Pregnancy and labour are stressful life events that may trigger depressive symptoms among women in the postnatal period(Reference Biaggi, Conroy and Pawlby1). The worldwide prevalence of postpartum depression ranges from 3 to 38 %(Reference Hahn-Holbrook, Cornwell-Hinrichs and Anaya2,Reference Gelaye, Rondon and Araya3) , depending on the country and region and on the diagnostic criteria that are chosen. There are higher prevalences among women with a previous history of mental health issues, those who are younger at delivery and those with lower socio-economic status, lower educational attainment and lack of social support during the pregnancy(Reference Gelaye, Rondon and Araya3,Reference Fisher, Cabral de Mello and Patel4) .
Untreated maternal depression can lead to consequences for women and their offspring’s health. In mothers, postpartum depression has been correlated with the development of depressive disorders later in life(Reference Kumar and Robson5). In the family environment, maternal depression may have a negative impact on the marital relationship, thus increasing family conflict(Reference Burke6). Maternal depressive symptoms such as sadness, increased hostility, negative interactions, less responsiveness and communication(Reference Lovejoy, Graczyk and O’Hare7,Reference Rahman, Harrington and Bunn8) have negative consequences for mother–child interactions. The lack of proper child stimulation and the mother’s reduced ability as a caregiver (which can impair the duration of breast-feeding and the child’s sleep routine, health care, diet quality, screen-viewing time and physical activity) can negatively affect the offspring’s proper development and growth(Reference Edwardson and Gorely9–Reference Surkan, Patel and Rahman12). Previous reviews of the literature on this matter have not been able to reach any conclusions regarding data on the growth and body composition of the offspring of mothers with depressive symptoms(Reference Farías-Antúnez, Xavier and Santos13–Reference Pierce, Hope and Kolade15). Weight- and height-deficient growth have been observed over the first year of life among the children of depressed mothers, while linear growth is possibly impaired for up to 5 years after birth(Reference Farías-Antúnez, Xavier and Santos13). Moreover, chronic, but not episodic maternal depression may increase the risk that the offspring might be overweight or obese during childhood(Reference Lampard, Franckle and Davison14).
Although there is evidence that the severity, timing and persistence of maternal depression are related to a variety of adverse child socio-emotional and behavioural outcomes(Reference Ahun, Geoffroy and Herba16–Reference Raskin, Easterbrooks and Lamoreau26), studies exploring the effect of maternal depression on child growth and body composition are scarce in the literature. In this light, the aim of the current study was to investigate the effect of maternal depressive symptom trajectories, from 3 months to 11 years postpartum, on the offspring’s body composition at 11 years of age.
Methods
Pelotas is a city with approximately 350 000 inhabitants located in southern Brazil. In the year 2004, all infants born alive between 1 January and 31 December, in any of the city’s five maternity hospitals, to mothers who lived within the urban area of Pelotas or in the Jardim America district (which currently belongs to the neighbouring municipality of Capão do Leão), were deemed eligible for the study.
A total of 4231 newborns (representing 99·2 % of all births in the city that year) were included in the cohort. Standardised interviews were conducted with the mothers during their stay at the hospital of delivery (perinatal study), to seek data on their socio-economic, demographic and behavioural characteristics and their use of antenatal care. The newborns were examined (weighed and measured) by the study team under the supervision of a paediatrician in the maternity hospitals, within the first 24 h after birth. Home visits were carried out when the newborns were at the ages of 3, 12, 24 and 48 months. When these children reached 6 and 11 years of age, they were interviewed together with their mothers at the study clinic. The follow-up rates ranged from 87 to 96 %. A detailed description of the methodology is given elsewhere(Reference Barros, da Silva dos Santos and Victora27,Reference Santos, Barros and Matijasevich28) . For the current study, all cohort participants with valid data from at least three time points maternal depression evaluations were included in the analyses.
Maternal depression
Maternal depressive symptoms were assessed using the Edinburgh Postnatal Depression Scale (EPDS)(Reference Cox, Holden and Sagovsky29). EPDS is an instrument based on a questionnaire that expresses the intensity of depressive symptoms over the preceding 7 d. The scale includes ten items; each item has four possible responses scored from 0 to 3, with a minimum total score of 0 and a maximum of 30. The Brazilian version of EPDS had previously been validated for evaluating depressive symptoms in adults(Reference Santos, Matijasevich and Tavares30,Reference Matijasevich, Munhoz and Tavares31) , with a cut-off point of ≥13. It showed sensitivity of 59·6 % (49·5–69·1 %) and specificity of 88·3 % (83·9–91·9 %) for identifying individuals who were at increased risk of depression in this population. At the 3-month follow-up, the EPDS was administered only to a subsample of 965 mothers, due to logistic arrangements (comprising all mothers whose infants were born between 1 October and 31 December 2004). However, at the 12-, 24- and 48-month and the 6- and 11-year follow-ups, the EPDS was applied to the whole cohort.
Offspring body composition
At the 11-year follow-up, body composition was assessed through anthropometric measurements and by means of air-displacement plethysmography (BOD POD®). The BOD POD is composed of a scale and a chamber. The participants’ weight is assessed, and they are placed inside the chamber wearing tight clothes, a swimming cap and no metal accessories (bracelets or rings). Using an air-displacement system, the body volume is estimated using the relationship between pressure and volume inside the equipment. Body density is later estimated through body volume and body mass assessments, thus enabling estimation of fat and fat-free mass percentages(Reference Dempster and Aitkens32).
The children’s weight was measured using an electronic high-precision scale with 0·01 kg resolution that was connected to the BOD POD® (model BWB-627-A; Tanita), in the study clinic. The Harpenden stadiometer with 1 cm precision was used for height measurements. The BMI was calculated as body weight (kg) divided by height in metres squared (m2) and was then standardised into z-scores according to the child’s age and sex. After the body density had been obtained through the BOD POD®, the body fat mass (FM) (kg) and fat-free mass (FFM) (kg) were determined, which enabled estimation of the fat mass percentage (FM%) and fat-free mass percentage (FFM%). The fat mass index (FMI in kg/m2) and the fat-free mass index (FFMI in kg/m2) were estimated through the general population equation(Reference Siri33).
Covariables
Information on the mother’s and the child’s characteristics, recorded during the perinatal interview, was included in the analyses as possible confounding factors. The mother’s characteristics comprised the following: age (years), self-reported skin colour (white or black/brown/other), family monthly income (in Brazilian currency – real), schooling (years), marital status (married/living with a partner or single/divorced/widowed), parity, defined as the number of viable prior gestations (1 or ≥2 children), smoking (at least one cigarette a day during any pregnancy trimester), alcohol consumption (any amounts of alcohol consumed during pregnancy), pre-gestational BMI and self-reported gestational weight gain (kg).
The child’s characteristics included the following: sex (male or female), gestational age (GA), birth weight and exclusive breast-feeding at 3 months of age. GA was estimated by means of the algorithm proposed by the National Center for Health Statistics(Reference Martin, Hamilton and Sutton34), which is based on the first day of the last menstrual period, whenever this is consistent with birth weight, length and head circumference, as described in the normal curves for these parameters for each week of GA. If the last menstrual period-based GA was unknown or inconsistent, the clinical maturity estimate based on the Dubowitz method(Reference Dubowitz, Ricciw and Mercuri35), which was performed on all newborns, was used. Preterm births were defined as <37 gestational weeks. Birth weight was determined using the hospital scales, with 10-g precision, which were regularly calibrated by the researchers. Birth weight was measured by the nursing professionals overseeing delivery. Newborns were classified as small for GA, when the birth weight according to GA was <10th percentile; appropriate for GA, if it was between the 10th and 90th percentiles; or large for GA, if the birth weight adjusted for GA was >90th percentile(36). Exclusive breast-feeding at 3 months was assessed through a dietary recall questionnaire that asked for information on breast-feeding and age of introduction of liquids and solid and semi-solid foods. Based on this information, the presence of exclusive breast-feeding (yes or no) was determined in accordance with the WHO definition, that is, no food or drink other than breast milk (not even water) administered to the child, with the exception of medications and vitamins(Reference Alexander, Himes and Kaufman37).
Data analyses
The characteristics of the participants, both those who were and those who were not included in the data analyses, were described according to the potential confounding variables. Maternal depression trajectories were identified through the Nagin & Tremblay(Reference Nagin and Tremblay38,Reference Nagin39) semi-parametric group-based modelling approach, using data on depressive symptoms covering the period from 3 months to 11 years postpartum, based on EPDS scores at each follow-up.
Group-based trajectory modelling identifies groups of individuals who follow similar developmental trajectories, using a finite mixture modelling approach. The relationship between an attribute and age or time is modelled through a polynomial function(Reference Nagin and Tremblay38–Reference Nagin and Odgers40).
Trajectories were created from continuous EPDS scores, from at least three time points, using a censored normal model (cnorm) to fit the data and the Stata procedure ‘traj’. The proportion of the women with complete EPDS information from at least three time points was 81·9 % (3467). Individuals with missing information were not excluded from the model, given the ability of group-based trajectory modelling to handle missing data using maximum likelihood estimation(Reference Nagin39). The number and shape of the trajectories were selected based on the best fit of the model estimated through the maximum Bayesian information criteria and the interpretability of the obtained trajectories. The trajectories thus selected were confirmed using the posterior probability score, which assesses the subject’s likelihood of belonging to a given trajectory group. This likelihood needed to be higher than 70 % for all groups(Reference Nagin39) (see online supplementary material, Supplementary Table S1).
Maternal depressive symptom trajectories were described according to the potential confounding factors, using ANOVA (continuous variables) and χ 2 tests (categorical variables). Linear regression was used to estimate the associations between the child’s body composition measurements (BMI z-score, FM, FFM, FM%, FFM%, FMI and FFMI) and the covariables. The means and sd relating to the association between the trajectory of depression and the offspring’s body composition measurements were calculated through variance analysis. All body composition parameters were included in the analyses as continuous variables.
Multivariable linear regression was used to investigate the association between maternal depression trajectories and the offspring’s body composition at 11 years (model 1). This was then firstly adjusted for maternal characteristics, that is, maternal age, skin colour, family income, schooling, marital status, parity, smoking and alcohol consumption during pregnancy, pre-gestational BMI and gestational weight gain (model 2), and secondly, for the mother’s and child’s characteristics, that is, model 2 + sex, GA, nutritional status at birth and exclusive breast-feeding at 3 months (model 3). A backward selection strategy was used, and variables at the 0·20 significance level were entered into each model. All analyses were performed using the Stata software, version 15.0 (StataCorp LP).
Results
A total of 3467 cohort participants were included in the current study. The mothers included in the current analyses had higher education levels (8·2 ± 3·4 v. 7·7 ± 3·7 years), were more likely to be married (84·5 % v. 79·5 %) and less likely to have smoked during pregnancy (26·7 % v. 30·9 %). The children included in the current study were less likely to be preterm (13·6 %) and more likely to have exclusively received breast milk at 3 months of age (27·3 % v. 22·7 %). The mothers who were included and those who were not included in the current analysis were of similar age, skin colour, monthly family income, parity, alcohol consumption during pregnancy and pre-gestational BMI. There were no differences regarding sex or nutritional status at birth between the children who were included and those who were not included (Table 1).
Table 1 Sample characteristics comparing cohort participants included and not included in the analyses*

AGA, appropriate for gestational age; SGA, small for gestational age; LGA, large for gestational age.
* Pelotas 2004 Birth Cohort.
† χ 2 test.
‡ ANOVA test.
Maternal depression trajectories
Five trajectory groups were identified (Fig. 1). Group 1 (‘Low’), with 1175 mothers (33·9 %) who scored 5 points or less in the EPDS throughout all evaluations; Group 2 (‘Moderate low’) comprising 1411 mothers (40·7 %) with EPDS scores lower than 10 points throughout the whole period; Group 3 (‘Increasing’) with 11·5 % of the mothers (n 399), included those with depressive symptoms that increased over time (mothers who scored <10 points until about 4 years into the postpartum period and thereafter presented increasing scores until reaching about 15 points at 11 years after childbirth); Group 4 (‘Decreasing’), with 9·0 % of the women (n 312), presenting the opposite pattern (scoring between 10 and 15 points over the first 4 years and with constantly decreasing EPDS scores after that time); and Group 5 (‘Chronic high’) brought together 170 women (4·9 %) who scored over 13 points on the EPDS scale at every follow-up. For all groups, the average posterior probability was above 0·7, as recommended (0·85, 0·81, 0·79, 0·80 and 0·88, for groups 1–5, respectively).

Fig. 1 Maternal depression trajectories. ![]() , ‘low’ 33·9 %;
, ‘low’ 33·9 %; ![]() , ‘moderate low’ 40·7 %;
, ‘moderate low’ 40·7 %; ![]() , ‘increasing’ 11·5 %;
, ‘increasing’ 11·5 %; ![]() , ‘decreasing’ 9·0 %;
, ‘decreasing’ 9·0 %; ![]() , ‘chronic high’ 4·9 %. EPDS, Edinburgh Postnatal Depression Scale
, ‘chronic high’ 4·9 %. EPDS, Edinburgh Postnatal Depression Scale
Maternal age was similar in all trajectory groups (Table 2). The mothers in the ‘Low’ depressive symptom group had higher family income and more years of formal education and were more likely to be white and to be married or living with a partner. Women in the ‘Chronic high’ group were more likely to have two or more children and to have smoked and consumed alcoholic beverages during pregnancy and had higher pre-gestational BMI. The children of mothers in the ‘Chronic high’ group were also less often exclusively breastfed at 3 months of age. The offspring of mothers in the ‘Increasing’ group were more often born preterm (17·8 %; Table 2).
Table 2. Maternal and child characteristics according to maternal depression trajectories
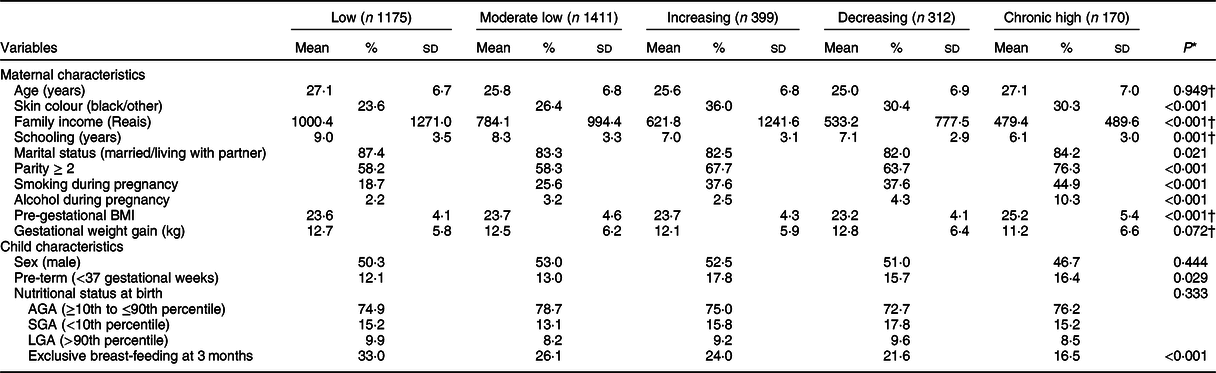
AGA, appropriate for gestational age; SGA, small for gestational age; LGA, large for gestational age.
* χ 2 test.
† ANOVA test.
Maternal depression trajectories and offspring body composition
There were no differences in any of the offspring body composition indices according to maternal depression trajectories (Table 3). However, BMI (z-score), FM (kg), FM (%) and FMI (kg/m2) were slightly higher among the children from mothers in the ‘Low’ and ‘Moderate low’ depression trajectories, whereas FFM% was slightly higher among the children from mothers with ‘Increasing’ and ‘Chronic High’ trajectories (Table 3).
Table 3 Offspring body composition indices at 11 years of age, according to maternal depression trajectories
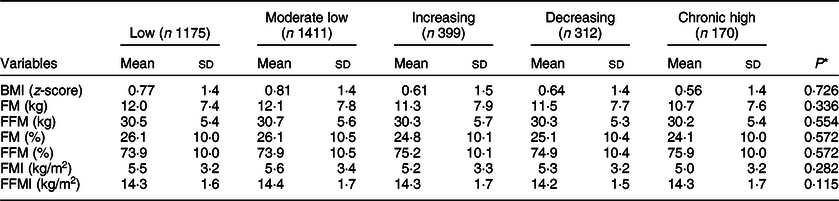
FM, fat mass; FFM, fat-free mass; FM (%), percentage fat mass; FFM (%), percentage fat-free mass; FMI, fat mass index; FFMI, fat-free mass index.
* ANOVA test.
Unadjusted regression analyses (Table 4) showed that BMI (z-score), FFM (%) and FMI (kg/m2) were 0·04 ± 0·06 kg/m2, 0·06 ± 0·40 percentage points and 0·05 ± 0·13 kg/m2, respectively, higher among the children from mothers in the ‘Moderate low’ depression trajectory, compared with those from mothers in the ‘Low’ trajectory. The children from mothers in the ‘Chronic high’ trajectory presented the lowest body composition parameters: BMI z-score (–0·21 ± 0·12), FM (–1·34 ± 0·64 kg), FFM (–0·40 ± 0·46 kg), FM% (–2·02 ± 0·85 percentage points) and FMI (–0·57 ± 0·27 kg/m2), compared with the children from mothers in ‘Low’ trajectory group. Unadjusted FFM% was 2·16 ± 0·85 percentage points higher among the children from mothers in the ‘Chronic high’ group, in comparison with the children from mothers in the ‘Low’ trajectory group. After adjusting for the mother’s and child’s characteristics, maternal depression trajectories were not associated with any of the offspring’s body composition indices investigated (Table 4).
Table 4. Effect of maternal depression trajectories on offspring’s body composition indices at 11 years of age

β, beta coefficient from linear regression; FM, fat mass; FFM, fat-free mass; FM (%), percentage fat mass; FFM (%), percentage fat-free mass; FMI, fat mass index; FFMI, fat-free mass index.
Model 1: unadjusted model.
Model 2: adjusted by maternal characteristics:
* Schooling, parity, smoking, alcohol consumption, pre-gestational BMI, gestational weight gain;
† Age, skin colour, schooling, parity, smoking, pre-gestational BMI, gestational weight gain;
‡ Skin colour, schooling, marital status, parity, smoking, alcohol consumption, pre-gestational BMI, gestational weight gain;
§ Age, skin colour, schooling, marital status, parity, smoking, alcohol consumption, pre-gestational BMI, gestational weight gain.
Model 3: adjusted by model 2 + child’s characteristics:
‖ Sex, gestational age, exclusive breast-feeding at 3 months;
¶ Gestational age, exclusive breast-feeding at 3 months;
** Gestational age.
Discussion
In the current study, it was possible to identify five maternal depressive symptom trajectories using data covering the period from 3 months to 11 years after the child’s birth (two low-symptom groups and groups with increasing, decreasing and permanently high depressive symptom scores). Adjusted analyses showed that sustained (‘Chronic high’) or transitory (‘Increasing’ or ‘Decreasing’) maternal depressive symptoms during the offspring’s childhood did not have any effect on the offspring’s body composition indices at 11 years of age.
As far as we know, this is the first study to explore the association between maternal depression and the offspring’s body composition by means of an indirect method. Previous studies assessed the association between maternal depression and offspring anthropometry using anthropometric indices to estimate the attainment of adequate weight and height or the risk of overweight and obesity(Reference Farías-Antúnez, Xavier and Santos13,Reference Lampard, Franckle and Davison14) . These studies gave rise to conflicting results(Reference Farías-Antúnez, Xavier and Santos13,Reference Lampard, Franckle and Davison14) . The children of depressed mothers were more likely to be underweight and become stunted before, but not after, their first year of life. These children would then continue to show stunting until the age of 5 years(Reference Farías-Antúnez, Xavier and Santos13). This might be explained by children’s higher dependence on maternal caregiving in their first year of life, since appropriate feeding is among children’s main needs during this period(Reference Thompson41). Additionally, in our study, maternal depressive symptoms were more severe, in the ‘Increasing’ and ‘Chronic high’ trajectory groups, in the first 4 years after childbirth, thus potentially accentuating the negative effect on children’s development over that period.
Furthermore, Stewart(Reference Stewart11) suggested that mothers’ mental health might have a greater effect on their behaviour towards their children, such as the duration of breast-feeding and early weaning, insituations of more hostile environments, such as those with poorer hygiene, lower income and reduced access to proper healthcare services.
In regions with more equitable access to healthcare services, with quality healthcare programmes that identify and treat possible growth deficiencies early on, it has become harder to observe the effects of exposure to maternal depressive symptoms(Reference Stewart11). This could help explain why studies with larger samples (those that include not only specific regions) and from developed countries tend to show no effect from maternal postnatal depression on the offspring’s growth(Reference Grote, Vik and von Kries42). Moreover, proper adjustment for potential confounding factors may also explain the lack of association found in papers with higher methodological quality(Reference Farías-Antúnez, Xavier and Santos13,Reference Lampard, Franckle and Davison14) .
Other studies have shown that the longer the exposure period is, the higher the risk will be that the offspring may present excessive weight in childhood and early adolescence(Reference Farías-Antúnez, Xavier and Santos13). Maternal depressive symptom trajectories have been found to increase the risk of overweight at 3 and 7 years of age among children whose mothers presented depression on at least three occasions after childbirth(Reference Audelo, Kogut and Harley43,Reference Wang, Anderson and Dalton Iii44) , although no effect has been found at age 4(Reference Santos, Matijasevich and Domingues45). Continuously depressed mothers tend to establish unhealthy interactions with their offspring that can negatively affect encouragement of these children’s physical activity practices(Reference Edwardson and Gorely9). The negative effects of chronically depressed mothers can also influence their offspring’s life in other behavioural aspects, such as unhealthy feeding practices(Reference Stewart11) and sedentary behaviour due to excessive screen time exposure(Reference Hoyos Cillero and Jago10).
In our study, at 11 years of age, the children from mothers in the ‘Chronic high’ trajectory group had the highest difference in BMI z-score, FM, FFM, FM% and FMI, compared with the children from mothers in the ‘Low’ trajectory group. These results indicate that the children from chronically depressed mothers are thinner than children from the reference group. Such differences were significant only in unadjusted analyses, thus indicating that other factors were positively confounding the association. After allowing for maternal antenatal characteristics and child characteristics at birth, maternal depression showed no effect on any of the body composition indices.
The current study has strengths and limitations. The population-based nature of the data is among its strengths. Additionally, the previously published studies on this subject mainly used doubly indirect methods to estimate body composition, such as BMI. In our study, air displacement plethysmography was used to assess body composition. Indirect methods provide more precise and more reliable information by using constants and rules based on direct methods (physical or chemical analysis of human cadavers, which is considered to be the gold standard)(Reference Deurenberg and Deurenberg-Yap46). The EPDS is an adequate instrument for evaluating maternal depressive symptoms that has previously been proven to be valid for use in this population(Reference Matijasevich, Munhoz and Tavares31). The maternal depression trajectory groups were built using EPDS information that was collected on seven occasions during the offspring’s childhood, from birth to 11 years of age, thus offering rich information on maternal mental health status during the child’s development.
However, the possibility that the mothers might only have been occasionally depressed, that is, at the times of the evaluations and not in between them, cannot be ruled out. The assessment of depression in only a subsample of the mothers at the 3-month follow-up may be considered to be a limitation to our findings. Nevertheless, the group-based trajectory modelling considers a minimum of three points in time when building the depressive symptom trajectories, and it is capable of handling the missing data using maximum likelihood estimation(Reference Nagin39). Also, maternal depression trajectories might be representing the effect of other behavioural variables included in the analyses, and not their own effect. Nonetheless, collinearity between the trajectories and alcohol consumption (variance inflation factor 1·02) and cigarette smoking during pregnancy (variance inflation factor 1·02) was tested and no evidence of collinearity between them was shown.
Conclusion
Although maternal depression has been linked to several negative health outcomes among these women’s offspring, our findings suggest that exposure to maternal depressive symptoms during childhood has no negative effects on the child’s body composition indices at age 11. In controlling for confounding, even the children who were raised by chronically depressed mothers had body composition indices similar to those of children whose mothers had never been depressed.
Acknowledgements
Acknowledgements: None. Financial support: The current study was funded by Wellcome Trust (grant no. GR 72403MA), Conselho Nacional de Pesquisa, Brazil (grant no. 476727/2003-0), Research Support Foundation of the State of Rio Grande do Sul (grant no. 2799-03), the Brazilian Ministry of Health (MS) (grant no. FNS 2799-03), Children’s Pastorate and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brazil – Finance Code 001. A.M., A.J.B. and I.S.S. are supported by Conselho Nacional de Pesquisa. Conflict of interest: None. Authorship: The manuscript authors S.F.-A., A.M. and I.S. conducted the data analyses. Authors S.F.-A. and I.S. wrote the manuscript. S.F.-A., A.M., A.B. and I.S. reviewed the manuscript. All authors contributed to and have approved the final manuscript. Ethics of human subject participation: The current study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects/patients were approved by the Federal University of Pelotas, Medical School Ethics Committee, associated with the National Research Ethics Committee (Comissão Nacional de Ética em Pesquisa). Written informed consent was obtained from all subjects.
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S1368980019005196








