Iodine is a vital component of the thyroid hormones required for normal growth, development and tissue metabolism. Iodine deficiency can cause widespread detrimental effects including spontaneous abortion, stillbirth, congenital anomaly and prenatal mortality in fetuses and impaired mental function, decreased educability, reduced work productivity, goitre and hypothyroidism in adults( Reference Dunn 1 – 4 ). Based on the WHO report, iodine deficiency still remains a major public health concern worldwide despite global progress in its elimination( Reference De Benoist, McLean and Andersson 5 ).
According to the WHO guidelines, an efficient way to estimate household iodine nutrition status is frequently assessment of urinary iodine concentration (UIC) in schoolchildren( 4 ). However, some studies have revealed that UIC in schoolchildren is not an adequate criterion to estimate whole population iodine status( Reference Nazeri, Mirmiran and Mehrabi 6 – Reference Soriguer, Garcia-Fuentes and Gutierrez-Repiso 8 ). The Islamic Republic of Iran is known as an area of iodine deficiency( Reference Emami, Shahbazi and Sabzevari 9 , Reference Azizi, Kimiagar and Nafarabadi 10 ). Production and nationwide consumption of iodized salt containing 40 ppm iodine began in 1990 and became mandatory for household consumption by 1994. Since then, national surveys conducted every 5 years have shown sustainable elimination of iodine-deficiency disorders in schoolchildren. Although Iran was declared to be free of iodine deficiency in the year 2000( Reference Azizi, Sheikholeslam and Hedayati 11 ), results of the last national survey and a recent study conducted in Tehranian adults were indicative of decreases in median UIC to <100 µg/l( Reference Nazeri, Mirmiran and Mehrabi 6 , Reference Delshad, Amouzegar and Mirmiran 12 ). Moreover, the public health education campaign regarding iodized salt consumption was conducted through the earlier years of implementation of the universal salt iodization programme from 1989 to 1994( Reference Sheikholeslam 13 ). After that there has been little or no public knowledge dissemination regarding iodized salt consumption via various mass media, measures that are essential to raise levels of public awareness and need to be prioritized in health policies.
On the other hand, it is well known that parents – especially mothers’ knowledge, attitude and behaviour – have a great influence on the health conditions, quality of life and nutritional status of other members of the family, mainly their children( Reference Vereecken and Maes 14 – Reference Tahirovic and Toromanovic 16 ). Close correlations have been observed between parents and their children in dietary patterns and nutrient intakes( Reference Ovaskainen, Nevalainen and Uusitalo 17 – Reference Vauthier, Lluch and Lecomte 19 ). However, only a few studies specifically address the effects of mothers’ knowledge, attitude and behaviour on the health and nutrition status of other family members, i.e. adult members. With respect to family structure in Iran, where family members live very close to each other and are very dependent upon the core, and also the ultimate aim of the salt iodization programme in Iran which is the provision of adequate dietary iodine to all members of families not only schoolchildren, the present study was designed to explore, for the first time, the influence of mothers’ knowledge, attitude and behaviour on the iodine nutrition status of adult family members and the relationship between iodine nutrition status of mothers and adult family members.
Methods
Participant sampling
The current cross-sectional study was conducted on the sample of a previous study of Tehranian adults in 2009, further details of which have been published elsewhere( Reference Nazeri, Mirmiran and Mehrabi 6 ). For selection of families, the health-care centres of eight districts from four distinct areas (i.e. the south, west, east and north) of Tehran were randomly selected. In each family, two adult members were selected for providing a 24 h urine sample; these included the mother who took responsibility for food preparation and one other adult member who was willing to participate in our study, was aged ≥19 years, came from the same family (couple/husband, or son or daughter, or others who lived in the joint family system) and who ate at least one meal with family members. After excluding incomplete urine samples, and pregnant and lactating mothers, 383 families including 639 adults (338 mothers and 301 one other adult members) remained for the analysis. Families that had only one adult member or provided only one 24 h urine sample were excluded. Hence, of 383 households, 290 families including 580 adults (290 mothers and 290 one other adult members) met the above-mentioned criteria. Personal information, medical history of thyroid diseases and hypertension, usage of medications and iodine-containing supplements were self-reported and documented using an interviewer-administered questionnaire. Households’ per capita income in their region of residence was chosen as a proxy for socio-economic position (based on report of the Statistical Center of Iran), as evidence suggests that the three most common indicators of socio-economic status (SES) are education, income and occupation. Informed written consent was obtained from all participants and the Ethical Committee of the Research Institute for Endocrine Sciences, Shahid Beheshti University of Medical Sciences, Tehran, approved the study.
Salt sample collection
During the first home visit, a sample of two tablespoons of salt was collected from each of the 290 families. The samples were kept in lightproof, closed plastic cans and labelled with the code for each family.
Urine collection
At the first home visit, written instructions and 2·5 litre labelled plastic containers were given to the mother and one other adult member of each family, who were asked to collect all urine passed during a 24 h period, beginning on Friday (a weekend day in Iran), starting from the second morning urine and ending with the first urine passed the following morning.
At the second visit, on the following Saturday, all samples were collected and sent to the iodine laboratory of the Endocrine Research Center, Research Institute for Endocrine Sciences, Shahid Beheshti University of Medical Sciences, Tehran, the reference laboratory of the Eastern Mediterranean region; total volume was measured and urine was transferred into screw-topped labelled plastic vials. The aliquots were kept frozen until iodine, Na and creatinine concentrations were measured.
Salt and iodine intake estimation
Salt intake was estimated by 24 h urinary Na excretion (1 g salt=17·1 mmol Na)( 20 – Reference Stamler 22 ). In contrast to European countries where 80 % of salt consumed is hidden( 23 ), in Iran a significant portion of salt intake comes from iodized salt, the main dietary iodine source which is used during cooking and as table salt. Hence iodine intake was estimated based on the amount of salt intake and the iodine content of salt.
Mothers’ knowledge, attitude and behaviour assessment
To test knowledge, attitude and practices of mothers, an appropriate questionnaire was developed considering the following aspects of iodine nutrition status: the importance of iodine as an essential nutrient; the consequences of iodine-deficiency disorders; and purchase, consumption and preservation conditions of iodized salt. The general content and specific items of the questionnaire were initially derived from an intensive review of literature available in national and international sources by the main researcher. Irrelevant and unsuitable items were eliminated or changed based on experts’ advice. This resulted in a questionnaire comprising twenty-four items that encompassed: (i) a Knowledge section with ten items; (ii) an Attitude section with eight items; and (iii) a Practice section with six items.
Validity of the questionnaire was confirmed by face and content validity. Face validity means that the instrument (i.e. questionnaire) covers the attribute it is intended to measure, on the face of it. Therefore, investigators seek experts or lay people to review the instrument for grammar, syntax, organization, appropriateness and confirmation that it appears to flow logically. Content validity indicates whether the items in the questionnaire complete the range of the attribute under study. After a researcher defines the construct of interest and its dimensions by searching the literature, seeking expert opinions, performing population sampling or through qualitative research, then a panel of content experts is asked to review the potential items and validate that they are appropriate indicators of the construct( Reference DeVon, Block and Moyle-Wright 24 ). To ensure face validity, ten mothers completed the questionnaire, which assessed their knowledge, attitude and behaviour on the importance of iodine consumption in their daily lives. Also, they were asked to review the appropriateness and clarity of each item on the questionnaire. To assess content validity, an expert panel including ten specialists in nutrition, endocrinology, health education and sociology reviewed the potential items of the questionnaire. They were asked to comment on individual items in relation to their relevancy, clarity and necessity; items were modified slightly based on expert reviews. In addition, Cronbach’s alpha was determined for internal consistency of the questionnaire (Cronbach’s α=0·83).
At the first home visit, mothers’ knowledge, attitude and practice regarding iodine nutrition and iodized salt were assessed using a questionnaire administered by a skilled interviewer in a face-to-face manner. Knowledge questions were focused on the preservation conditions, time of salt addition during the cooking process and signs of iodine-deficiency disorders. Responses were ‘yes’, ‘no’ and ‘do not know’ options or three choices including ‘true’, ‘false’ and ‘do not know’. The scoring system for each ‘yes’ and ‘true’ answer was 3 points, ‘no’ and ‘false’ answer scored 1 point, and ‘do not know’ was 2 points. The concept of the attitude questions was based on the importance of consumption of iodine and iodized salt. The answers were ‘strongly disagree’, ‘disagree’, ‘have no idea’, ‘agree’ and ‘strongly agree’, which were scored based on the Likert criteria from 1 point, as the undesirable, to 5 points, as the desirable attitude. Questions related to behaviour included the use of iodized salt, preservation conditions of iodized salt (e.g. exposure to light, humidity and storage in uncovered containers), time of salt addition during the cooking process and considerations during salt purchase. Each question was scored 1 point. To assess the level of knowledge, attitude and practice regarding iodine nutrition and iodized salt, the scores in each section were calculated. Total score was 26 (range: 9–26) points for knowledge, 40 (range: 24–40) points for attitude, 6 (range: 0–6) points for practice and 68 (range: 38–68) points for the overall knowledge, attitude and practice questionnaire. Total score in each section was then categorized into three levels according to score tertiles. In each section of knowledge, attitude and practice, mothers who had scores below the lower, in the middle and above the higher tertiles were defined as having low, intermediate and high scores, respectively.
Laboratory measurements
Iodine concentration of salt samples was determined using the iodometric titration method with 1 ppm sensitivity and CV of 1 %( Reference DeMayer, Lowenstein and Thilly 25 ), and the results are expressed in parts per million. The iodine concentration in urine samples was analysed using the Sandell–Kolthoff (acid digestion) reaction( Reference Sandell and Kolthoff 26 ) and results are expressed as micrograms of iodine per litre of urine. The intra- and inter-assay CV were 9·6 % and 10·4 %, respectively and the sensitivity was 2 µg/l. Completion of 24 h urine sample collection was confirmed with creatinine concentration. Urine samples with creatinine levels below 500 mg/d were considered incomplete. Urinary creatinine was measured by the kinetic Jaffé method (Creatinine Colorimetric Kit; Pars Azmoun, Tehran, Iran). The assays were run using an autoanalyser (Vitalab Selectra-2; Vital Scientific NV, Dieren, the Netherlands). The inter-assay sensitivity and CV were 0·2 mg/dl and 2·1 %, respectively. Na excretion in 24 h urine collections was analysed by flame emission photometry (Corning 480 Flame Photometer; Dow Corning, Corning, NY, USA).
Statistical analyses
Means and standard deviations, medians and interquartile ranges (IQR), and numbers and proportions (%) were computed according to continuous numerical and qualitative ordinal variables. The Wilcoxon and Kruskal–Wallis tests were used to compare non-parametric variables between mothers and adult family members, and among different age groups, education levels and SES categories. To identify influential factor(s) associated with UIC<100 µg/l in adult family members, multiple logistic regression with the backward stepwise method was employed. The χ 2 test at significance level P<0·25 was used to screen potential variable(s) to be included in the model. Statistical analyses were done using the statistical software package SPSS for Windows version 16·0, with P<0·05 being considered as significant.
Results
General characteristics
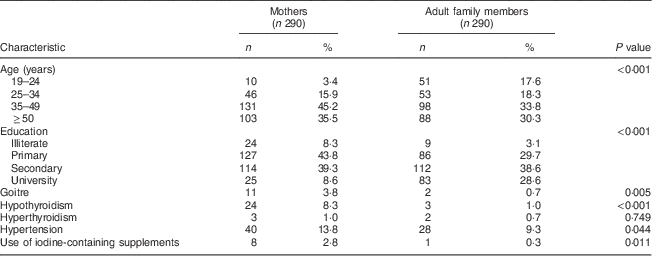
Two hundred and ninety mothers (mean age 44·3 (sd 11·2) years) and adult family members (mean age 41·5 (sd 15·0) years) completed the study. Adult family members included 203 (70 %) husbands (mean age 47·7 (sd 12·3) years), fifty-two (18 %) daughters (mean age 25·4 (sd 6·1) years), twenty-nine (10 %) sons (mean age 24·3 (sd 4·4) years) and six (2 %) other individuals living with the family (mean age 53·1 (sd 18·2) years). The adults who were excluded did not differ significantly in basic characteristics from those included (data not shown). General characteristics of the participants in the two groups, mothers and adult family members, are shown in Table 1. Significant differences in age, education, history of goitre, hypothyroidism, hyperthyroidism, hypertension and use of iodine-containing supplements were observed between the two groups.
Table 1 Basic characteristics (age, education level, medical history and use of iodine-containing supplements) of participants in the two groups, mothers and adult family members, Tehran, Iran
Urinary iodine concentration
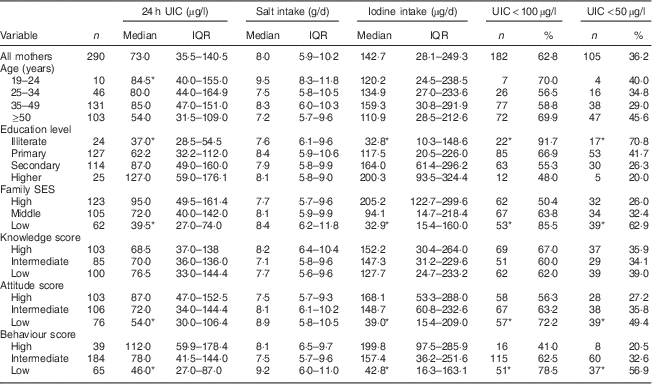
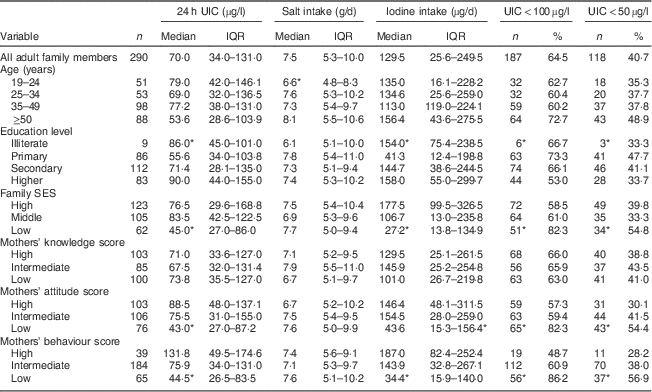
Median 24 h UIC in mothers and adult family members was 73 (IQR 36–141) µg/l and 70 (IQR 34–131) µg/l, respectively (P>0·05). Frequency distribution of UIC<100 µg/l and UIC<50 µg/l was 63 % and 36 % in mothers and 65 % and 41 % in adult family members, respectively. Values of median 24 h UIC in mothers with limited education and the oldest age group were lower than those in mothers with higher education levels and the youngest age group, respectively (P<0·05); whereas no significant difference was observed for adult family members. Median 24 h UIC was lower in participants with low SES than in those with high SES: 40 (IQR 27–74) µg/l v. 95 (IQR 50–161) µg/l in mothers (P<0·05) and 45 (IQR 27–86) µg/l v. 77 (IQR 30–169) µg/l in adults (P<0·05), respectively (Tables 2 and 3).
Table 2 Median and interquartile range of 24 h urinary iodine concentration (UIC), salt intake and iodine intake, and the number percentage of mothers with UIC<100 µg/l and UIC<50 µg/l, according to age group, education level, family socio-economic status (SES), and knowledge, attitude and behaviour categories, Tehran, Iran
*P<0·05.
Table 3 Median and interquartile range of 24 h urinary iodine concentration (UIC), salt intake and iodine intake, and the number and percentage of adult family members with UIC<100 µg/l and UIC<50 µg/l, according to age group, education level, family socio-economic status (SES), and mothers’ knowledge, attitude and behaviour categories, Tehran, Iran
*P<0·05.
Dietary iodine intake
Median dietary iodine intake in mothers and adult family members was 143 (IQR 28–249) µg/d and 130 (IQR 26–250) µg/d, respectively (P>0·05). Daily iodine intakes >150 µg (the RDA) were found in 47·0 % and 44·9 % of mothers and adult family members, respectively. We found significant differences in dietary iodine intake among mothers with different education levels and in different SES groups (P=0·001; Table 2).
Iodine content of household salt
Median iodine content of salt was 3 (IQR 2–20) ppm, 17 (IQR 2–30) ppm and 29 (IQR 19–36) ppm in low, middle and high SES, respectively (P<0·001). In mothers with the lowest education levels, the median iodine content of salt was significantly lower than in those with the highest education levels (6 ppm v. 32 ppm).
Dietary salt intake
Median daily salt intake in mothers and adult family members was 8·0 (IQR 5·9–10·2) g and 7·5 (IQR 5·3–10·0) g, respectively (P>0·05). The proportion of mothers and adult family members with daily salt intake <5 g (recent WHO recommendation) was 16 % and 22 %, respectively. Median salt intake among mothers in the youngest v. the oldest age group was 9·5 g/d and 7·2 g/d, respectively, whereas corresponding values were 6·6 g/d and 8·1 g/d in adult family members. No significant differences in median salt intake according to education level and SES were found for both mothers and adult family members.
Mothers’ knowledge, attitude and behaviour scores
Scores obtained by mothers for knowledge, attitude and behaviour were as follows: for knowledge 34·5 %, 29·3 % and 35·5 % of mothers had low, intermediate and high scores, respectively; corresponding values for attitude were 27·2 %, 36·6 % and 35·5 %; and for behaviour were 22·6 %, 63·9 % and 13·5 %. There were significant differences in attitude and behaviour among different SES groups and education levels (P<0·001). In adult family members whose mothers had higher attitude and behaviour scores, the iodine content of salt, median 24 h UIC and dietary iodine intake were significantly higher than in adult family members whose mothers had lower scores for attitude and behaviour (P<0·05). Significant correlations were observed between the median 24 h UIC, dietary iodine intake and salt iodine content of adult family members and mothers’ attitude (r=0·17, P=0·003; r=0·20, P=0·001; r=0·22, P<0·001, respectively) and behaviour (r=0·15, P=0·008; r=0·24, P<0·001; r=0·22, P<0·001, respectively). There were no significant associations between mothers’ knowledge, attitude and behaviour and adult family members’ dietary salt intake.
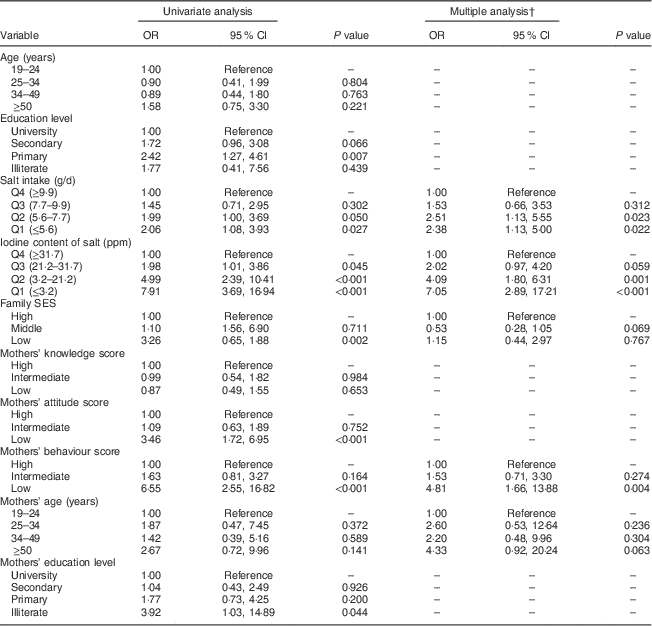
The results of univariate and multiple logistic regression analyses are shown in Table 4. In multiple analysis, lower quartiles of salt iodine content and salt intake increased the risk of UIC<100 µg/l as compared with higher quartiles. Inappropriate behaviour scores in mothers increased the risk of UIC<100 µg/l in adult family members as compared with higher scores. There was no statistically significant association between UIC<100 µg/l in adult family members and mothers’ age and family SES.
Table 4 Factors associated with UIC<100 µg/l in adult family members by univariate and multiple logistic regression analyses, Tehran, Iran
UIC, urinary iodine concentration; Q, quartile; SES, socio-economic status.
† Multiple analysis was run with the backward stepwise method.
Discussion
The current research indicated that dietary iodine status among Tehranian mothers and adult family members was marginally suboptimal and was significantly correlated. Mothers’ behaviour, but not knowledge and attitude, had a significant effect on the dietary iodine status of adult members of the family. The adult family member whose mother had higher behaviour score had better iodine intake and iodine nutritional status and consumed more salt with optimal iodine content.
Lack of nutritional knowledge is parallel to individuals’ dietary iodine insufficiency. In various countries such as India, Nigeria, South Africa, Kazakhstan and Australia, a low level of knowledge is one of the most important obstacles hindering successful elimination of iodine deficiency( Reference Abuye and Berhane 27 – Reference Charlton, Yeatman and Lucas 30 ). However, similar to our previous study on the association between iodine nutrition status and knowledge, attitude and behaviour in Tehranian women( Reference Mirmiran, Nazeri and Amiri 31 ), in the current study no association was found between mothers’ knowledge and attitude and iodine nutrition status in adult family members. This contradiction may be attributable to the impact of different factors on nutrition knowledge, i.e. age, education, socio-economic position and occupation( Reference De Vriendt, Matthys and Verbeke 32 ). Moreover, high nutritional knowledge and positive attitude do not necessarily lead to behavioural outcome and dietary changes, as indicated in our study( Reference He, Piche and Beynon 33 ).
Two common ideas exist about the association between attitude and behaviour. As the primary idea, attitude more than knowledge motivates behaviour and differences in behaviour are reflected by differences in attitude. The second idea demonstrates that educational background and overall SES rather than attitude influence health and health-related behaviour( Reference Stenhammar, Sarkadi and Edlund 34 – Reference Vereecken, Keukelier and Maes 36 ). Our findings indicated that despite generally having positive attitude towards the importance of iodine and iodized salt, vulnerability to iodine deficiency and inadequate dietary iodine intake, low iodine content of salt and high salt intake were observed more in lower SES groups than in those with higher SES. These results support the second idea that as compared with those of a higher socio-economic background, individuals with lower socio-economic levels have a less healthy dietary pattern, consume more foods with high energy density and lower nutrient density, and fewer fruits and vegetables and more high-fat and high-salt snacks( Reference Aranceta, Perez-Rodrigo and Ribas 37 , Reference Inglis, Ball and Crawford 38 ).
Familial correlation in dietary intakes has been found especially between parents and their children( Reference Ovaskainen, Nevalainen and Uusitalo 17 – Reference Vauthier, Lluch and Lecomte 19 , Reference Klesges, Stein and Eck 39 ). However in spite of close correlation in families such as Iranian family members observed here, as compared with younger children the impact of parental roles might be diminished in older children and adolescents due to their increasing independence as they grow older; consequently, dissimilarities in dietary intakes could be expected between parents and other adult members of the family( Reference Vereecken, Haerens and De Bourdeaudhuij 15 ). In a study by Ategbo et al., there was no correlation in iodine status between schoolchildren and pregnant mothers from the same household( Reference Ategbo, Sankar and Schultink 40 ). For example, in families using salt with optimal iodine content, children and pregnant mothers had median UIC of 171 µg/l and 126 µg/l, indicating iodine sufficiency and deficiency, respectively; a difference which, of course, can be explained by the physiological status of pregnant women, who need an additional dietary iodine intake. However, in agreement with other studies conducted among parents( Reference Oliveria, Ellison and Moore 18 , Reference Vauthier, Lluch and Lecomte 19 ), the findings of the present study confirm this similarity in patterns of dietary iodine and salt intakes between mothers and adult members of the family.
Different factors other than knowledge, attitude and behaviour have an impact on iodine nutrition status. As seen in a French study conducted on an adult population, dietary iodine intake is influenced by age, education, energy intake and smoking( Reference Valeix, Faure and Peneau 41 ). The present study revealed that iodized salt, used during cooking and as table salt, is the main dietary iodine source providing almost all the iodine intake in Iran, where due to the low iodine content of its soil, many locally grown plants and animal foods have iodine concentrations too low to serve as constant contributors to the iodine supply( Reference Azizi, Sheikholeslam and Hedayati 11 , Reference Azizi, Mehran and Sheikholeslam 42 ). Furthermore, as stated in our previous study( Reference Nazeri, Mirmiran and Mehrabi 6 ), the illegal production and distribution of less expensive or inadequately iodized brands of salt is another major destabilizing factor in sufficient dietary iodine intake. Our findings that individuals with lower daily salt intake and iodine content of salt were more susceptible to iodine deficiency are consistent with WHO reports( 4 , 23 , Reference Beard 43 ). In addition, no associations were observed between salt intake and mothers’ knowledge, attitude and behaviour regarding iodine and iodized salt, which can be explained by the fact that salt intake is strongly determined by environmental issues such as cultural factors and palatability( Reference Mattes 44 ). Also, taste preference and satisfaction rather than health aspects are a major contributor in salt intake, data confirmed in our study( Reference Rasanen, Niinikoski and Keskinen 45 ).
The present study has a few potential limitations that need to be considered. First, we could not measure the dietary iodine intake through iodine content of foodstuffs, although iodized salt is the main dietary of iodine source in Iran. Second, iodine concentration in a 24 h urine sample is considered a gold standard for determination of dietary iodine intake. Yet, a single 24 h urine sample is an inappropriate indicator of habitual iodine intake, because of variation in daily dietary iodine intakes( Reference Vejbjerg, Knudsen and Perrild 46 ). Third, causality relationships cannot be established due to the cross-sectional design of the study.
Conclusion
Marginally suboptimal iodine status was observed in both mothers and adult family members of the Tehranian families studied. Furthermore, there was a significant correlation between UIC of mothers and adult members of the family. It seems that maternal behaviour has a significant effect not only on children, but also on other adult members of the family. Our study findings emphasize the importance of continuous public education regarding iodine nutrition in areas that have iodine sufficiency, following effective universal salt iodization. Appropriate social marketing and encouraging the willing cooperation of families, in particular mothers, could guarantee effective, long-lasting adequate iodine nutrition in the community.
Acknowledgements
Financial support: This study was supported by financial grants from the Research Institute for Endocrine Sciences, Shahid Beheshti University of Medical Sciences and the National Nutrition and Food Technology Research Institute, Faculty of Nutrition and Food Technology, Shahid Beheshti University of Medical Sciences, Tehran, Islamic Republic of Iran. Both funders had no role in the design, analysis or writing of this article. Conflict of interest: None. Authorship: P.N. contributed to the design, data analysis and writing of the manuscript; P.M. contributed to the design of the study and writing, reading and final approval of the manuscript; G.A. contributed to the design of the study and writing of the manuscript; N.S. contributed to the design of the study and writing of the manuscript; Y.M. contributed to the statistical analysis and writing of the manuscript; F.A. contributed to the design of the study and writing, reading and final approval of the manuscript. Ethics of human subject participation: Informed written consent was obtained from all participants and the Ethical Committee of the Research Institute for Endocrine Sciences approved this study.






