Iodine-deficiency disorders (IDD) have a negative effect on a country’s overall health and development potential( Reference Zimmerman 1 ). The identification that IDD is a major public health problem throughout the world has led to a global acceleration of efforts to combat IDD( 2 ). A meta-analysis of nineteen studies conducted in severely iodine-deficient areas showed that iodine deficiency is associated with a mean intelligence quotient loss of 13·5 points in the population( Reference Bleichrodt and Born 3 ). The most significant effects are degrees of mental impairment leading to poor school performance, reduced intellectual ability and impaired work capacity( Reference Stanbury 4 ). Iodine deficiency is the greatest cause of preventable brain damage in childhood, which is the motivation behind the current worldwide drive for elimination( 5 ).
The Aseer region, with a population of 1 200 000, covers more than 80 000 km2 in south-western Saudi Arabia. Sharing its border with Yemen, the area extends from the high Aseer Mountains, almost 3200 m above sea level, down to the Red Sea. In 1995, a national epidemiological survey of schoolchildren for IDD( Reference Al-Nuaim, Al-Mazrou and Kamel 6 ) reported a moderate degree of IDD in all provinces of Saudi Arabia with relatively higher figures for the Aseer region. Forty-five per cent of schoolchildren having a urinary iodine concentration (UIC) of less than 10 μg/dl were found in the Aseer region. The study also reported an overall total goitre rate (TGR) of 30 %. Based upon the WHO criteria for endemicity of goitre, the national survey considered the Aseer region as an endemic area of goitre. The contributing factors for IDD are poor iodine content of soil and limited consumption of food items with adequate iodine content( 7 ).
By the end of 1996, sixteen countries in the Eastern Mediterranean Region had identified IDD as a public health problem and decided to start universal salt iodization (USI)( 8 ). An epidemiological study in the Aseer region in 2000( Reference Abu-Eshy, Abolfotouh and Al-Naggar 9 ) revealed an overall prevalence of goitre of 24 % among elementary-school students. The prevalence was significantly higher in high-altitude areas (27 %) than in low-altitude areas (13 %).
In view of the improved iodine status of children in other countries after the introduction of USI programmes( Reference Kavishe 10 ), it is important to investigate the effectiveness of introducing iodization of salt in the Aseer region. Iodine has a rapid turnover in the body and 2 years of exposure to iodized salt is sufficient to assess the effectiveness of salt exposure( Reference Jooste, Weight and Lombard 11 ).
Based on these factors, we believe that an updated, comprehensive and meticulous study of IDD in the Aseer region is essential in overcoming these preventable disorders and their effects. The objectives of the current work were to study the current prevalence of IDD and their environmental determinants in the Aseer region.
Materials and methods
Design
The study was a cross-sectional study on a representative sample of schoolchildren in the Aseer region, south-western Saudi Arabia.
Target population
Children of the age category 8–10 years living in the Aseer region were the target group for screening for IDD because of their high vulnerability( Reference Bagchi 12 ). WHO recommends the inclusion of an additional community-based sample if the proportion of children attending schools is less than 50 %( 5 ). In Saudi Arabia, school enrolment is above 90 %. Therefore, there was no need to include samples from outside school settings.
Sampling and field activities
Using the WHO manual Sample Size Determination in Health Studies ( Reference Lwanga and Lemeshow 13 ), at 95 % confidence interval with a conservative estimate of the anticipated population proportion of 45 %( Reference Al-Nuaim, Al-Mazrou and Kamel 6 ), and with an absolute precision of 2 %, the minimal sample size required for the study was calculated to be 2377 children. To compensate for a possible loss of cases, a total sample of 3000 children was planned to be included in the study.
A stratified proportional allocation sample of schools was selected taking into consideration sex, the relative size of the population in each district, rural/urban differences, altitude and social differences (governmental and private).
Each of the chosen schools was visited twice. During the first visit two classes (including students aged 8–10 years) from each selected school were randomly chosen. Confidential letters were sent to their parents explaining the purpose of the study and asking for their written consent. Children lacking parental consents were not included. The letters also asked parents to bring with their children a sample of table salt used in their home. Similarly, the letter included a simple questionnaire to be filled by parents regarding sociodemographic conditions of their children. Two days later, the school was revisited for field activities.
Measurement of location parameters
In each school altitude, latitude and longitude were measured using a global positioning system (GPS Magellan model; Meridian Color, USA).
Clinical goitre
Clinical examination of the thyroid gland was carried out through inspection and palpation techniques. Grading was based on the criteria endorsed by the WHO( 2 ) as follows: grade 0=no palpable or visible goitre; grade 1=a mass in the neck that is consistent with an enlarged thyroid that is palpable, but not visible when the neck is in the neutral position. It also moves up in the neck on swallowing; grade 2=a swelling in the neck that is visible when the neck is in a neutral position and is consistent with an enlarged thyroid when the neck is palpated. The TGR is the sum of these two grades (1+2). To avoid inter-observer variation, intensive training and standardized procedures were adopted. At the beginning of work in each school a group of children (not included in the present study) was examined independently by field physicians. Grading was recorded independently by each physician. Screening of target children started if more than 95 % agreement was observed. The schoolchildren suffering from goitre in the present survey were referred through school health authorities to Ministry of Health facilities. The objective was to assess the thyroid function, treat them and follow them closely to prevent further neurodevelopment delay.
Thyroid ultrasonography
Ultrasound scanning was carried out using a portable ultrasound machine (LOGIC Book XP; General Electric, Jiangsu, China) with a standard 5·0 MHz transducer (GE 8 L-RS ultrasound probe). Longitudinal and transverse scans were performed, allowing the measurement of the thickness, width and length of each lobe. The volume (V) of each lobe was calculated by the WHO formula( Reference Zimmermann, Hess and Molinari 14 ): V (ml)=0·000479×length (mm)×width (mm)×thickness (mm).
Thyroid volume was calculated as the sum of the volumes of both lobes. Thyroid glands were classified as normal or enlarged using the revised criteria published in 2004 (thyroid volume for age). Thyroid volumes greater than the 97th percentile values were considered abnormally large and those less than or equal to the 97th percentile values were regarded as normal( Reference Zimmermann, Hess and Molinari 14 ).
Measurement of urinary iodine concentration
Casual urine samples were obtained from each child. The samples were analysed for iodine concentration using ammonium persulfate to digest the samples, followed by the Sandel–Kolthoff reaction( Reference Sullivan, May and Maberly 15 ). WHO criteria( 2 ) for assessing the severity of iodine nutrition status and which are based on the median UIC were used.
Iodine content in water
Samples of water used for drinking were taken from each school. The samples were taken from public desalinated tap water of water tanks filled regularly by school authorities. The same method as used for UIC was followed for water analysis. The sample volume was raised from 200 μl to 500 μl. The technique mentioned by WHO( 2 ) was followed.
Iodine content in table salt
The family of each child was asked to provide a spoonful of table salt used in their kitchen. The salt samples were brought in standard, small, self-sealed plastic bags. The technique mentioned by WHO( 2 ) for salt was followed.
Quality control of iodine measurements
A precision study for urine and water was done using two samples with different iodide levels analysed twenty times in duplicate in a single batch (intra-assay) and on different days (inter-assay). The CV values (which ranged from 5 % to 7 %) were well within the acceptable limits, indicating good intra- and inter-assay precision. A recovery study was carried out to assess the accuracy. The recovery was 97 % with a range from 92·3 % to 102·8 %. Similarly, precision and accuracy studies were done for salt and similar results were obtained.
Data analysis
Data were coded, validated and analysed using the statistical software package IBM SPSS Statistics 22. Frequency, percentage, arithmetic mean, median, standard deviation and 95 % confidence interval were used to present the data. Appropriate tests of significance (χ 2, Student’s t, F and Mann–Whitney U tests) were applied wherever necessary at the 5 % level of significance. To measure agreement between clinical and ultrasound results, Cohen’s κ was used.
Results
The present study included 3046 school-age children (1501 boys and 1545 girls). The age of the study sample ranged from 8 to 10 years with a mean of 8·77 (sd 0·67) years and a median of 9 years.
Location parameters
The altitude of the study sample of schools ranged from 370 m (1213 ft) to 2735 m (8974 ft) above sea level, with a median of 2225 m (7301 ft) and a mean of 2068 (sd 465) m (6786 (sd 1526) ft). Similar to the Aseer region, all schools of the study sample were above sea level. Ten per cent of the studied schools were at low altitude levels ranging from 305 m (1000 ft) to less than 1524 m (5000 ft), 20 % of schools were at middle altitude levels ranging from 1524 m (5000 ft) to less than 2134 m (7000 ft), and the rest of the schools were at high altitude levels of more than 2134 m (7000 ft).
Overall clinical goitre rate
The study showed that 18·5 % (n 563) of the study sample had grade 1 goitre and 5·5 % (n 167) had grade 2 goitre. The TGR amounted to 24·0 %.
Goitre rate and gender
Figure 1 and Table 1 show that 18·2 % (n 273) of boys had grade 1 goitre and 5·3 % (n 80) of boys had grade 2 goitre. The TGR among boys amounted to 23·5 %. Similarly, 18·8 % (n 290) of the girls had grade 1 goitre and 5·6 % (n 87) had grade 2 goitre. The TGR among girls amounted to 24·4 %. The gender difference was not statistically significant (χ 2=0·344, P=0·842).
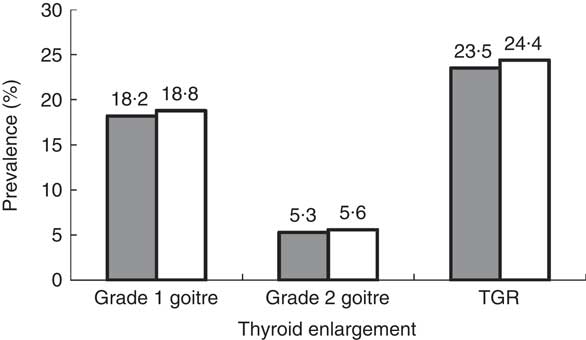
Fig. 1 Prevalence of thyroid enlargement by gender (![]() , boys;
, boys; ![]() , girls) among schoolchildren (n 3046) aged 8–10 years, Aseer region, south-western Saudi Arabia (TGR, total goitre rate)
, girls) among schoolchildren (n 3046) aged 8–10 years, Aseer region, south-western Saudi Arabia (TGR, total goitre rate)
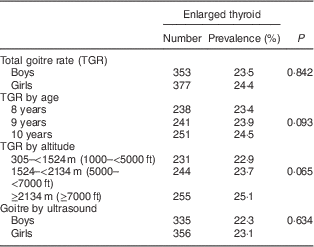
Table 1 Prevalence of enlarged thyroid among schoolchildren (n 3046) aged 8–10 years, Aseer region, south-western Saudi Arabia
Goitre rate and age
The TGR increased from 23·4 % among children aged 8 years to 23·9 % among those aged 9 years. The TGR reached 24·5 % among children aged 10 years. Yet, the age difference was not statistically significant (χ 2=4·72, P=0·093; Table 1).
Goitre rate and altitude
The TGR increased from 22·9 % among schoolchildren living at an altitude of 305–<1524 m (1000–<5000 ft) to reach 25·1 % among those living at an altitude of ≥2134 m (≥7000 ft). Yet, the difference was not statistically significant (χ 2=5·745, P=0·065; Table 1).
Ultrasound thyroid enlargement rate
The thyroid volume of the study sample ranged from 0·9 ml to 5·3 ml with a mean of 3·2 (sd 1·7) ml and a median of 2·4 ml. Using age- and sex-specific 97th percentiles of thyroid volume measured by ultrasound as cut-off points( Reference Zimmermann, Hess and Molinari 14 ), enlarged thyroid states were identified. The study showed that 22·3 % (n 335) of boys and 23·1 % (n 356) of girls had enlarged thyroid volume by ultrasound. The overall prevalence of thyroid enlargement by ultrasound amounted to 22·7 %. The difference between boys and girls was not statistically significant (χ 2=0·227, P=0·634).
Concordance of clinical and ultrasound examination of goitre
Table 2 shows a strong significant agreement between clinical examination of the thyroid and ultrasound examination results (Cohen’s κ=0. 93, 95 % CI 0·91, 0·94; P=0·001).
Table 2 Concordance of clinical and ultrasound examination of the thyroid gland among schoolchildren (n 3046) aged 8–10 years, Aseer region, south-western Saudi Arabia

κ=0·93 (95 % CI 0·91, 0·94), P=0·001.
Urinary iodine concentration
The UIC of the study sample of schoolchildren ranged from 0·3 to 96 µg/l with a mean 19·03 (sd 11·1) µg/l. The median UIC of the study sample of schoolchildren amounted to 17·0 µg/l. The study showed that more than half of the children (n 1714, 56·3 %) had UIC of less than 20 µg/l. Results showed that none of the study sample of school-age children had UIC of more than 100 µg/l.
Median urinary iodine concentration by gender
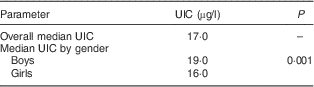
The median UIC of girls (16·0 µg/l) was less than the median UIC of boys (19·0 µg/l). The difference was statistically significant (Mann–Whitney U test: z value=6·109, P=0·001; Table 3).
Table 3 Meadian urinary iodine content (UIC) among schoolchildren (n 3046) aged 8–10 years, Aseer region, south-western Saudi Arabia
Iodine content of table salt samples used in students’ houses
It was observed that some table salt samples collected from students were yellowish coarse granules (similar to rock salt). The rest of the samples were white and fine. The level of iodine content of table salt samples ranged from 0 to 112 mg/kg with a mean 47·8 (sd 27·9) mg/kg and a median of 55 mg/kg. Figure 2 shows the distribution of samples of table salt by iodine content. Thirty-three samples (1·1 %) showed iodine content of 0 mg/kg, 652 samples (21·4 %) showed iodine content of 0·1–14·9 mg/kg. The results indicated that 22·5 % of the samples of table salt were below the recommended iodine content (15–40 mg/kg) set by WHO.
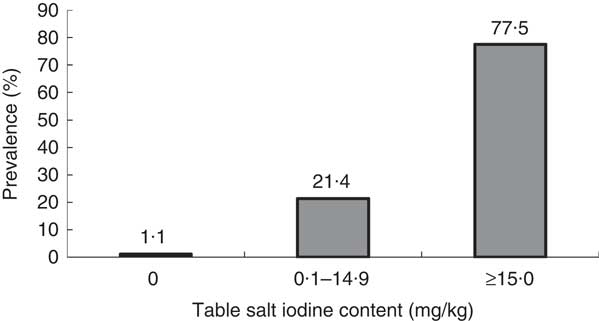
Fig. 2 Distribution of iodine content of table salt in samples from the homes of schoolchildren (n 3046) aged 8–10 years, Aseer region, south-western Saudi Arabia
Total goitre rate by iodine content of table salt
Figure 3 shows that the TGR increased from 19·8 % among children using table salt with iodine content ≥15 mg/kg to reach 48·5 % among children using table salt of zero iodine content. The difference was statistically significant (χ 2=102·8, P=0·001).
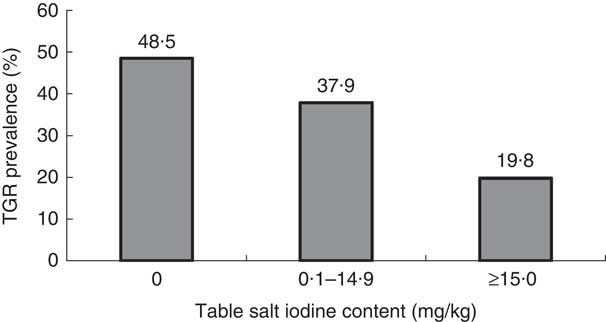
Fig. 3 Prevalence of total goitre rate (TGR) by iodine content of table salt in samples from the homes of schoolchildren (n 3046) aged 8–10 years, Aseer region, south-western Saudi Arabia
Salt iodine content and median urinary iodine concentration
The median UIC of children using table salt with iodine content <15 mg/kg (13 µg/l) was less than that of children using table salt with ≥15 mg iodine/kg (19 µg/l). The difference was statistically significant (Mann–Whitney U test: z value=10·988, P=0·001). Children using table salt with <15 mg iodine/kg were at significantly more risk to have UIC<20 µg/l compared with children using table salt having iodine content ≥15 mg/kg (OR=2·136, 95 % CI 1·779, 2·566).
Iodine content of drinking water
Iodine content ranged from 0 to 12 µg/l with a mean of 1·45 (sd 3·25) µg/l and a median of 0 µg/l. The majority of water samples (78·8 %) showed iodine content of 0 µg/l.
Discussion
Iodine deficiency is the major preventable cause of brain damage and mental retardation in childhood( 5 ). The results of the present study among schoolchildren aged 8–10 years in the Aseer region of Saudi Arabia showed a TGR prevalence of 24·0 % by clinical examination and a 22·7 % prevalence of enlarged thyroid by ultrasound examination. The study revealed IDD to be a moderate public health problem (rate ranging between 20 % and <30 %) by WHO criteria( 2 ). The national Saudi survey in 1995 reported a goitre prevalence rate of 30 % in the Aseer area. On the other hand, a recent study in 2012 reported a goitre prevalence rate in Jazan (a coastal south-western region of Saudi Arabia) of 11 %( Reference Alsanosy, Gaffar and Khalafalla 16 ). The present study showed that 18 years after the national Saudi study, the IDD problem is still endemic in the Aseer region.
The TGR among females in the present study was not significantly different from that in males. On the other hand, the median UIC of the girls was significantly lower than the median UIC of boys. Several studies have reported inconsistent findings for the effect of gender on goitre prevalence. Although some authors found no differences in goitre prevalence between genders( Reference Chanoine, Toppet and Lagasse 17 ), larger thyroid glands in females have been reported by others( Reference Azizi, Malik and Bebars 18 , Reference Colak, Ozkan and Kececi 19 ). A recent meta-analysis study concluded that goitre is more frequent in females and that the gender difference in goitre prevalence is more prominent in iodine-deficient areas, and with grade 2 of goitre, notably after puberty( Reference Malboosbaf, Hosseinpanah and Mojarrad 20 ). Our figures showed no gender difference. Considering that this sex difference exists only after puberty, sex steroids may play a role in volume of the thyroid. An animal study showed that testosterone decreased thyroid enlargement and serum free thryroxine levels in iodine-deficient castrated rats, which may explain the lower incidence of goitre in men than women in iodine-deficient regions( Reference Bahrami, Hedayati and Taghikhani 21 ).
Altitude and goitre have been addressed by many researchers( Reference Al-Nuaim, Al-Mazrou and Kamel 6 , Reference Abu-Eshy, Abolfotouh and Al-Naggar 9 , Reference Abuye, Berhane and Ersumo 22 ). Iodine, usually found in the top layer of the soil, can easily be leached away by erosion mainly in high-altitude and high-gradient areas. If not conserved, the topsoils at high altitudes are more likely to be exposed to erosion than those in low lands. The crops grown in iodine-deficient soils are poor in iodine content. Hence the leaching away of iodine in the top layer of the soil may contribute to the higher prevalence of goitre at high altitudes than in lowland areas. The high prevalence of IDD and the non-significant altitude difference found in the present study may be explained by the fact that the entire Aseer region is considered a high-altitude area.
The present study showed a strong significant agreement between clinical examination of the thyroid and ultrasound examination results. A study comparing different indicators of IDD among schoolchildren from study sites in Bangladesh, Indonesia, Guatemala and the USA reported similar results, and concluded that palpation and thyroid volume by ultrasound are similarly consistent in identifying IDD( Reference Sullivan, May and Maberly 15 ).
As approximately 90 % of absorbed iodine is eventually excreted in the urine( Reference Cavalieri 23 ), UIC is a good indicator of changes in dietary iodine intake over preceding days or weeks. The UIC is a biomarker of recent iodine intake. UIC is the recommended indicator for assessing iodine status in populations. Although UIC data do not provide direct information on thyroid function, a low value suggests a population is at higher risk of developing thyroid disorders( 2 ). The median UIC (17·0 µg/l) of the present study sample revealed a severe iodine deficiency situation( 2 ) among schoolchildren in the Aseer region. The present results showed an increase in median UIC compared with the previous national figure( Reference Al-Nuaim, Al-Mazrou and Kamel 6 ) for the Aseer region (11·0 µg/l). Yet, the current figure is still denoting severe iodine deficiency status (<20·0 µg/l). The median UIC of the present study reveals minor progress, if any, in eliminating IDD in the Aseer region. Similar results were observed in Isfahan, Iran( Reference Aminorroaya, Amini and Hovsepian 24 ). After 15 years of successful USI in Isfahan, goitre was still endemic (TGR of 19 % and median UIC of 18 µg/l). The authors stated that this endemicity may be due to thyroid autoimmunity and other environmental or genetic factors( Reference Aminorroaya, Amini and Hovsepian 24 ).
The results of the present study showed that the majority of drinking water samples taken from desalinated tap water of water tanks in schools had an iodine content of 0 ppm. Water for consumption in Aseer region is provided mainly by desalination plants in Shagig. The process of desalination entails removing materials and soluble ions including iodine.
Since people universally consume salt in small, fairly constant amounts daily, it is an ideal vehicle to deliver physiological amounts of micronutrients like iodine to the population at large. The concept of salt fortification is not new. Iodization of salt has been practised successfully in several countries for over 60 years. USI is a strategy to ensure sufficient intake of iodine by all individuals( 25 ). Although USI started a long time ago in Saudi Arabia, the availability of non-iodized rock salt in the markets of the Aseer region, which is very cheap compared with manufactured iodized salt and is used by local communities, has hindered the progress of IDD elimination in the region. In the present study, researchers asked children and their parents to bring a spoonful of table salt used in their houses. No attempt was made by researchers to identify type and manufacturer of the salt. The results of the present study indicated that 22·5 % of the study sample used table salt with iodine content below 15 mg/kg, the recommended level set by WHO( 2 ). The TGR increased significantly from 19·8 % among children using table salt with ≥15 mg iodine/kg to reach 48·5 % among children using table salt with zero iodine content. The high TGR (19·8 %) in the children from households using adequately iodized salt cannot be the residual effect of previous deficiency since USI was introduced more than a decade ago.
The low iodine content of table salt observed in the present study can be attributed to many factors. First, it seems that local people are still using table salt of rock origin available in the local market, which is very cheap but not iodized at all. Second, cooking, processing and storing conditions (in humid, very hot and sunny conditions) play an important role( Reference Comandini, Cerretani and Rinaldi 26 ). Different cooking methods, utensils and cooking conditions affect the stability of iodine in iodized salt. Garlic, fresh chili, pepper and green curry paste cause high loss of iodine( Reference Chavasit, Malaivongse and Judprasong 27 ).
Conclusion
The results of the present study showed that 18 years after the Saudi national study, and after more than a decade of USI in Saudi Arabia, the IDD problem is still endemic among schoolchildren in the Aseer region. To address this problem, recommendations should focus on fostering advocacy and communication and ensuring the availability of adequately iodized salt.
Advocacy and communication play an essential role in strategies to eliminate iodine deficiency through encouraging and teaching people at all levels about the importance of iodine and iodized salt. Successful communication efforts need to reach out to specific audiences, including community leaders, the media, teachers, the general public and parents of schoolchildren.
Universal access to iodized salt is mandatory for IDD elimination. The availability and easy accessibility of iodized salt can be achieved through marketing and sales of iodized salt must reach geographically and socially isolated communities in the Aseer region. Municipalities in the Aseer region should play an active role in banning non-iodized salt in the local markets in the Aseer region.
Acknowledgements
Acknowledgements: The investigators wish to sincerely express their thanks and gratitude to King Khalid University for the support during the study. Thanks go to the Director General of Education of the Aseer region, and the staff of boys’ and girls’ school health units in Abha, Ahad Rufeida, Sarat Ebeida and Muhayeel. The full cooperation and support of the study schools directors and teachers are highly appreciated. The meticulous and skilful technical and administrative assistance of Mr Riyad Eisa and Mr Allan Agaton are highly appreciated. Financial support: This study was funded by King Abdulaziz City for Science and Technology, Saudi Arabia (grant number APR-29-42). The funders approved the design, analysis and reporting of the project. The authors alone are responsible for the content and writing of the paper. Conflict of interest: None. Authorship: F.I.A., S.A.A.-E. and A.A.M. revised the literature and designed the study. S.A.A.-E., S.A.A.-F. and A.A.M. carried out the data collection and clinical examinations. S.M.A. and H.E.-W. carried out the micro-environmental data collection. M.G.M. carried out the ultrasound examinations. C.S.D. and A.P. carried out the laboratory analyses. A.A.M. drafted the manuscript. All authors contributed to the data interpretation and critical revision of the manuscript. Ethics of human subject participation: The study was conducted in accordance with ethical standards and approved by King Khalid University Ethical Committee.









