Mutebe, Cate Kafeero, Enos Baghendaghe, William Lwebuge, Freddie Ssentogo, Hellen Birungi, Josephine Tegamanyi, Paul Wangiri, Christine Nabanoba, Phiona Namulondo, Richard Tumusiime, Ezra Musingye, Christina Nanteza, Joseph Wandege, Michael Waiswa, Evelyn Najjuma, Olive Maggaga, Isaac Kato Kenoly and Barbara Mukanza; from the AFRICOS South Rift Valley, Kenya team: Rither Langat, Aaron Ngeno, Lucy Korir, Raphael Langat, Francis Opiyo, Alex Kasembeli, Christopher Ochieng, Japhet Towett, Jane Kimetto, Brighton Omondi, Mary Leelgo, Michael Obonyo, Linner Rotich, Enock Tonui, Ella Chelangat, Joan Kapkiai, Salome Wangare, Zeddy Bett Kesi, Janet Ngeno, Edwin Langat, Kennedy Labosso, Joshua Rotich, Leonard Cheruiyot, Enock Changwony, Mike Bii, Ezekiel Chumba, Susan Ontango, Danson Gitonga, Samuel Kiprotich, Bornes Ngtech, Grace Engoke, Irene Metet, Alice Airo and Ignatius Kiptoo; from the AFRICOS Kisumu, Kenya team: Valentine Sing’oei, Winne Rehema, Solomon Otieno, Celine Ogari, Elkanah Modi, Oscar Adimo, Charles Okwaro, Christine Lando, Margaret Onyango, Iddah Aoko, Kennedy Obambo, Joseph Meyo and George Suja; from the AFRICOS Abuja, Nigeria Group: Michael Iroezindu, Yakubu Adamu, Nnamdi Azuakola, Mfreke Asuquo, Abdulwasiu Bolaji Tiamiyu, Afoke Kokogho, Samirah Sani Mohammed, Ifeanyi Okoye, Sunday Odeyemi, Aminu Suleiman, Lawrence C. Umeji, Onome Enas, Miriam Ayogu, Ijeoma Chigbu-Ukaegbu, Wilson Adai, Felicia Anayochukwu Odo, Rabi Abdu, Roseline Akiga, Helen Nwandu, Chisara Sylvestina Okolo, Ogundele Taiwo, Otene Oche Ben, Nicholas Innocent Eigege, Tony Ibrahim Musa, Juliet Chibuzor Joseph, Ndubuisi C. Okeke; from the AFRICOS Lagos, Nigeria Group: Zahra Parker, Nkechinyere Elizabeth Harrison, Uzoamaka Concilia Agbaim, Olutunde Ademola Adegbite, Ugochukwu Linus Asogwa, Adewale Adelakun, Chioma Ekeocha, Victoria Idi, Rachel Eluwa, Jumoke Titilayo Nwalozie, Igiri Faith, Blessing Irekpitan Wilson, Jacinta Elemere, Nkiru Nnadi, Francis Falaju Idowu, Ndubuisi Rosemary, Amaka Natalie Uzoegwu, Theresa Owanza Obende, Ifeoma Lauretta Obilor, Doris Emekaili, Edward Akinwale and Inalegwu Ochai; from the AFRICOS Mbeya, Tanzania team: Lucas Maganga, Samoel Khamadi, John Njegite, Connie Lueer, Abisai Kisinda, Jaquiline Mwamwaja, Faraja Mbwayu, Gloria David, Mtasi Mwaipopo, Reginald Gervas, Doroth Mkondoo, Nancy Somi, Paschal Kiliba, Gwamaka Mwaisanga, Johnisius Msigwa, Hawa Mfumbulwa, Peter Edwin, Willyhelmina Olomi.
Financial Support
This work was supported by the President’s Emergency Plan for AIDS Relief via a cooperative agreement between the Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc. and the U.S. Department of Defense [W81XWH-18-2-0040].
Conflict of Interest
The authors have no conflicts of interest to disclose.
Author Contributions
JAA and CSP designed the research study. JO, JM, EB, HK and MI collected the research data. AE and ND analysed the data. CCO, RUN, MI, AE, ND and TAC wrote the paper. All authors have read and approved the final manuscript.
Ethics of Human Subject Participation
This study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving research study participants were approved by the institutional review boards of the Walter Reed Army Institute of Research, Silver Spring, MD, USA; Makerere University School of Public Health, Kampala, Uganda; Kenya Medical Research Institute, Nairobi, Kenya; Tanzania National Institute of Medical Research, Mbeya, Tanzania; and Nigerian Ministry of Defence, Abuja, Nigeria. Written informed consent was obtained from all participants.
Disclaimer
The views expressed are those of the authors and should not be construed to represent the positions of the US Army, the Department of Defense or the Department of Health and Human Services. The investigators have adhered to the policies for protection of human subjects as prescribed in AR-70.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S136898002100361X









