Eighty-four per cent of the 130 countries affected by iodine-deficiency disorders (IDD) have either national legislation or a draft on salt iodization in place(Reference de Benoist, Andersson, Egli, Takkouche and Allen1). Worldwide, the sustainability of IDD control programmes has become a major focus. In several countries in which IDD was eliminated by universal salt iodization (USI), control programmes faltered, IDD recurred(Reference Maberly, Haxton and van der Haar2, Reference Anderson, Takkouche, Egli, Allen and de Benoist3) and new cases of cretinism have also appeared(Reference Markou, Georgopoulos and Makri4, Reference Cetin, Kisioglu, Gursoy, Bilaloglu and Ayata5).
On recommendations of the Central Council of Health in 1984, the Government of India took a policy decision with the goal of ‘universal iodization of salt’. Despite remarkable progress in the control of IDD, it remains a significant public health problem in India(Reference Vir6–Reference Sankar, Moorthy, Pandav, Tiwari and Karmarkar8). It has been estimated that 200 million people in India are exposed to the risk of iodine deficiency and more than 71 million suffer from goitre and other IDD(9). The central notification restricting the sale of non-iodized salt was relaxed in late 2000 by removing the punitive clause. Although the restriction on the sale of non-iodized salt was re-imposed in November 2005, it did not take effect until May 2006(Reference Sankar and Pandav10). Assessing the severity of IDD and monitoring the progress of salt iodization programmes remains the cornerstone of any control strategy.
The National Family Health Survey-III revealed that 51 % of households were using adequately iodized salt (iodine content ≥15 ppm) and approximately 25 % of the population was using non-iodized salt(11). The benefits of USI have often accrued to the urban population due to better coverage and availability of packaged, fine iodized salt(Reference Cetin, Kisioglu, Gursoy, Bilaloglu and Ayata5, 11). This is often the case with big metropolises situated in mild iodine-deficient areas. USI monitoring teams often overlook the poor coverage of the programme in severely affected rural communities. Seventy-seven per cent of households in large cities, 72 % in small cities and towns and only 41 % in rural areas use salt with the recommended level of ≥15 ppm iodine(11). It is reported that though globally iodine nutrition has greatly improved, 20 % to 30 % of pregnancies and thus newborns still do not fully benefit from the use of iodized salt(Reference Maberly, Haxton and van der Haar2).
The principal indicators of an effective programme are the household availability of iodine through edible salt and the median urinary iodine concentration (UIC) in a population, because it is highly sensitive to recent changes in iodine intake(12). Goitre rate that reflects thyroid size is a poor secondary indicator, not only because it indicates just the history of iodine malnutrition but also because of subjective discrepancies that may creep in among the evaluators(12). Most often, however, it is the only indicator that is assessed in endemic areas due to constraints on financial and trained human resources. Moreover, persisting goitre in the first few years of childhood often takes a long time to regress(Reference Zimmerman13, Reference Agheni-Lombardi, Antonangeli and Pinchera14).
The present study was undertaken to objectively assess the status of the IDD control programme in Gonda District, located in the sub-Himalayan region of northern India, which had a reported goitre prevalence of 69 % and high neonatal chemical hypothyroidism in the year 1982(Reference Pandav and Kochupillai15, Reference Kochupillai, Godbole, Pandav, Karmarkar and Ahuja16).
Material and methods
Subjects
We carried out the cross-sectional field survey in Gonda District of Uttar Pradesh province, situated in the sub-Himalayan region approximately 120 km north-east of Lucknow, the provincial capital. The study was carried out from 28 April 2007 to 14 July 2007. The most recent census data in 2001 showed that the district has four sub-districts, 1815 villages and a total population of 2·7 million.
The sample size was calculated to be 2875 subjects on the basis of an assumed goitre prevalence of 50 %, 95 % confidence interval, a design effect of 1·5, a relative precision of 10 % and a non-response rate of 3 %. We randomly selected fifty-three villages and ten houses in each village.
All subjects underwent evaluation of goitre size by palpation. Goitre was graded as per the WHO classification (grade 0, no goitre; grade 1, palpable but not visible; grade 2, clearly visible with the neck in normal position)(Reference de Benoist, Andersson, Egli, Takkouche and Allen1). Before undertaking the goitre prevalence survey, the members of the clinical teams checked their techniques of grading of goitre for inter-observer variation. Three teams of two clinical thyroidologists each carried out the goitre grading. Initially, unacceptably high variations in goitre grading were observed among the three teams deployed for the field survey. To resolve the problem, all clinicians in the three teams examined twenty-five children (aged 6–14 years) in a primary school and recorded the thyroid grading in a blinded fashion. A consensus was arrived at, ensuring uniform goitre grading. In the situation of variation in goitre grade between two members in a given subject, the immediate lower goitre grade was assigned. Since the area that was surveyed had high prevalence of cretinism a few decades ago, the prevalence of deaf-mutism, spastic diplegia, squint and dwarfism in subjects older than 20 years was recorded. To assess salt purchasing habits, its household processing, storage and use, a ‘knowledge, attitude and practice’ (KAP) questionnaire was utilized and administered to the woman of each house. Informed consent was obtained from all study subjects. The project was approved by the Institute’s Ethics Committee.
Biochemical analysis
Spot urine (10–15 ml) was collected from every second subject and preserved in a plastic bottle containing a few drops of toluene. A sample of salt (approx 100 g) was collected in a self-sealing plastic pouch from 70 % of surveyed households. A blood sample (10 ml) was drawn from every tenth individual; serum separated after centrifugation (500 g) at room temperature was preserved on ice. Serum samples were transported to the laboratory on ice and stored at −20°C until the time of assay.
UIC was measured using a kit (Bioclone Australia Pty Limited, Sydney, Australia) based on the Sandell–Koltoff reaction(Reference Ohashi, Yamaki, Pandav, Karmarkar and Irie17). The sensitivity of the method was 10 μg/l, with a range of 10–400 μg/l. The intra- and inter-assay CV were 6·5 % and 10·5 %, respectively, for iodine content of medium range (130–200 μg/l).
Iodine content of salt was estimated by iodometric titration(Reference Tyabji18) and is expressed in parts per million. Serum free thyroxine (FT4) and thyroid-stimulating hormone (TSH) were measured by radioimmunoassay and immunoradiometric assay, respectively, using commercially available kits (Diagnostic Products Corporation, New York, NY, USA).
Statistical analysis
Statistical analysis of data was carried out using the SPSS statistical software package version 14·0 (SPSS Inc., Chicago, IL, USA). Goitre prevalence is presented as percentage of the study population. The iodine status of subjects was determined using the recommended WHO/UNICEF/International Council for the Control of Iodine Deficiency Disorders criteria(Reference de Benoist, Andersson, Egli, Takkouche and Allen1). Salt samples were categorized according to iodine content. Results are expressed as medians for UIC and salt iodine content, means and standard deviations for serum FT4 and TSH. Categorical variables were compared using the χ 2 test. P values less than 0·05 were considered statistically significant.
Results
We surveyed 2860 subjects (1415 males, 49·5 %; 1445 females, 50·5 %) in fifty-three geographically well distributed villages. As shown in Table 1, the goitre prevalence in the study population was 27·7 % (95 % CI, 26·0, 29·3 %). Goitre prevalence was significantly higher (P < 0·001) among females (33·7 %) compared with males (21·5 %). Goitre prevalence in the youngest age group of females (1–17 years) was 29·1 %, increasing to 40·5 % in the oldest age group (>40 years, P < 0·001). There was a significant difference observed in the goitre prevalence among males of different age groups (P = 0·001). Children in age the group of 6–12 years showed a goitre prevalence of 31·0 %. Strikingly, study subjects in 34 % of villages still suffered from severe iodine deficiency (goitre rate of >30 %).
Table 1 Goitre prevalence in the study population: subjects (n 2860) residing in fifty-three villages of Gonda District, northern India
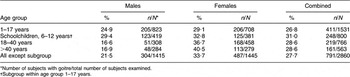
*Number of subjects with goitre/total number of subjects examined.
†Subgroup within age group 1–17 years.
Table 2 summarizes UIC levels. Severe iodine deficiency (UIC < 20 μg/l) was present in 8·0 % of the study population while moderate (20–49 μg/l) and mild deficiency (50–99 μg/l) was present in 12·5 % and 27·3 % of the population, respectively. The median UIC was 100 μg/l (0·79 μmol/l) with range of 5·0 to 560 μg/l.
Table 2 Urinary iodine concentration (UIC) in the study population: subjects (n 1236) residing in fifty-three villages of Gonda District, northern India

UIC in μmol/l = UIC in μg/l × 0·0079.
Table 3 shows median and range of UIC and salt iodine content, as well as mean and standard deviation of circulating FT4 and TSH levels. The median iodine content of household salt of 6·3 ppm indicates an ineffective USI programme in several pockets of the district. Table 4 shows that only 21 % of households were consuming adequately iodized salt (≥15 ppm). Thus 79 % of households were still consuming salt with either inadequate or almost negligible iodine content.
Table 3 Median urinary iodine concentration (UIC) and salt iodine content, and mean free thyroxine (FT4) and thyroid-stimulating hormone (TSH) levels in the study population: subjects residing in fifty-three villages of Gonda District, northern India

*Normal range: FT4 = 0·7–2·0 ng/dl.
†Normal range: TSH = 0·5–5·0 μU/ml.
Table 4 Household (n 338) salt iodine content in fifty-three villages of Gonda District, northern India

The wide range of UIC values prompted us to undertake a detailed village-wise analysis of goitre prevalence, UIC and salt iodine contents. The results indicated a significant presence of goitre and inadequate iodine intake in many villages (Table 5).
Table 5 Village-wise analysis of analysis of goitre prevalence, urinary iodine concentration (UIC) and salt iodine content in fifty-three villages of Gonda District, northern India

The results of the KAP questionnaire revealed the vital information that more than 90 % of subjects purchased locally available salt with average crystal size range of 25–50 mm (1–2 in) from roadside vendors and washed their salt with water to remove dust particles before pulverization and use. Women from the majority of households confirmed that salt forms hard lumps on prolonged storage in powdered form under the humid conditions prevalent in this area for most of the year.
Associated subclinical hypothyroidism (TSH>5·0 μU/ml and FT4 < 0·7 ng/dl) was present in 10 % of the study population (data not shown). Although we recorded eight children with one or more cretinoid features, we did not record any cretin in the age group of >20 years (data not shown).
Discussion
The study shows that the overall goitre prevalence rate in Gonda District has undergone a significant decrease over the past two decades (last survey done in the year 1982)(Reference Pandav and Kochupillai15, Reference Kochupillai, Godbole, Pandav, Karmarkar and Ahuja16). The outcome indicators such as UIC, TSH and FT4 levels point to a satisfactory implementation of the USI programme in this district. However, the moderate degree of goitre prevalence and the low iodine content of salt depict a less than ideal implementation of USI in certain pockets of the district.
The persistence of higher goitre prevalence in females seen in the present study has also been reported in other endemic areas(Reference Sarkar, Mohanty and Basu19–Reference Laway and Zargar21). The documented failure to meet females’ requirements in a crucial period of growth, pregnancy and lactation also holds true for iodine nutrition(11). This may be further accentuated by the higher circulating thiocyanate levels reported earlier in females from the same area(Reference Marwaha, Tandon, Gupta, Karak, Verma and Kochupillai22). The goitre prevalence and UIC data in schoolchildren are in agreement with earlier reports, suggesting only partial success of the programme(Reference Sankar, Moorthy, Pandav, Tiwari and Karmarkar8, Reference Marwah, Tandon, Karak, Gupta, Verma and Kocupillai23, Reference Kapil, Sharma and Singh24).
Village-wise analysis of goitre prevalence, UIC and salt iodine content further indicates that satisfactory iodine nutrition is achieved in only one-third of the villages (Table 5). Strikingly, in 85 % of villages, the goitre prevalence rate is well above the internationally accepted norm. The situation in these groups of villages is more complex and heterogeneous. Half of the villages in this group had a much higher goitre prevalence of 49 % despite median UIC levels being satisfactory. In contrast, subjects from an equal number of villages had a much lower goitre prevalence of 29 %, albeit higher than the internationally acceptable norm, despite UIC levels showing unsatisfactory iodine nutrition. This heterogeneous picture could in part be explained by: (i) a possible contribution of thiocyanate and other goitrogens as reported previously from the same area(Reference Marwaha, Tandon, Gupta, Karak, Verma and Kochupillai22); and (ii) single point analysis may not reflect the USI failure at earlier times(Reference Zimmerman13).
The non-significant correlation between goitre rate and urinary iodine (data not shown) indicates it as a poor indicator of success of USI because it reflects a population’s history of iodine nutrition and not its present status. The results are in agreement with earlier studies showing that goitre rates may not become normal for months or years following implementation of USI(Reference Delange, de Benoist, Pretel and Dunn25, Reference Zimmermann, Moretti, Chaouki and Torresani26). A number of recent epidemiological surveys have shown that, in spite of satisfactory urinary iodine levels, goitre rates are still on the high side(Reference Kapil, Sharma, Singh, Dwivedi and Kaur7, Reference Sankar, Moorthy, Pandav, Tiwari and Karmarkar8, Reference Zimmerman13).
However, poor availability of iodine through edible salt in rural areas (only 21 % of households received adequate iodized salt compared with 41 % according to the NFHS-III survey) remains the main factor accounting for IDD in Gonda District. The use of unpacked crystalline salt and its washing with water before use is a standard culinary practice in the majority of households and is the major cause of persisting severe iodine deficiency in many villages. This salt is available only in bigger crystal size and powdered salt displays a tendency for lump formation due to high Ca content. A large population still suffers from moderate to severe iodine deficiency due to insufficient iodine consumption at household level for the following reasons: (i) the practice of washing salt before use; (ii) inability to afford adequately iodized packaged salt for financial reasons; and (iii) lack of health awareness.
Identification of hot spots of severe persisting IDD calls for constant monitoring and evaluation of IDD control in USI implementation programmes. This also indicates a strong need to formulate alternative strategies in specific areas. Similar hot spots might be present in other underdeveloped countries having USI programmes. Emphasis should be given to pregnant women, lactating mothers and children up to 3 years of age to evaluate the impact of iodization. This becomes particularly important in Indian communities, as the only source of food up to 1 year in infants is mother’s milk. Adequate iodine should be ensured to sustain brain development during this period.
USI seems to have worked to prevent further cretins being born; however, it has not helped to reduce goitre prevalence rates to a desirable level of <5 % among most susceptible populations/villages. The impact of lapses in implementation of USI on the learning potential of children from the most affected villages needs to be assessed.
The first reason for the partial failure of USI seems to be inability to teach the rural masses, to adapt to the situation wherein washing of the iodized crystal salt should be discouraged. The matching health education essential to achieve results is grossly lacking. The USI programme in Uttar Pradesh should have a massive education component built in for it to succeed. Second, it was found that the districts receiving iodized salt by road had a higher number of salt samples with negligible iodine content as compared with the districts receiving iodized salt by rail transport. The inspectors of the salt department vigilantly monitor the quality of salt transported by railways before it is loaded to the train. However, no monitoring of salt quality is done for transportation of salt by road. The issue assumes importance in view of startling figures from the UNICEF regional office in Lucknow that, since the freight hike by railways, approximately 30 % of salt reaching Uttar Pradesh is coming through road transport(Reference Kapil, Tondon, Pathak and Pradhan27). Finally, other state governments have successfully adopted the supply of smaller crystalline salt with less Ca content and better iodine retention(Reference Gerasimov, Pandav, Benmiloud, Delange and Todd28). The Uttar Pradesh Government as well as wholesale traders need to follow this to fulfil their social responsibility towards the rural population.
The results suggest the need for formulating a mission approach for the state of Uttar Pradesh with two clear components: (i) action on the demand side to make communities aware of the need to use adequately iodized salt; and (ii) action on the supply side to ensure the distribution and sale of adequately iodized salt. The state government’s mission should be to seek cooperation of the salt commissioner, traders, transporters, the UNICEF regional office and independent monitoring agencies to formulate strategies for time-bound implementation of USI.
Acknowledgements
Sources of funding: The study was funded by financial support from the Council for Science and Technology, Government of Uttar Pradesh, India to M.M.G. (no. CST/SERPD/D-10) and an intramural grant of the Sanjay Gandhi Postgraduate Institute of Medical Sciences, Lucknow to S.Y. (no. PGI/DIR/RC/588/2005). Conflict of interest declaration: None of the authors had a conflict of interest. Authors’ contributions: S.Y. designed the study, collected and analysed the data, and wrote the manuscript. S.K.G. designed the study, collected and analysed the data. M.M.G. designed the study, collected and analysed the data, and wrote the manuscript. M.J. performed the clinical evaluation of IDD. U.S. conducted the sample size design of the study and analysed the data. P.V.P performed the clinical evaluation of IDD. R.B. performed the clinical evaluation of IDD. A.M. performed the clinical evaluation of IDD. A.S. undertook specimen collection. A.T. performed the clinical evaluation of IDD. M.O. performed the clinical evaluation of IDD. A.C. performed the IDD clinical evaluation. M.S. performed the hormone analysis. N.Y. performed the salt analysis. S.B. performed the urine analysis. M.D. performed the hormone analysis. P.K.A. performed the hormone analysis. Acknowledgments: We gratefully acknowledge officers and staff of Indian Telecom Industry (ITI), Mankapur and the District Hospital Gonda for their help in the field. The cooperation and willingness of the subjects of study population is gratefully acknowledged.







