The metabolic syndrome is a co-occurrence of cardiovascular risk factors that identifies patients at increased risk for CVD and type 2 diabetes. It is defined as a combination of increased waist circumference or central obesity, lipid abnormalities typified by elevated TAG or low levels of HDL-cholesterol (HDL-C), abnormal fasting glucose and high blood pressure( Reference Zimmet, Alberti and Kaufman 1 ).
Among adolescents, metabolic syndrome has a prevalence of 2–9 % depending on the case definition used( Reference Cook, Auinger and Li 2 – Reference de Ferranti, Gauvreau and Ludwig 4 ). Several definitions of metabolic syndrome have been used in the literature. Most case definitions include an increase of central adiposity, namely a waist circumference ≥90th percentile. Dyslipidaemia is part of the definition and can include TAG above 100–110mg/dl (1·13–1·24mmol/l) or HDL-C below 35–40mg/dl (0·9065–1·036mmol/l). Elevated blood pressure is also part of the definition and most commonly defined as a systolic or diastolic blood pressure ≥90th percentile for age, sex and height( Reference Zimmet, Alberti and Kaufman 1 ).
Adolescents with metabolic syndrome have higher markers of inflammation and show signs of vascular remodelling as reflected in measurements of carotid intima-media thickness( Reference Ford, Ajani and Mokdad 5 – Reference Toledo-Corral, Ventura and Hodis 7 ). Because of the propensity for childhood metabolic risk to track into adulthood( Reference Magnussen, Raitakari and Thomson 8 ), there is a great need to identify risk factors for childhood metabolic syndrome which could be targeted through early lifestyle modification.
Dietary items scrutinized for their association with cardiovascular risk in young people have included factors linked to higher risk such as sugary drinks( Reference Kosova, Auinger and Bremer 9 , Reference Ambrosini, Oddy and Huang 10 ) and added sugar( Reference Rodriguez, Madsen and Cotterman 11 ), and those with no effect or lower risk such as fruit juices( Reference Wang, Lloyd and Yang 12 ), fibre( Reference Carlson, Eisenmann and Norman 13 ), vegetables and fruits( Reference Pan and Pratt 6 , Reference Yoo, Nicklas and Baranowski 14 ), and other foods( Reference O’Neil, Nicklas and Zanovec 15 ). While a link between nut consumption and metabolic health is emerging in adults, little is known about this relationship in adolescents. Interest in nuts as a highly nutritive food found footing with the recognition that an eating pattern rich in nuts is associated with improved health outcomes. A 6-year cohort study of Seventh Day Adventists in California found that higher levels of nut intake were associated with reduced risk of CHD( Reference Fraser, Sabate and Beeson 16 ). These findings were subsequently corroborated in different populations of women and men( Reference Hu, Stampfer and Manson 17 – Reference Albert, Gaziano and Willett 19 ). Clinical trials of a Mediterranean diet, of which nuts constitute an important part, also indicated a diet rich in their intake has a potential benefit for the prevention of CVD in tertiary and primary prevention( Reference de Lorgeril, Salen and Martin 20 , Reference Estruch, Ros and Salas-Salvado 21 ). Candidate nutrients in nuts which are hypothesized to provide a mechanistic link with vascular health include fibre, monounsaturated fats, protein, Ca, Mg, Cu, phenols and phytosterols( Reference Ros 22 ). Despite the accumulating evidence supporting a role for nuts in a healthy diet, little is known about the association between nut intake and cardiovascular risk factors in adolescents. Elucidating this relationship with greater clarity could suggest future avenues for clinical trial design and healthy diet recommendations. The main objective of the present study was to determine whether nut consumption in US adolescents is associated with prevalence of metabolic syndrome.
Participants and methods
We analysed data from the National Health and Nutrition Examination Survey (NHANES), a study of the health status of children and adults in the USA. The sampling and data collection methods used in NHANES are available online( 23 ). A complex, multistage sampling procedure was used. The USA was divided into primary sampling units which consisted of counties or clusters of counties. Each sampling unit was divided into segments of approximately equal population. The probability of selection of a sampling unit and segment was based on area. Within a segment, households and individuals within households were sampled at a rate to yield a national sample that achieved the desired number of sampled participants for each demographic domain including race/ethnicity, age, sex and income. Oversampling of under-represented minority groups was performed to increase the sampling of groups of particular public health interest, including African Americans, Mexican Americans, low-income White Americans, adolescents, and people over 60 years of age( Reference Johnson, Dohrmann and Burt 24 ). NHANES was approved by the National Center for Health Statistics Institutional Review Board and all participants provided informed consent or assent( 25 ).
We used data from five 2-year cycles from 2003–2004 through 2011–2012. Adolescents were included if they were 12–19 years of age, and excluded if they were pregnant, did not complete the diet survey, did not fast for at least 8 h or had insufficient data to determine their metabolic syndrome status. Data were extracted from anthropometric measurements, fasting blood tests, 24 h diet recall surveys and responses pertaining to each participating adolescent’s sociodemographic background. Per NHANES protocol, adolescents were weighed on a digital scale while wearing underwear, a paper gown and foam slippers. Height was measured using a fixed stadiometer with a movable headboard. BMI was defined as weight in kilograms divided by the square of height in metres, and for analysis was converted to a Z-score specific to each adolescent’s age and sex using standards from the Centers for Disease Control and Prevention( Reference Kuczmarski, Ogden and Guo 26 ). Waist circumference was measured at the uppermost border of the lateral ilium( 27 ). Blood pressure was obtained after the adolescent was seated for 5 min, using a mercury sphygmomanometer and a digital blood pressure monitor. The blood pressure used for analysis was the average of the last two measurements, or a single measurement if only one was obtained. NHANES laboratories complied with the Clinical Laboratory Improvement Act, and quality control was maintained by random repeat testing performed in a reference laboratory of 2 % of samples as well as split tube parallel testing in practice sessions( 28 ). The CV, an indicator of precision, for this repeat testing of within-assay and between-assay duplicates had to be within 5 % or the specimen was re-analysed( 29 ). Glucose was determined by the glucose oxidase method. HDL-C and TAG were determined by an enzyme-linked colorimetric assay. Assays were performed on a Roche laboratory platform at designated laboratories.
Twenty-four-hour dietary recall data were collected by trained interviewers who first performed an in-person private interview. Children aged 12 years or older provided their own diet recall. To better capture ‘usual’ dietary intake, a second interview was conducted by telephone 3–10d later to help reduce intrasubject variability( Reference Ahluwalia, Dwyer and Terry 30 ). Interviewers were trained in the use of a multiple-pass method to enhance consistency and completeness of data capture. The dietary recall method used by NHANES has been validated( Reference Ahluwalia, Dwyer and Terry 30 ). Nut intake data were reviewed for plausibility. Nut intake was defined as the average of the nut intake on the two diet recalls.
We defined metabolic syndrome using the paediatric adaptation of the Adult Treatment Panel III guidelines from the National Cholesterol Education Program( Reference Li, Ford and McBride 31 , Reference Cook, Weitzman and Auinger 32 ). Specifically, metabolic syndrome occurred when three or more of the following were present: waist circumference ≥90th percentile for race, age and sex( Reference Fernandez, Redden and Pietrobelli 33 ), HDL-C <40 mg/dl (1·036mmol/l), TAG ≥110mg/dl (1·24mmol/l), fasting glucose ≥100mg/dl (5·5mmol/l) and/or elevated blood pressure defined as systolic or diastolic blood pressure ≥90th percentile normalized for age, height and sex( 34 ).
Because of their nutritional similarity and phylogenetic overlap, both nuts (tree nuts and peanuts) and seeds were included in the daily intake total. For clarity they are referred to simply as ‘nuts’ unless otherwise specified. Nut and seed butters were included as part of the total daily consumption. Nuts and seeds found in candy bars or other sweets were not included. For analytic purposes, nut exposure was defined as an intake of ≥5g/d, which was the mean intake.
Statistical analysis
Analyses were performed according to published NHANES analytic guidelines using the SURVEY procedures in the statistical software package SAS version 9.4. To adjust the weights of the analytic sample to reflect its definition as the intersection of two sub-samples, a propensity score was calculated for inclusion in the analytic sample predicted by demographic variables; the inverse of the propensity score was multiplied by the fasting laboratory sub-sample weight to produce a final analytic sample weight( Reference Tilert, Dillon and Paulose-Ram 35 ). Descriptive statistics, including means, standard errors and percentages, were computed with analytic sample weights. Weighted logistic regression was used to assess the association of metabolic syndrome with nut consumption ≥5g/d after adjustment for demographic, clinical and dietary variables. Nutrients and macronutrients in the model were treated as continuous variables. Demographic, clinical and dietary variables were selected for inclusion in the adjusted models model based on hypothesized clinical relevance and tested for multicollinearity prior to inclusion. Interaction effects between nut consumption and demographic variables were entered into the model and removed if not statistically significant with a significance criterion of 0·05. All main effects were included in the final models. Unadjusted and adjusted odds ratios with 95 % confidence intervals were presented. All tests were two-tailed and performed at a significance level of 0·05.
Results
Study population
There were 50 912 individuals in the NHANES database from years 2003–2012, of whom 8360 were non-pregnant and aged 12–19 years. Following exclusions for not fasting, incomplete diet information or indeterminate metabolic syndrome status, the remaining 2805 adolescents constituted the analytic sample. We compared the analytic sample with the excluded adolescents and found no significant differences in their age, sex, BMI or metabolic syndrome prevalence (data not shown). The excluded sample had a mean nut intake of 4 g/d compared with 5 g/d in the analytic sample (P=0·021).
Factors associated with nut consumption ≥5g/d
We then compared anthropometric, biochemical and demographic traits in adolescents consuming nuts ≥5g/d v. those eating <5g/d. The prevalence of metabolic syndrome was higher in those eating <5g/d (8 v. 2 %, P <0·001). Adolescents eating nuts ≥5g/d were more likely non-Hispanic White, had higher income:poverty ratio, and had greater intakes of sugar, energy, fibre, total fat, SFA, MUFA and PUFA (Table 1).
Table 1 Data summaries, overall and by nut intake, among US adolescents aged 12–19 years (n 2805), National Health and Nutrition Examination Survey (NHANES) 2003–2012
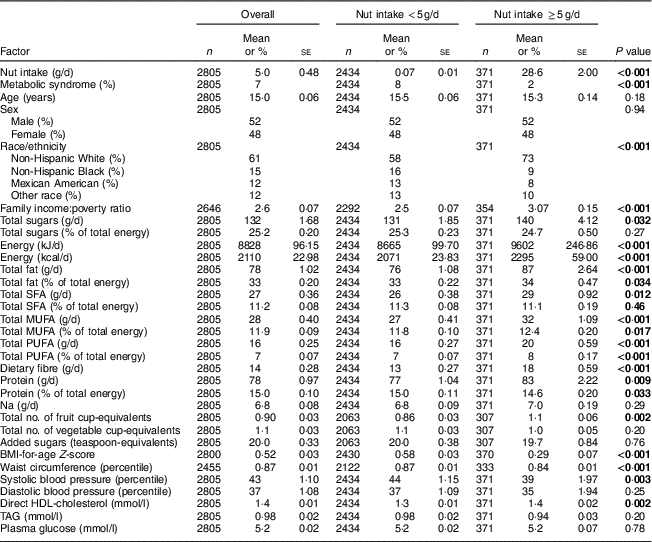
Descriptive statistics were computed with analytic sample weights (weights = fasting weights × 1/propensity score).
P values were obtained from weighted univariable logistic regression models; significant P values are indicated in bold font.
Logistic regression
Using logistic regression, the odds for metabolic syndrome was determined for sociodemographic and dietary factors. Of these, nut intake ≥5g/d, sex (female v. male), race/ethnicity (Black v. White) and family income:poverty ratio were significantly associated with metabolic syndrome risk (Table 2). Interaction terms for race and nut intake were not significant (data not shown).
Table 2 Logistic models of factors associated with metabolic syndrome among US adolescents aged 12–19 years (n 2805), National Health and Nutrition Examination Survey (NHANES) 2003–2012
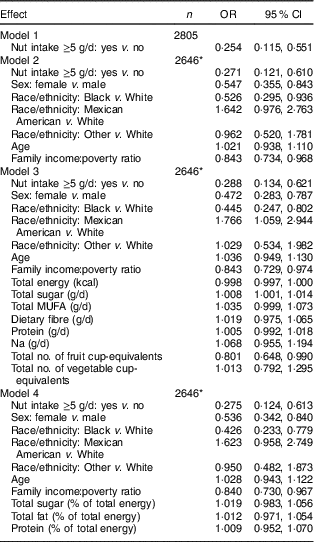
Models were computed with analytic sample weights (weights = fasting weights × 1/propensity score).
Model 1: unadjusted OR.
Model 2: adjusted for age, sex, race/ethnicity and income:poverty ratio.
Model 3: includes Model 2 plus intakes of sugar, Na, PUFA, fibre, fruits and vegetables, protein and total energy.
Model 4: includes Model 2 plus sugar, fat and protein as percentages of total energy.
*Missing data on income:poverty ratio.
Metabolic syndrome and nut intake
Adolescents who ate nuts ≥5g/d had lower odds for metabolic syndrome compared with those eating lesser amounts (unadjusted OR=0·25; 95 %CI 0·11, 0·55; P <0·001). Because of the known relationship between metabolic syndrome and sociodemographic and dietary factors, we generated additional models in which we adjusted for pertinent covariates. In Model 2, we adjusted for age, sex, race/ethnicity and family income:poverty ratio. In Model 3, we added adjustments for dietary factors including intakes of sugar, fat, SFA, fibre, fruits and vegetables. Model 4 included factors from Model 2, plus intakes of sugar, fat and protein as percentages of total energy. The models are shown in Table 2.
Because physical activity could confound the association between nut intake and metabolic syndrome, we repeated the analysis while limiting the sample to the NHANES cycle years in which physical activity data were uniformly and quantitatively collected (2007–2012). We designated as physically active those adolescents who reported performing work or recreational physical activity of moderate or vigorous intensity for ≥150min/week. Inclusion of this measure of physical activity into the adjusted model continued to show a significantly lower odds of metabolic syndrome associated with nut intake ≥5g/d (Table 3). Because BMI Z-score was highly correlated with metabolic syndrome, as well as nut intake, we tested for interaction by adding an interaction term to the unadjusted and adjusted regression models. No evidence for interaction was found (data not shown).
Table 3 Crude and adjusted odds ratios for metabolic syndrome among adolescents consuming nuts ≥5 g/d compared with non-consumers in those with available physical activity data: US adolescents aged 12–19 years (n 2805), National Health and Nutrition Examination Survey (NHANES) 2003–2012
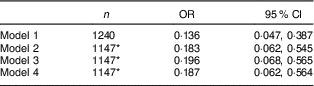
Models were computed with analytic sample weights (weights=fasting weights×1/propensity score).
Model 1: unadjusted OR.
Model 2: adjusted for age, sex, race/ethnicity, income:poverty ratio and physical activity≥150 min/week.
Model 3: includes Model 2 plus intakes of sugar, Na, PUFA, fibre, fruits and vegetables, protein and total energy, and physical activity ≥150 min/week.
Model 4: includes Model 2 plus sugar, fat and protein as percentages of total energy, and physical activity ≥150 min/week.
*Missing data on income:poverty ratio.
Nut intake in US adolescents
Because nut consumption was associated with metabolic syndrome status, we examined nut consumption patterns in US adolescents. Mean daily nut intake was 5 (se 0·48) g/d. The median and 75th percentile of nut intake was zero. Eighty per cent of all participants ate no nuts at all. Among those eating any nuts, median intake was 8·0g/d. Overall, these indicated a highly skewed distribution of nut intake.
Discussion
In US adolescents, we found that nut consumption ≥5g/d is associated with lower odds for metabolic syndrome. This relationship remained significant after controlling for age, sex, race/ethnicity, household income, and several dietary characteristics including sugar, total energy, fruit and vegetable intakes. We also found that nut intake is low among adolescents and a majority reported consuming no nuts at all during the interview days. We found that all races/ethnicities and both sexes ate few nuts. Nut intake was associated with lower odds for metabolic syndrome in Blacks v. Whites, and higher odds for Mexican Americans v. Whites; however, the number of individuals in each subgroup was small, which should temper overinterpretation of these differences. Females and those with higher income:poverty ratio also had lower odds for metabolic syndrome. Overall, our finding that a small amount of daily nut intake is associated with lower odds of metabolic syndrome in adolescents was still significant after controlling for pertinent dietary, lifestyle and sociodemographic characteristics.
The present study has several limitations. First, because it is a cross-sectional study, the findings are correlative and from them one cannot infer causation. Second, although we adjusted for important covariates, residual confounding cannot be excluded. However, we did attempt to control for dietary practices that could covary with nut intake, for example as part of a healthy eating pattern and lifestyle. To this end we included intakes of fruits, vegetables, fibre, fat, saturated fat, total energy and physical activity. Third, in the current analysis we were not able to ascertain if there were specific types of nuts or seeds that were more strongly associated with metabolic well-being. However, the published literature suggests that health benefits are observed with a range of types including peanuts, walnuts, almonds, pistachios and others. The use of 24h diet recalls has been criticized as not capturing usual intake compared with other methods, such as FFQ. However, it also has advantages over competing methods in that it is open-ended, tends to be more quantitative, and requires less recall of foods eaten weeks and months before the assessment. Moreover, using two 24h diet recalls as we did captures usual intake more accurately than a single survey and reduces intrasubject variability( Reference Shim, Oh and Kim 36 ).
In adults, nut consumption has been linked with several positive health outcomes( Reference Hu, Stampfer and Manson 17 – Reference Albert, Gaziano and Willett 19 , Reference Jaceldo-Siegl, Haddad and Oda 37 , Reference Brown, Tey and Gray 38 ). In large cohort studies, greater nut consumption is associated with a lower risk for sudden cardiac death( Reference Albert, Gaziano and Willett 19 ), myocardial infarction( Reference Hu, Stampfer and Manson 17 ), metabolic syndrome( Reference Jaceldo-Siegl, Haddad and Oda 37 ) and diabetes( Reference Jiang, Manson and Stampfer 39 , Reference Kochar, Gaziano and Djousse 40 ). Nuts are a major component of the Mediterranean diet, which has been shown to result in a 30 % reduction in combined cardiovascular morbidity in a primary prevention trial( Reference Estruch, Ros and Salas-Salvado 21 ).
In contrast to the literature available in adults, the relationship between nut consumption and cardiometabolic risk factors in children and adolescents has received scant attention in the literature, and the published reports have been limited with respect to age group, geographic location or sex( Reference Moreno, Johnston and El-Mubasher 41 , Reference Maranhao, Kraemer-Aguiar and de Oliveira 42 ). O’Neil et al. found that nut intake greater than 7·1g/d correlated with higher daily intakes of energy, MUFA, PUFA, fruits and vegetables. Among adolescents in their study, nut intake above 7·1 g/d was associated with lower body weight and a lower prevalence of overweight or obese status. The study was limited however by use of only a single day’s dietary recall, finding only a small number of adolescents with a nut intake above 7·1g/d, not appropriately reporting blood pressure as a percentage based on height, weight and sex, and lack of biochemical data in any non-adults( Reference O’Neil, Keast and Nicklas 43 ). Therefore, our study goes beyond previous findings, fills a significant knowledge gap, and is the first to examine the relationship between nut consumption and metabolic syndrome in adolescents.
Nielsen et al. have reported that 40 % of nuts consumed by children aged 2–19 years is as isolated nuts, whereas about 60 % comes in a combination such as in baked grain products like cakes or cookies, in candy, or mixed in with other foods such as ice cream, cheese or other food items( Reference Nielsen, Herrick and Akinbami 44 ). We omitted nuts consumed in these mixed forms since these foods tend to be high in sugar, fat and energy. Concern about allergies could be a factor limiting nut intake in children; however, recent advances may lessen fears about childhood nut allergies in the future.
Mechanistically, nutrients within nuts appear to modify circulating cardiometabolic risk factors. Human trials have shown that nut consumption is linked to reduced fasting insulin( Reference Tapsell, Batterham and Teuss 45 ) and glucose( Reference Parham, Heidari and Khorramirad 46 ), and an improved lipid profile( Reference Mercanligil, Arslan and Alasalvar 47 ). These effects may be mediated by their high concentrations of polyphenols and mono- and polyunsaturated fatty acids( Reference Dominguez-Avila, Alvarez-Parrilla and Lopez-Diaz 48 , Reference Jones, Jew and AbuMweis 49 ). Pistachios, for example, have been found to enhance endothelial function( Reference Kendall, West and Augustin 50 ), lower inflammation( Reference Gulati, Misra and Pandey 51 ), reduce atherogenic LDL-cholesterol( Reference Holligan, West and Gebauer 52 ), and improve blood pressure and measures of heart rate variability( Reference Sauder, McCrea and Ulbrecht 53 ). Walnuts exhibit similar attributes, improving lipids( Reference Rajaram, Haddad and Mejia 54 ) and enhancing endothelial function as assessed by flow mediated dilation( Reference Katz, Davidhi and Ma 55 ). The vascular benefits in particular could be mediated by their constituent polyphenols, the urinary excretion of which has been shown to correlate with plasma nitric oxide( Reference Medina-Remon, Tresserra-Rimbau and Pons 56 ). Nuts are also relatively high in fibre, which may improve glucose homeostasis and satiety( Reference Tan and Mattes 57 ). The nut consumers in our study had significantly higher intakes of MUFA, PUFA and fibre, in comparison to non-consumers.
Nut consumers had a significantly higher daily energy intake compared with non-eaters yet had a significantly lower BMI Z-score, a finding not previously reported in adolescents( Reference O’Neil, Keast and Nicklas 43 ). As metabolic syndrome prevalence increases with BMI, it would be reasonable to surmise that BMI may mediate favourable health outcomes associated with nut consumption. Our analysis did not support this however, since a test for interaction between BMI Z-score and nut intake v. the odds of metabolic syndrome was non-significant in all regression models. Moreover, after controlling for potential lifestyle confounders including dietary sugar, fruit and vegetable intakes, total energy and physical activity, we found that the adjusted odds for metabolic syndrome remained significant. Potential mechanisms for nut eaters to have a lower BMI include increased energy expenditure and incomplete energy absorption( Reference Sabaté 58 ); however, these hypotheses require further study.
In summary, greater nut consumption among adolescents is associated with lower odds for metabolic syndrome and an improved cardiovascular risk factor profile. Despite the growing literature supporting the health benefits of nuts, most adolescents consume no or very few nuts each day. Prospective research is needed to determine whether promotion of nut consumption among adolescents would result in improved health outcomes.
Acknowledgements
Acknowledgements: The authors acknowledge Anne Tang for support with data acquisition and analysis. Financial support: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors. Conflicts of interest: None. Authorship: R.J.K. developed the hypothesis and research plan, contributed to data acquisition and analysis, and had primary responsibility for writing and the final content. S.W., L.W. and D.L. retrieved the data and performed statistical analysis. All authors contributed to writing and approved the final manuscript. Ethics of human subject participation: This study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects were approved by the Institutional Review Board of the National Center for Health Statistics. Written informed consent or assent was obtained from all subjects.





