Preconception and pregnancy are considered sensitive periods for obesity development for both mother and offspring( Reference Ross and Desai 1 ). Detrimental effects linked to excessive weight during pregnancy include gestational diabetes, pre-eclampsia, emergency caesarean delivery, preterm delivery and congenital defects( Reference Poston, Harthoorn and Van Der Beek 2 – Reference McDonald, Han and Mulia 4 ). Women with obesity before pregnancy have higher risk of excessive gestational weight gain( Reference Chu, Callaghan and Bish 5 , Reference Holowko, Mishra and Koupil 6 ) and gaining excessive gestational weight is associated with a higher postpartum weight retention( Reference Nehring, Schmoll and Beyerlein 7 ). This vicious circle could have negative consequences on subsequent pregnancies as interpregnancy weight gain has been linked to negative pregnancy outcomes( Reference Cnattingius and Villamor 8 , Reference Villamor and Cnattingius 9 ).
Obese women, on average, give birth to larger offspring, who have an increased risk of obesity( Reference Cnattingius, Villamor and Lagerros 10 , Reference Yu, Han and Zhu 11 ) and higher proportion of fat mass( Reference Reynolds, Osmond and Phillips 12 – Reference Henriksson, Löf and Forsum 16 ) later in life. The heritability of BMI and fat mass are reported to be 46–75 %( Reference Elks, den Hoed and Zhao 17 ) (all ages) and ~60 %( Reference Elder, Roberts and McCrory 18 ) (adults), respectively, indicating that some of these effects may be genetically mediated. However, birth order may also be of importance as lower birth order has been linked to higher BMI( Reference Jelenkovic, Silventoinen and Tynelius 19 ), fat mass( Reference Reynolds, Osmond and Phillips 12 ), and overweight or obesity( Reference Derraik, Ahlsson and Lundgren 20 ).
To our knowledge, there are no studies investigating if birth order modifies the association between mother’s pre-pregnancy BMI and her offspring’s body composition and whether any such association differs between daughters and sons. The purpose of the present study was to investigate if the association between maternal pre-pregnancy BMI and offspring’s body composition in late adolescence or young adulthood, defined as percentage fat mass (%FM) and percentage lean mass (%LM), varies by offspring birth order and sex.
Methods
Data used in the present study come from the Uppsala Family Study (UFS)( Reference Leon, Koupil and Mann 21 ), which includes families with two consecutive singleton offspring delivered at term (38–41 weeks of gestation) within 36 months of each other at the Uppsala Academic Hospital (Uppsala, Sweden) between 1987 and 1995. Families were recruited for a clinical examination in 2000–2002, with 602 sibling pairs (mean age 10·1 years) and their parents participating (31 % of eligible families invited to participate). Information regarding maternal and child characteristics related to pregnancy and delivery was obtained from the Swedish Medical Birth Register.
All 602 families were invited to a second clinical examination in 2010–2012. Members from 301 of the 602 families participated, including 168 sibling pairs (mean age 20·2 years, range 15·3–24·6 years). The participants completed health and lifestyle questionnaires and underwent physical examinations including anthropometric measurements and body composition measurement by dual-energy X-ray absorptiometry (DXA; Lunar Prodigy, GE Healthcare Lunar, Madison, WI, USA). Of the 168 sibling pairs with body composition measurements in the second clinical examination, 113 first- and second-born siblings also had data on their mothers’ pre-pregnancy BMI, as well as complete information on other child and maternal relevant covariates. These 113 sibling pairs (n 226) comprised the analytical sample for the study.
Our exposure variables were maternal pre-pregnancy BMI and pre-pregnancy overweight/obesity (BMI≥25·0 kg/m2) for each offspring, which were estimated from mothers’ height and self-reported pre-pregnancy weight recorded during their first antenatal care visit and obtained from the Swedish Medical Birth Register. Our outcome variables were offspring %FM and %LM based on DXA readings relative to the total body mass; LM is a composite of non-fat and non-bone tissue. Covariates included offspring’s age (years), height (cm) and puberty stage at the time of DXA measurements; and mothers’ age at offspring birth (years) and education level at the time of DXA measurements (some university education v. less). Puberty stage was estimated based on menarche (assessed by questionnaire) for girls and self-assessed testicular volume by orchidometer for boys( Reference Rollof and Elfving 22 ). Girls post-menarche were considered post-puberty, while boys were considered post-puberty if their orchidometer values were ≥12 or if they were older than 18 years. All first-born offspring and all but six boys and two girls in the second-born group were post-puberty at the time of DXA measurement.
Statistical analysis
We present characteristics of the study sample as means and standard deviations, or as frequencies and percentages, stratified by birth order and sex. We ran multivariable linear regression models with maternal pre-pregnancy BMI or pre-pregnancy overweight/obesity as exposure and offspring %FM or %LM as outcome. We further assessed the association between mothers’ pre-pregnancy BMI and overweight/obesity from her first pregnancy and the body composition of her second-born offspring. All models were stratified by birth order and offspring sex. Model 1 was adjusted for offspring’s age at DXA measurement. Model 2 was further adjusted for height at DXA measurement and, among later-born sons, puberty stage. Model 3 further included maternal age and educational level. The statistical software package SAS version 9.4 was used for all statistical analysis and a P value of <0·05 was used to denote statistical significance.
Results
Participants’ characteristics are shown in Table 1. First-born offspring were on average 21·2 years old and second-born offspring 19·2 years old at the time of DXA measurements. Girls had higher %FM and lower %LM than boys, with %FM differing by approximately 2 percentage points by birth order for boys (no difference for girls). The mean maternal pre-pregnancy BMI did not differ much by birth order or offspring sex. However, maternal pre-pregnancy overweight/obesity was more common among second-born daughters (23·6 %) compared with the rest (11–12 %).
Table 1 Characteristics of the offspring (born 1987–1995 and examined in 2010–2012) and their mothers from the Uppsala Family Study (113 sibling pairs; n 226)
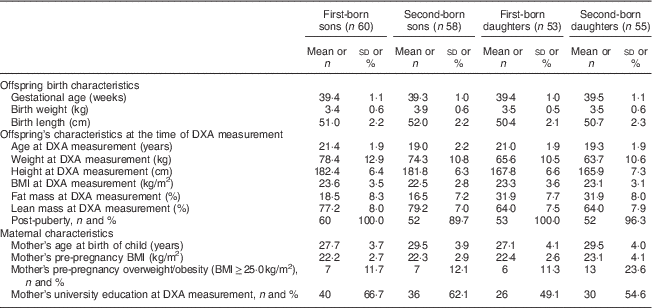
DXA, dual-energy X-ray absorptiometry.
Data are presented as mean and sd unless indicated otherwise.
Mothers’ pre-pregnancy BMI was positively and negatively associated with %FM and %LM, respectively, among daughters (Table 2). The association was present for both first- and second-born, but appeared to be stronger among first-born daughters. In the fully adjusted model, a one-unit increase in maternal pre-pregnancy BMI was associated with an increase of 0·9 and 0·6 percentage points of FM among first-born and second-born daughters, respectively. Associations with %LM mirrored those with %FM albeit in the opposite direction. In fully adjusted models, having a mother who was overweight/obese pre-pregnancy was not associated with offspring %FM and was associated with %LM among first-born daughters only (Table 2, Model 3). Mothers’ pre-pregnancy BMI and overweight/obesity were not significantly associated with their sons’ %FM or %LM.
Table 2 Associations of mother’s pre-pregnancy BMI and overweight/obesity (BMI ≥ 25·0 kg/m2) with offspring’s body composition determined by DXA among a sample of 113 first- and second-born sibling pairs, stratified by birth order and sex, from the Uppsala Family Study
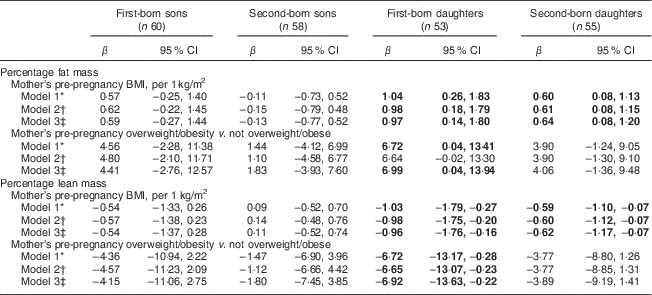
DXA, dual-energy X-ray absorptiometry.
Significant results are shown in bold.
* Model 1: adjusted for offspring’s age at DXA measurement.
† Model 2: Model 1+offspring’s height at DXA measurement and for later-born sons also puberty stage.
‡ Model 3: Model 2+mothers’ age at birth of offspring and mothers’ education at DXA measurement.
We further examined the association between mother’s pre-pregnancy BMI before her first child was born with the body composition of her second-born offspring. Mothers’ BMI before the first pregnancy was associated with %FM and %LM of their second-born daughters but not of their second-born sons (Table 3). These estimates for the daughters were stronger than those for the pregnancy-specific pre-pregnancy BMI. Interpregnancy weight change was not associated with the second-born offspring’s body composition (results not shown). We also tested interactions between maternal pre-pregnancy BMI and birth weight on offspring’s body composition, with no interaction effects found (results not shown).
Table 3 Associations of mother’s pre-pregnancy BMI and overweight/obesity (BMI≥25·0 kg/m2) before her first pregnancy with her second-born offspring’s body composition determined by DXA (n 113), stratified by sex, Uppsala Family Study
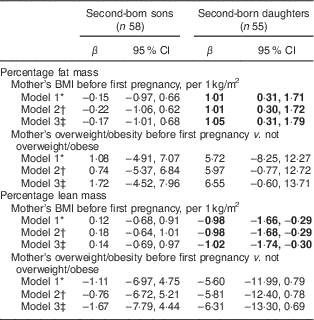
DXA, dual-energy X-ray absorptiometry.
Significant results are shown in bold.
* Model 1: adjusted for offspring’s age at DXA measurement.
† Model 2: Model 1+offspring’s height at DXA measurement and for later-born sons also puberty stage.
‡ Model 3: Model 2+mothers’ age at birth of offspring and mothers’ education at DXA measurement.
Discussion
Among a sample of Swedish adolescents and young adults, there was a differential association between mothers’ pre-pregnancy BMI and her offspring’s body composition by birth order and sex. The association between maternal pre-pregnancy BMI (and overweight/obesity) and offspring body composition was stronger for first-born compared with second-born daughters, with weaker and non-significant estimates for sons.
Other studies have observed positive associations between maternal pre-pregnancy BMI and %FM in offspring at ages 6( Reference Castillo, Santos and Matijasevich 13 ), 9( Reference Gale, Javaid and Robinson 14 ) and 30 years( Reference Reynolds, Osmond and Phillips 12 ) although few have carried out sex-specific analysis( Reference Gale, Javaid and Robinson 14 ). Maternal FM or BMI was associated with offspring’s FM also at infancy (≤1 week) but results regarding sex differences were inconsistent( Reference O’Tierney-Ginn, Presley and Minium 15 , Reference Henriksson, Löf and Forsum 16 ). The body composition of 9-year-old girls and boys in the UK was dependent on their mothers’ pre-pregnancy BMI, with a stronger association in girls( Reference Gale, Javaid and Robinson 14 ), similar to our results in offspring aged 15–25 years. Sex differences in the genetics of BMI have been observed, especially after adolescence( Reference Ortega-Alonso, Pietilainen and Silventoinen 23 , Reference Silverntoinen, Jelenkovic and Sund 24 ). Such differences could potentially manifest as stronger mother–daughter compared with mother–son BMI or FM correlations( Reference Han, Hart and Haig 25 , Reference Blain, Vuillemin and Jeandel 26 ) and might contribute to the sex differences observed in our results. In addition, cultural factors may also play a role: mothers may have a stronger influence on their daughters than on their sons in terms of dietary and lifestyle patterns, as well as attitudes and norms towards body image.
Animal studies have also found a differential sex effect of maternal nutrition during pregnancy on offspring’s body composition, with the female offspring being more affected( Reference Rattanatray, MacLaughlin and Kleemann 27 , Reference Aiken and Ozanne 28 ). Female animals seem to be more sensitive to the intra-uterine environment than males as they seem more responsive to placental changes( Reference Aiken and Ozanne 28 ). The sex of the offspring appears to influence both the size of the placenta and its ability to respond to adverse stimuli( Reference Gabory, Roseboom and Moore 29 ).
Previous research has already suggested that first-borns have a higher BMI compared with later-borns( Reference Reynolds, Osmond and Phillips 12 , Reference Jelenkovic, Silventoinen and Tynelius 19 , Reference Derraik, Ahlsson and Lundgren 20 ). However, most of these studies were based on population samples, with only one study based on siblings( Reference Derraik, Ahlsson and Lundgren 20 ), like ours. It is possible that the differences we observed between first- and second-born siblings were due to age or sexual maturation stage as opposed to birth order. A study of 10–17-year-old children in Lebanon found correlations in FM parameters for mother–children pairs but only among late pubertal sons and post-menarcheal daughters( Reference Nabulsi, Mahfoud and El-Rassi 30 ). All first-born and most second-born offspring (90 % of boys and 96 % of girls) in our study were post-puberty. We were therefore unable to adjust for puberty stage in the analysis among second-born daughters but did account for pubertal status of second-born sons. Interestingly, studies also indicate that the heritability of BMI increases during childhood, reaching a peak at age 18–19 years( Reference Elks, den Hoed and Zhao 17 , Reference Silverntoinen, Jelenkovic and Sund 24 ) – close to the mean age of the second-born offspring in our study – before decreasing again during adulthood( Reference Elks, den Hoed and Zhao 17 ).
Our finding that pre-pregnancy BMI before the first pregnancy had a stronger association with the body composition of second-born daughters (but no association for second-born sons) than the pregnancy-specific BMI deserves further attention. This result implies that the weight status of the mother at the start of her reproductive life is of importance not only for the first-born but also for the second-born, particularly among daughters.
Strengths of our study include its family design, which allowed us to investigate the effects of maternal pre-pregnancy BMI on first- v. second-born full siblings. In addition, we assessed the offspring’s actual body composition measured by DXA instead of using BMI as a proxy. As for limitations, pre-pregnancy BMI was recorded 8–12 weeks into pregnancy and may be different from the true pre-pregnancy BMI. Moreover, our sample is relatively small and homogeneous in terms of ethnicity, including only full siblings delivered at term with a maximum interpregnancy interval of 36 months. This is a strength for internal validity, but may limit the generalizability of the results to mothers and siblings with larger interpregnancy intervals, children not born at term, or children of mothers who remained primiparous. Only half of the original UFS families participated in the follow-up examination, and parents who did not come for the second wave were on average younger, less educated and had a higher BMI. Moreover, most mothers in our sample had a normal BMI; this limits our ability to test the hypothesis that overweight/obesity pre-pregnancy influences offspring’s body composition. On the other hand, if we observed effects of pre-pregnancy BMI on offspring’s body composition even in this small sample with a normal BMI range, we speculate that the effects could be higher and stronger among populations with higher-than-normal BMI. Finally, puberty stage was self-reported albeit with valid methods, with some of the second-born children in the sample not having gone through puberty at the time of DXA measurement. We controlled for puberty stage in our analysis of second-born sons, but it is possible that our results reflect pubertal age rather than birth order.
Conclusion
In conclusion, mothers’ pre-pregnancy BMI was associated with daughters’ body composition in late adolescence and young adulthood, with stronger effects among the first-born. The mechanisms behind the reported associations and the apparently absent effects among sons remain to be clarified. Moreover, mothers’ BMI before their first pregnancy was associated with their second-born daughter’s body composition, with stronger associations than those observed for pregnancy-specific BMI. This result implies that preventing overweight and obesity at the start of women’s reproductive life may prevent obesity in the first and subsequent offspring. Larger studies employing family designs with multiple offspring are needed to further disentangle the effects of pre-pregnancy BMI on offspring body composition from the possible effects of gestational weight gain and interpregnancy weight change.
Acknowledgements
Acknowledgements: The authors would like to thank the study participants as well as the research nurses who helped with the data collection. In addition, they wish to thank Amy Heshmati for her assistance with some of the statistical analyses. Financial support: The Uppsala Family Study was funded by the Medical Research Council, UK (MRC; grant number G9828126); the Swedish Research Council for Health, Working Life and Welfare (FORTE; grant number 2009–1322); and Uppsala University. M.P.C. and I.K. were partly supported by the Swedish Research Council for Health, Working Life and Welfare (FORTE grant number 2006–1518). The funders had no role in the design, analysis or writing of this article. Conflict of interest: None. Authorship: M.P.C., I.K. and L.B. conceptualized and designed the study. M.P.C. carried out the statistical analysis, drafted the initial manuscript, and approved the final manuscript as submitted. I.K. helped with the interpretation of data, reviewed and revised the manuscript, and approved the final manuscript as submitted. L.B. was involved with data collection, helped with the interpretation of data, reviewed and revised the manuscript, and approved the final manuscript as submitted. Ethics of human subject participation: This study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects/patients were approved by the regional ethics board in Uppsala, Sweden. Written informed consent was obtained from all subjects aged 18 years or above. For children below 18 years of age, written informed consent was obtained from both the children and their parents.






