Vitamin D, a fat-soluble secosteroid, has crucial roles mainly in accelerating intestinal absorption in humans(Reference DeLuca and Schnoes1). Under normal physiological conditions, vitamin D is transported from the blood to the liver tissue, is further hydroxylated to form 25-hydroxyvitamin D (25(OH)D) in the liver and is finally released into the blood.
In addition to its traditional skeletal adverse effect(Reference Lips2), vitamin D deficiency has been now recognised to exert abnormal biological effects on several other organ systems (e.g. cardiovascular risk(Reference Lavie, Dinicolantonio and Milani3), diabetes(Reference Pittas, Lau and Hu4), metabolic syndrome(Reference Ford, Ajani and McGuire5), some common cancers and autoimmune diseases(Reference Holick6)) in humans. Recently, some studies have found that vitamin D deficiency is common in patients with chronic liver disease and vitamin D levels are inversely correlated with the severity of chronic liver disease(Reference Arteh, Narra and Nair7,Reference Stokes, Volmer and Grunhage8) . For instance, epidemiological studies have found that non-alcoholic fatty liver disease patients have a marked decrease in serum 25(OH)D concentrations(Reference Barchetta, Angelico and Del Ben9). In addition, lower vitamin D status in patients is closely associated with histopathological features of non-alcoholic fatty liver disease(Reference Targher, Bertolini and Scala10). These results suggest potential associations between 25(OH)D levels and liver function. However, currently the information on associations between vitamin D status and liver enzymes is lacking.
Herein, we performed the first cross-sectional study exploring the relationship between serum 25(OH)D levels and serum levels of four enzymes commonly used in liver function tests (alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase (ALP), and gamma-glutamyl transaminase (GGT)) in US adults. Elucidation of these relationships may be of clinical importance in formulating preventative or therapeutic strategies for liver dysfunction.
Method
Study population
The National Health and Nutrition Examination Survey (NHANES), a study of a nationally representative sample of the US population(11), was conducted by the National Center for Health Statistics of the Centers for Disease Control and Prevention. A detailed description of the study design has been published elsewhere(Reference Xu, Liu and Zhang12,Reference Xu, Liu and Zhang13) . To explore whether a potential relationship is present between low 25(OH)D levels and serum hepatic enzyme levels in adults, we used a database including all available serum 25(OH)D levels from 2001 to 2006. We excluded data from participants who lacked serum 25(OH)D levels and liver enzyme concentrations, those who were pregnant, those younger than 18 years old or those who reported suffering from osteoporosis. The final sample included 24 229 participants. The study was approved by the National Center for Health Statistics Research Ethics Review Board.
Assessment of serum 25-hydroxyvitamin D levels
The DiaSorin RIA kit was used to measure serum 25(OH)D concentrations from the NHANES 2001–2006 at the National Center for Environmental Health. A detailed description is available on the website(14). It is noteworthy that before October 2015, there was excessive method bias and imprecision in the methods used to measure 25(OH)D levels in the NHANES. Thus, a model according to RIA quality control pool data was selected based on the idea that the results should be independent of any empirical trend in the sample participant data(15). As a consequence, the 25(OH)D data from the NHANES 2003–2004 and 2005–2006 were generally adjusted to lower and higher values, respectively(15). According to the Institute of Medicine report defining vitamin D deficiency and insufficiency in the USA(Reference Ross, Taylor and Yaktine16), the three categories of deficiency were as follows: (a) adequate vitamin D level (serum 25(OH)D ≥ 50 nmol/l), (b) insufficient vitamin D level (serum 25(OH)D ≥ 30 nmol/l but below 50 nmol/l) and (c) deficient vitamin D level (serum 25(OH)D < 30 nmol/l).
Liver enzyme measurement
Serum biochemical parameters, such as alanine aminotransferase, aspartate aminotransferase, ALP and gamma-glutamyl transaminase, were measured using a Hitachi model 704 multichannel analyser in 2001–2002. Starting in 2003, NHANES used the Beckman Synchron LX20 analyser to detect the biochemistry profile. Although two different test methods are presented in our data, the distribution of the liver enzyme levels was nearly identical in the whole study period 2001–2006. As a consequence, cut-off points were recommended in 2001–2010 to define an abnormal status of alanine aminotransferase (>47 IU/l in men or >30 IU/l in women), aspartate aminotransferase (>33 IU/l in men and women), ALP (>113 IU/l in men and women) and gamma-glutamyl transaminase (>65 IU/l in men or >36 IU/l in women).(Reference Xiao, Sinha and Graubard17)
Covariates
A wide range of sociodemographic variables was collected during the NHANES 2001–2006, such as age, gender, race and ethnicity, education and poverty-income ratio. Behavioural risk factors, such as alcohol drinking, were obtained from the questionnaire. In addition, NHANES participants reported medical conditions, including osteoporosis. BMI (body weight divided by height squared, kg/m2) was measured by trained examiners. Serum cotinine levels, a marker of smoking status, were measured from laboratory examinations. Additionally, hepatitis B infection status (infection defined as surface antigen- or core antibody-positive) and hepatitis C infection status (infection defined as antibody-positive) were obtained from laboratory examinations.
Statistical methods
To investigate the relationship between serum 25(OH)D concentrations and abnormal liver enzyme levels, we used weighted multiple variable logistic regression and listed the associations with the OR and 95 % CI, as well as the interaction between gender, BMI, alcohol consumption and the categories of serum 25(OH)D levels. The associations between serum 25(OH)D concentrations and continuous enzyme levels were assessed using weighted multiple variable linear regression models. We calculated two multivariate adjusted geometric means and 95 % CI of liver enzyme levels by categories of serum 25(OH)D levels. As liver enzyme levels are influenced by patients’ hepatitis condition, we conducted separate analyses stratified by hepatitis status. The unadjusted and two multivariate models were conducted after adjusting for model a, which encompassed age, gender, race and ethnicity, and model b, which included age, gender, race and ethnicity, education, BMI, cotinine, alcohol intake and poverty-income ratio. The highest level (adequate concentration) was used as the reference value. In addition, we performed a test for the trend of the OR to assess the association between elevated liver enzyme levels and 25(OH)D levels and 25(OH)D coded as an ordinal variable. Moreover, we present the magnitudes of association as the average percentage difference in liver enzyme levels for an interquartile (75th/25th percentiles) contrast of serum vitamin D, calculated as ((interquartile ratio^Beta)–1) × 100. Confirmed healthy population refers to those who are negative for hepatitis B and hepatitis C antibodies and those who chose ‘No’ to the questionnaire question related to ‘any liver condition’. Then, according to whether or not the individual drank, the group was further divided into a non-alcoholic healthy population and an alcoholic healthy population. The subjects who were positive for hepatitis B and hepatitis C antibodies were categorised as viral hepatitis. The above statistical analyses were performed with the Statistical Analysis Software package, version 9.2 (SAS Institute, Inc.). A P value <0·05 was designated as the cut-off for statistical significance.
Result
The final analytic population included 24 229 participants (12 487 males and 11 742 females; Fig. 1). Table 1 presents the baseline characteristics and mean concentrations of serum 25(OH)D, alanine aminotransferase, aspartate aminotransferase, ALP and gamma-glutamyl transaminase levels (adequate, inadequate and deficient status) among the participants included in the present study from the NHANES 2001–2006 database. Overall, female (59·0 %), non-Hispanic black (57·3 %) and obese (45·9 %) participants represented the greatest proportion of vitamin D-deficient individuals in the sample.

Fig. 1 Eligible participants and those included in the analyses of the associations between serum 25-hydroxyvitamin D and alkaline phosphatase (ALP) in adults. NHANES, National Health and Nutrition Examination Survey; ALT, alanine aminotransferase; AST, aspartate aminotransferase; GGT, gamma-glutamyl transaminase
Table 1 Selected characteristics of study sample by serum 25-hydroxyvitamin D (25(OH)D) category in adults, National Health and Nutrition Examination Survey (NHANES) (2001–2006)
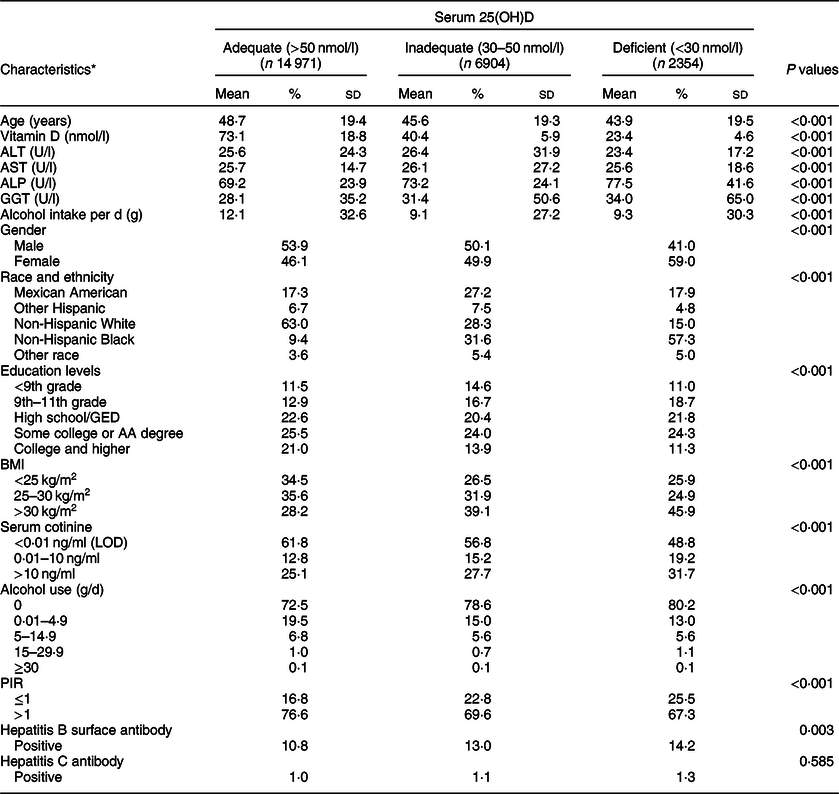
ALT, alanine aminotransferase; AST, aspartate aminotransferase; ALP, alkaline phosphatase; GGT, gamma-glutamyl transaminase; GED, General Educational Development; AA, Associate in Arts; LOD, limit of detection; PIR, poverty-income ratio.
* Weighted percentage.
Considering that osteoporosis and age may be confounding factors, we excluded people who had osteoporosis. At the same time, we present the relationship between age and ALP, and the proportion of vitamin D deficiency in the elderly population was not larger than that in the other subgroups in our study (Supplemental Fig. 1).
A scatter diagram showed an approximately inverse relationship between log-transformed 25(OH)D levels and log-transformed ALP levels (Fig. 2). We further found that lower 25(OH)D levels were associated with higher odds of abnormal ALP (Table 2), and the results were largely similar after adjusting for the two models. Compared to those with adequate 25(OH)D concentrations, those who had inadequate and deficient statuses had an OR of 1·41 (95 % CI 1·12, 1·77) and 2·67 (95 % CI 1·98, 3·59), respectively, for abnormal levels of ALP, while the other three liver enzymes were not significantly different in model b. According to a previous study, liver enzyme levels are affected by alcohol intake and hepatitis status(Reference Xiao, Sinha and Graubard17). In our study, negative results were found in both the non-alcoholic healthy population and alcoholic healthy population. In the viral hepatitis subgroup analysis, an abnormal level of ALP was inversely associated with low 25(OH)D levels in both the confirmed healthy subpopulation (ORdeficient v. adequate 3·02, 95 % CI 2·25, 4·07; P for trend < 0·001) and in the viral hepatitis subpopulation (ORinadequate v. adequate 2·13, 95 % CI 1·34, 3·39; ORdeficient v. adequate 2·87, 95 % CI 1·52, 5·44; P for trend: 0·006).

Fig. 2 Scatter plot and a fitted line with a 95 % CI of the relationship between serum 25-hydroxyvitamin D and alkaline phosphatase in males (a) and females (b)
Table 2 Adjusted associations of two models between categories of serum 25-hydroxyvitamin D and abnormal levels of ALT, AST, ALP and GGT, NHANES (2001–2006)
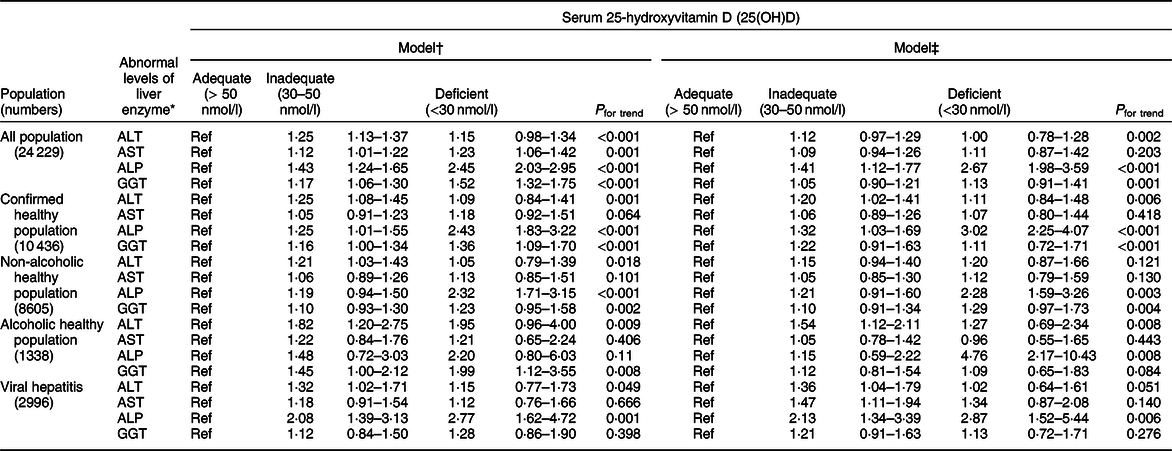
ALP, alkaline phosphatase; AST, aspartate aminotransferase; ALT, alanine aminotransferase; GGT, gamma-glutamyl transaminase; NHANES, National Health and Nutrition Examination Survey.
* Defined as >47 IU/l in men or >30 IU/l in women for ALT, >33 IU/l in men and women for AST, >113 IU/l in men and women for ALP and >65 IU/l in men or >36 IU/l in women for GGT.
† Adjusted for age (continuous), gender (male, female), race and ethnicity (Mexican American, other Hispanic, non-Hispanic White, non-Hispanic Black and other).
‡ Adjusted for age (continuous), gender (male, female), race and ethnicity (Mexican American, other Hispanic, non-Hispanic White, non-Hispanic Black and other), education (<9th grade, 9–11th grade, high school graduate/GED, some college or AA degree, college graduate or above), poverty:income ratio (≤1 and >1), BMI (<25, 25–30 and >30 kg/m2), alcohol use (0, 0·01–4·9, 5–14·9, 15–29·9 and ≥30) and cotinine level (<0·01 ng/ml (LOD), 0·01–10 ng/ml and >10 ng/ml).
For each doubling to tripling (interquartile ratio contrast) in serum vitamin D levels, the percentage difference in ALP levels in the various groups ranged from –3·8 to –5·3 % (entire sample), –3·3 to –5·1 % (confirmed healthy), –3·9 to –8·4 % (non-alcoholic healthy), –0·7 to –7·2 % (alcoholic healthy) and –3·7 to –7·4 % (viral hepatitis) (Fig. 3).

Fig. 3 For each doubling to tripling (interquartile ratio contrast) in serum vitamin D levels, the percentage difference in alkaline phosphatase (ALP) levels in the various groups
In the subgroup analysis by gender, BMI and alcohol intake, the inverse association between low serum 25(OH)D concentrations and abnormally high OR of ALP levels appeared to be somewhat stronger among those who were male, obese and drank alcohol (Supplemental Fig. 2). Similar to the interquartile ratio-contrasting serum vitamin D levels, the percentage difference in ALP levels in the sample ranged from –3·5 to –5·3 % in the different subgroup analyses (Supplemental Fig. 3). However, there were no significant interactions between serum 25(OH)D levels and gender, BMI, alcohol consumption for the ALP levels in either the logistic regression model or linear regression model.
Discussion
In this large sample of US adults, we first found that low serum 25(OH)D levels were inversely associated with a higher risk of having abnormal levels of liver enzymes, mainly ALP. Inverse associations with liver enzymes largely persisted in participants with viral hepatitis. In addition, stronger associations were found between 25(OH)D and ALP among individuals who were male, obese and drank alcohol habitually.
Among all commonly measured enzymes in the liver function tests, ALP has been less extensively studied. From a traditional perspective, serum ALP has mostly been reported to be associated with osteoporosis. Although we excluded participants with osteoporosis, age may be another influencing factor that is highly correlated with osteoporosis. However, in the present study, we found that the prevalence of vitamin D deficiency did not increase with age. Thus, age was not the main factor affecting the relationship between vitamin D and ALP in our study.
Previous epidemiological studies have shown that there is no difference in ALP levels between those with and without 25(OH)D deficiency among a Pakistan population(Reference Nadeem Saqib, Rafique and Hayder18). Similarly, results among Australians showed that there were no differences in ALP levels among different vitamin D concentration classifications. Further, there was no difference in ALP levels between the vitamin D supplement group and the placebo group(Reference Naderpoor, Mousa and de Courten19). It is worth mentioning that the sample size of the above studies was small. A meta-analysis showed that vitamin D supplementation reduces ALP levels in people with nonalcoholic fatty liver disease(Reference Mansour-Ghanaei, Pourmasoumi and Hadi20). In addition, one study showed that maternal vitamin D deficiency can cause increased ALP levels in offspring(Reference Al Alwan, Al Badi and Badri21). Jesudason et al. reported that vitamin D insufficiency is inversely related to serum ALP in postmenopausal women(Reference Jesudason, Need and Horowitz22). Our results are consistent with previous studies, but the associations are presented in general adults. Welz et al. (Reference Welz, Childs and Ibrahim23) found that HIV-infected patients who were treated with efavirenz had severe vitamin D deficiency and increased ALP levels simultaneously. Although the authors did not explain the coexistence of vitamin D deficiency and increased ALP levels, several studies have suggested that efavirenz leads to hepatotoxicity in humans(Reference Martin-Carbonero, Nunez and Gonzalez-Lahoz24,Reference Sulkowski, Thomas and Mehta25) and slight bone toxicity(Reference Cassetti, Madruga and Suleiman26). Thus, it is essential to explore the relationships between 25(OH)D levels and liver health. In general, higher ALP levels occur if the bile ducts are obstructed(Reference Kaplan and Righetti27) or if bone conditions are present(Reference Garnero and Delmas28). One of the articles showed that ALP levels were higher in consecutive patients with 25(OH)D concentrations of ≤10 nM(Reference Need, O’Loughlin and Morris29), and the authors speculated that the elevated ALP may be due to increased bone formation. In the present study, we excluded individuals with osteoporosis to some extent to eliminate confounding factors in our analysis.
The relationship and mechanism between vitamin D deficiency and ALP levels are rarely reported in mouse, rat or cell studies. However, it has been found that adding vitamin D to cells can increase ALP activity(Reference Xiong, Zhang and Xin30,Reference Ozeki, Aoki and Fukui31) . In addition, supplementation of 1α, 25(OH)D (3) could increase ALP concentrations in mice with low ALP levels(Reference Poon, Li and Seto32). More mechanism studies are needed to explore the relationship between vitamin D deficiency and ALP concentrations.
In the present study, we found strong associations between vitamin D deficiency and higher ALP levels in individuals with viral hepatitis. Petta et al. reported that chronic hepatitis C subjects had low 25(OH)D serum concentrations as well as decreased expression of cytochrome P450, family 27, subfamily A, polypeptide 1 (CYP27A1)(Reference Petta, Camma and Scazzone33). Furthermore, scientists discovered that vitamin D supplementation in patients with hepatitis C virus could improve the viral response(Reference Abu-Mouch, Fireman and Jarchovsky34). Therefore, low serum 25(OH)D levels may be associated with the occurrence of viral hepatitis. Notably, our study suggests that ALP may play an important role in hepatitis caused by vitamin D deficiency.
In our study, we found no interaction between alcohol consumption and ALP. However, alcohol has side effects on the liver. It has been found that there is a significant interaction between Mn exposure and alcohol consumption in the relationship between Mn exposure and liver function(Reference Deng, Liu and Li35). Although no interaction was observed in our results, the reason may be the small number of cases (n 38, 2·8 %) in the alcoholic healthy population; however, the number of cases of abnormal levels of ALP in other populations varies from 4·4 to 4·9 %, which may induce low statistical power for inference. This phenomenon indicates that the number of people who drink alcohol with abnormal ALP levels is less than that of other subgroups, suggesting that there is a negative association between alcohol consumption and ALP, which is supported by previous studies(Reference Tolstrup, Gronbaek and Tybjaerg-Hansen36,Reference Rex, Krarup and Laurberg37) , although other studies have found no association between alcohol and ALP levels(Reference Gluud, Andersen and Dietrichson38,Reference Shimizu, Nakazato and Sekita39) . Further studies are needed with a larger sample size to demonstrate this association.
This study has some critical strengths. A large and representative sample of the US population ensures the reliability of the results. Moreover, the inverse relationships between serum 25(OH)D levels and ALP remained after adjusting for confounders related to liver disease, including smoking, alcohol drinking, obesity and hepatitis B and C infection.
There are several limitations to our study. First, because of the cross-sectional nature of the present study, we could not determine whether 25(OH)D levels affect ALP concentrations or viceversa. Second, although we adjusted for potential covariates, residual contributing factors remain a possibility, such as genetic predisposition.
Conclusions
In summary, we found evidence of an inverse association between serum vitamin D and serum ALP levels in the US general population, and the correlation was strong among individuals who had viral hepatitis. These findings suggest the need for an ongoing evaluation of the possible protective role of vitamin D supplementation for liver function. Future studies are needed to confirm these findings and to explore the potential mechanisms.
Acknowledgements
Acknowledgements: N/A. Financial support: N/A. Conflict of interest: None declared. Authorship: J.T., C.X. and Y.J.S. contributed to the conception and design of the study. C.X. performed the analyses and drafted the manuscript. The major contribution for writing of the manuscript was from X.W.H. and C.X. Y.J.S., Z.H.L. and X.Z.F. helped in drafting the manuscript. All authors approved the final manuscript. Ethics of human subject participation: This study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving research study participants were approved by the National Center for Health Statistics Research Ethics Review Board. Written informed consent was obtained from all subjects.
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S1368980020000348







