Breast-feeding provides numerous immunological, psychological, social, economic and environmental benefits, is a natural first food and an ideal nutrition for the infant( Reference Dewey, Heinig and Nommsen 1 ). Therefore the WHO expert consultation recommends full breast-feeding for 6 months, with introduction of complementary foods and continued breast-feeding thereafter( 2 ). Nevertheless, the prevalence of exclusive breast-feeding for 6 months is low in most countries( 3 ) and various studies worldwide are aiming to identify predictors of short exclusive breast-feeding duration( Reference Scott and Binns 4 ). Both maternal type 1 diabetes (T1D) and gestational diabetes mellitus (GDM) have been associated with shorter breast-feeding duration( Reference Hummel, Winkler and Schoen 5 – Reference Sparud-Lundin, Wennergren and Elfvin 7 ). This may be explained by factors that are associated with maternal diabetes, such as the increased frequency of caesarean sections and pre-term delivery( Reference Sorkio, Cuthbertson and Bärlund 8 ). However, there have also been studies that did not find an association between maternal diabetes and breast-feeding duration( Reference Webster, Moore and McMullan 9 , Reference Stage, Norgard and Damm 10 ).
Little is known on the effect of maternal diabetes and a T1D family history on the timing of introduction of complementary food. Findings from the German BABYDIET study indicated that offspring of mothers with T1D are introduced to complementary foods earlier than offspring of fathers and/or siblings with T1D( Reference Pflüger, Winkler and Hummel 11 ).
In addition to the beneficial effects of exclusive breast-feeding on maternal and offspring health mentioned above, prospective studies in children at increased risk for T1D have suggested that the timing of initial exposure to complementary foods may influence the risk of islet autoimmunity and T1D. Among candidate risk factors for islet autoimmunity are early introduction to cow's milk and to solid foods such as gluten-containing cereals, fruits/berries and roots( Reference Norris, Barriga and Klingensmith 12 – Reference Kimpimäki, Erkkola and Korhonen 15 ). Based on these findings it is important to identify determinants of infant feeding patterns in children at increased risk for T1D.
Therefore the aim of the current analysis was to assess the association of maternal T1D or GDM and non-maternal T1D in the family with infant feeding patterns. The patterns of interest in the present study were duration of exclusive and any breast-feeding and introduction ages of cow's milk and gluten-containing cereals (wheat, rye or barley). Breast-feeding duration and timing of introduction of complementary foods are strongly affected by country-specific socio-cultural factors and dietary guidelines. The TEDDY (The Environmental Determinants of Diabetes in the Young) study, which is an international, multicentre birth cohort study with standardized recruitment, dietary collection methodologies and analytical approaches, offers the opportunity to stratify for country( 16 ).
Patients and methods
Information on early infant feeding practices was obtained for 7026 children participating in the prospective TEDDY birth cohort study, a multicentre study comprising six clinical centres located in the USA and Sweden, Finland and Germany with the aim of identifying environmental factors that may trigger islet autoimmunity and T1D in children at increased genetic risk for T1D( 16 ). All of the children in TEDDY who were born between 2004 and 2010 and were followed for at least 1 year from 3 months of age (n 7540) were included. Of the children, 514 were excluded from the analysis because of missing data on diabetes status of the mother (n 257), multiple births (n 252) and pre-existing type 2 diabetes in the mother (n 5), resulting in a total of 7026 children in the analysis. Of the 7026 children, 292 (4·2 %) had a mother with T1D, 404 (5·8 %) had a mother with GDM, 464 (6·6 %) had a mother without T1D but a father and/or sibling with T1D and 5866 (83·4 %) had no diabetes family history. Due to the small numbers, offspring with first-degree relatives with type 2 diabetes were not included in the analysis.
To assess the duration of breast-feeding and the age at introduction of new foods, families were asked to record the age at introduction of all new foods in a specific booklet that was given to the parents at study entry (TEDDY book). The TEDDY book also included information on the use of infant formulas. These records were reviewed at all clinical visits (at 3, 6, 9, 12, 18 and 24 months of age). The definition of exclusive breast-feeding included small amounts of non-nutritious drinks such as tea, water and water-based drinks, and nutritional supplements. To assess age at introduction of cow's milk, cow's milk-based infant formulas as well as partially hydrolysed infant formulas were included but extensively hydrolysed infant formulas were excluded. This definition was based on the hypothesis that cow's milk proteins may trigger islet autoimmunity( Reference Knip, Virtanen and Seppä 17 ). The TEDDY study did not provide any recommendations or advice on infant feeding to the families.
Data on Apgar score at 5 min (categorized as ≥9 or <9), mode of delivery (categorized as normal vaginal, caesarean section, vaginal including instruments), gestational age, birth weight, birth order (categorized as first child yes or no), maternal BMI before pregnancy (reported by the mothers), maternal age at delivery, maternal education (categorized as high school or less or more than high school) and maternal smoking during pregnancy (categorized as smoking or non-smoking) were obtained by either questionnaires or structured interviews during one of the follow-up visits in the first year of the study.
Written informed consent was obtained from the parents. The TEDDY study was approved by the ethical review board of each site.
Data analysis
Data were analysed using the statistical software package SAS version 9·2. Categorical variables were analysed using Pearson's χ 2 test or Fisher's exact test. Continuous variables were tested using the t test for differences in means or the Wilcoxon rank-sum test for differences in medians. Mean differences were tested using ANOVA. Data were summarized using mean and standard deviation or median and interquartile range (IQR). All tests for significance were two-tailed. Kaplan–Meier life tables were used to describe age at end of breast-feeding and age at introduction of cow's milk products and gluten-containing foods; and groups were compared using the log-rank χ 2 statistic. Cox proportional hazard regression analysis was used to assess whether specific types of diabetes in the family were associated with infant feeding behaviours. The age of the child at the time when exclusive or any breast-feeding was ended or when cow's milk/gluten-containing foods were introduced was used as the time to event. We examined models with and without clinical factors (i.e. delivery mode, gestational age, Apgar score and birth weight) to determine whether they explained these associations.
Results
Characteristics of the study cohort
The proportion of infants with a 5 min APGAR score ≥9, with normal vaginal delivery and who were first-born children differed by the presence of diabetes in the family member (Table 1). Also, there were differences in gestational age, birth weight, maternal BMI before pregnancy, maternal weight gain during pregnancy, maternal age at delivery and maternal education by the presence of diabetes in a family member (Table 1).
Table 1 Characteristics of the children by presence of diabetes in the family: The Environmental Determinants of Diabetes in the Young (TEDDY) birth cohort
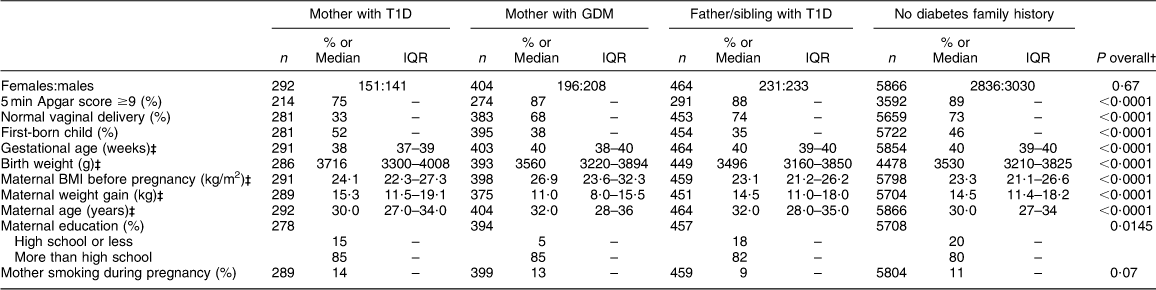
T1D, type 1 diabetes; GDM, gestational diabetes mellitus; IQR, interquartile range.
†P value for test across all four diabetes family history groups.
‡Data are presented as median and IQR.
Association of diabetes in a family member with breast-feeding behaviour
The initiation of breast-feeding was comparable between groups: 96 % of infants with T1D mothers, 98 % of infants with a father or sibling with T1D, 97 % of infants with GDM mothers and 98 % of infants without diabetes in the family were breast-fed during the first days of life. Univariately, exclusive breast-feeding duration was significantly shorter in offspring of mothers with T1D and GDM compared with children without diabetes in the family (P < 0·003, Fig. 1(a); median 0 month, IQR 0–1 months and median 0·2 months, IQR 0–1·8 months v. median 0·7 months, IQR 0–3·2 months). In contrast, exclusive breast-feeding duration was significantly longer in children with a T1D father or sibling (median 0·9 months, IQR 0–4 months) compared with children without diabetes in the family (Fig. 1(a)).
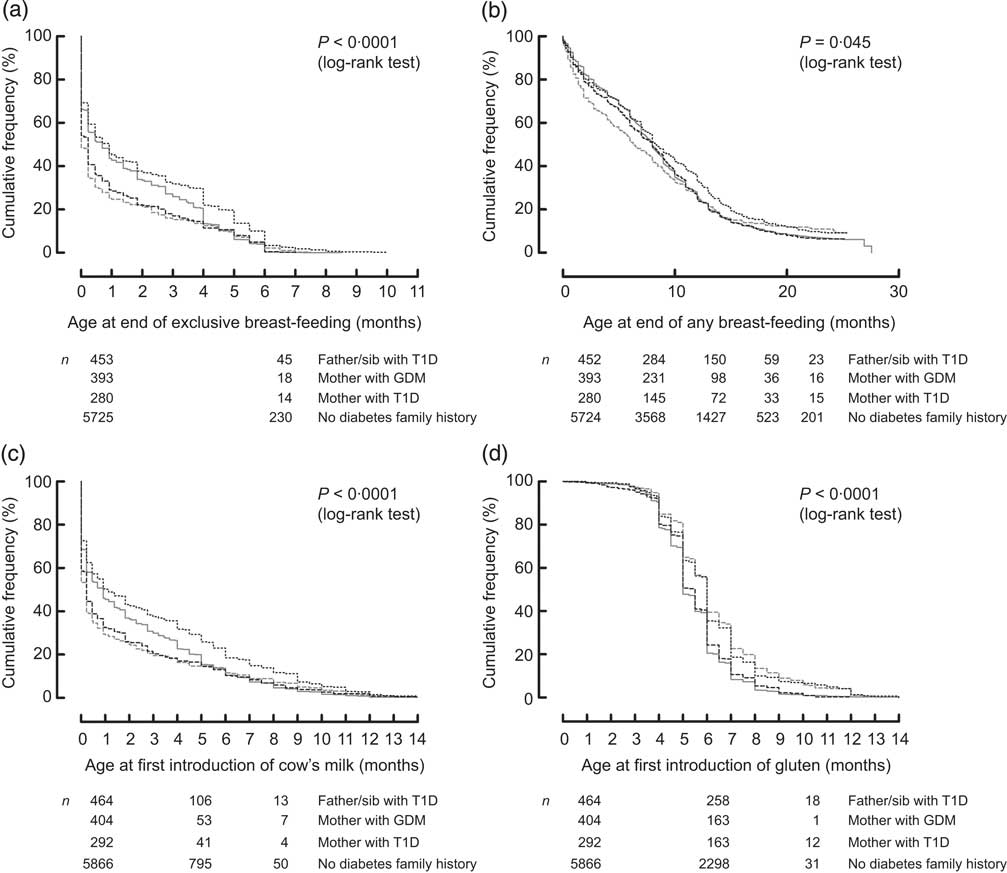
Fig. 1 Life-table analysis of age at end of exclusive breast-feeding (a), age at end of any breast-feeding (b), age at first introduction of cow's milk (c) and age at first introduction of gluten-containing cereals (d) in relation to the presence of diabetes in the family (![]() , father/sib with T1D;
, father/sib with T1D; ![]() , mother with GDM;
, mother with GDM; ![]() , mother with T1D;
, mother with T1D; ![]() , no diabetes family history), all participating countries: The Environmental Determinants of Diabetes in the Young (TEDDY) study (sib, sibling; T1D, type 1 diabetes; GDM, gestational diabetes mellitus)
, no diabetes family history), all participating countries: The Environmental Determinants of Diabetes in the Young (TEDDY) study (sib, sibling; T1D, type 1 diabetes; GDM, gestational diabetes mellitus)
The association between age at exclusive breast-feeding end and maternal T1D (hazard ratio (HR) = 1·18; 95 % CI 1·04, 1·33), maternal GDM (HR = 1·13; 95 % CI 1·01, 1·26) and T1D in the father and/or sibling (HR = 0·82; 95 % CI 0·74, 0·91) remained significant after adjusting for maternal smoking during pregnancy, maternal pre-pregnancy BMI, maternal weight gain during pregnancy, child's gender, maternal age, child's birth order, maternal education level and country (Model 1, Table 2). T1D in the father and/or sibling was significantly associated with later end of exclusive breast-feeding after additional adjustment for clinical factors (Model 2; HR = 0·81; 95 % CI 0·71, 0·92). The association between age at end of exclusive breast-feeding and maternal T1D and GDM was no longer significant after additional adjustment for clinical factors (Model 2, Table 2).
Table 2 Infant feeding patterns in relation to the presence of diabetes in the family, all participating countries: The Environmental Determinants of Diabetes in the Young (TEDDY) study
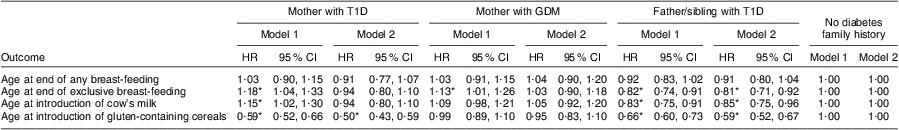
T1D, type 1 diabetes; GDM, gestational diabetes mellitus; HR, hazard ratio.
Model 1: adjusted for mother's smoking status, mother's pre-pregnancy BMI, mother's weight gain during pregnancy, child's gender, maternal age, birth order, country and mother's education level.
Model 2: Model 1 plus adjustment for delivery mode, gestational age, Apgar sore and birth weight.
Introduction of cow's milk is defined as cow's milk including cow's milk-based infant formulas as well as partially hydrolysed infant formulas and excluding extensively hydrolysed infant formulas.
*Significant at the P < 0·05 level.
Median age at end of any breast-feeding was 6·3 months (IQR 1·8–12·1 months) in infants with mothers with T1D, 8·0 months (IQR 2·8–12·0 months) in infants with mothers with GDM, 8·0 months (IQR 3·8–12·0 months) in children with a T1D father and/or sibling and 8·3 months (IQR 3·5–13·4 months) in children without diabetes in the family (Fig. 1(b)). There were no statistically significant differences in age at end of any breast-feeding with respect to the presence of diabetes in the family after adjusting for sociodemographic (Model 1) and clinical factors (Model 2, Table 2).
Because the feeding patterns varied from country to country, we examined whether the association between the presence of diabetes in the family and feeding behaviour differed by country by testing for interaction. The association between age at end of exclusive breast-feeding and presence of diabetes in the family was clearly different in different TEDDY countries (interaction P = 0·04, Fig. 2). In order to explore the age at end of exclusive breast-feeding by country interaction further, multivariate models were analysed separately for the USA, Finland, Germany and Sweden (Table 3). A strong association of maternal T1D and GDM with younger age at exclusive breast-feeding end was observed in Sweden (Table 3). In the USA, exclusive and any breast-feeding end was significantly earlier in offspring of mothers with T1D (Table 3) and exclusive breast-feeding end was earlier in offspring of mothers with GDM compared with infants without presence of diabetes in the family, while exclusive breast-feeding end was later in offspring with a father and/or sibling with T1D. These associations were attenuated by adjusting for clinical factors (Table 3). In Germany and Finland, age at end of exclusive or any breast-feeding was not associated with the presence of diabetes in the family (Table 3).
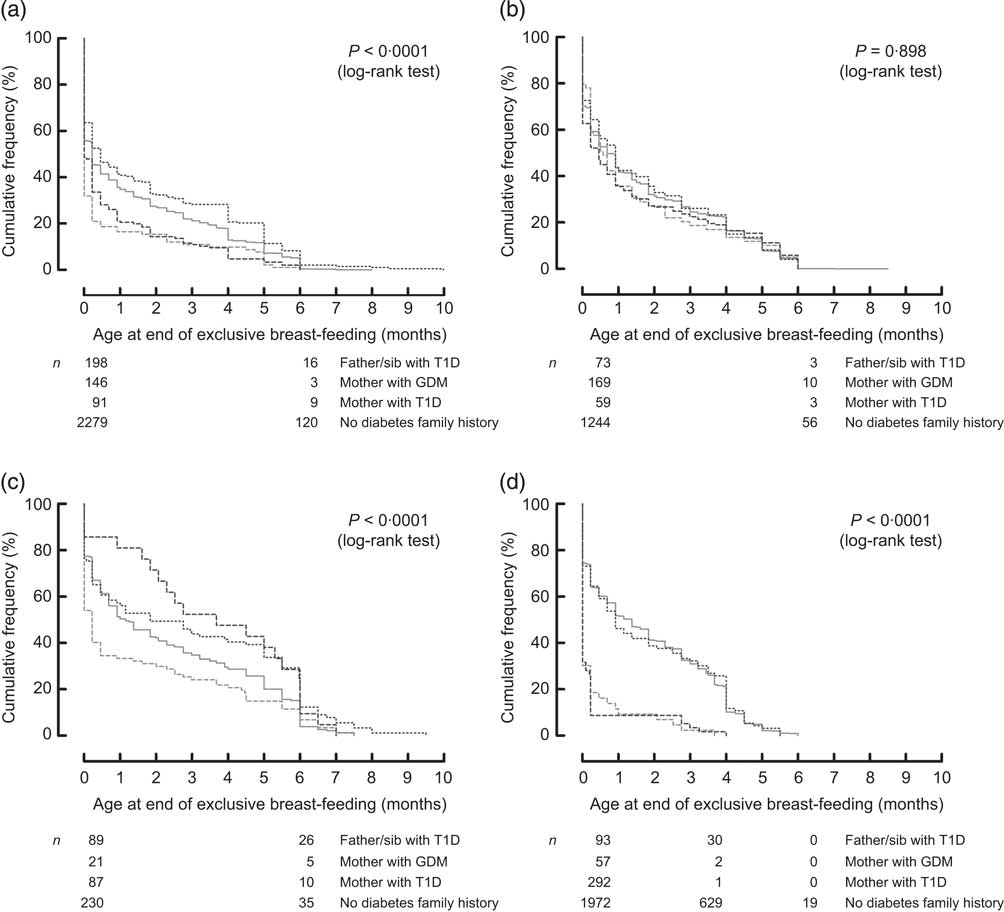
Fig. 2 Country-specific life-table analysis of age at end of exclusive breast-feeding in infants from the USA (a), Finland (b), Germany (c) and Sweden (d) in relation to the presence of diabetes in the family (![]() , father/sib with T1D;
, father/sib with T1D; ![]() , mother with GDM;
, mother with GDM; ![]() , mother with T1D;
, mother with T1D; ![]() , no diabetes family history): The Environmental Determinants of Diabetes in the Young (TEDDY) study (sib, sibling; T1D, type 1 diabetes; GDM, gestational diabetes mellitus)
, no diabetes family history): The Environmental Determinants of Diabetes in the Young (TEDDY) study (sib, sibling; T1D, type 1 diabetes; GDM, gestational diabetes mellitus)
Table 3 Country-specific analysis of infant feeding patterns in relation to the presence of diabetes in the family: The Environmental Determinants of Diabetes in the Young (TEDDY) study
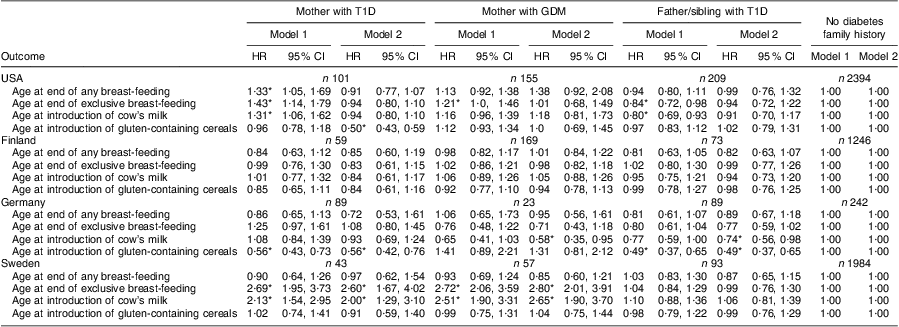
T1D, type 1 diabetes; GDM, gestational diabetes mellitus; HR, hazard ratio.
Model 1: adjusted for mother's smoking status, mother's pre-pregnancy BMI, mother's weight gain during pregnancy, child's gender, maternal age, birth order and mother's education level.
Model 2: Model 1 plus adjustment for delivery mode, gestational age, Apgar score and birth weight.
Introduction of cow's milk is defined as cow's milk including cow's milk-based infant formulas as well as partially hydrolysed infant formulas and excluding extensively hydrolysed infant formula.
*Significant at the P < 0·05 level.
Association of family history of diabetes with the age at introduction to cow's milk and gluten-containing cereals
The univariate analysis of the total cohort showed that offspring of mothers with T1D and GDM were introduced to cow's milk earlier (median age 0·23 months, IQR 0–2 months and median age 0·23 months, IQR 0–2·5 months, respectively) and infants with a father and/or sibling with T1D were introduced to cow's milk later (median age 1·2 months, IQR 0–5·5 months) compared with infants without diabetes in the family (median age 0·9 months, IQR 0–4 months; Fig. 1(c)). The association of age at cow's milk introduction with non-maternal T1D family history remained significant in both multivariate models (HR = 0·83; 95 % CI 0·75, 0·91 and HR = 0·81; 95 % CI 0·71, 0·92 respectively), while the associations with maternal T1D and GDM were attenuated after adjusting for sociodemographic (Model 1) and clinical factors (Model 2, Table 2).
By performing separate multivariate analysis for each country, strong significant associations of early age at first introduction of cow's milk and maternal T1D and GDM were shown for Sweden after adjusting for sociodemographic and clinical factors (HR = 2·00; 95 % CI 1·29, 3·10 and HR = 2·65; 95 % CI 1·90, 3·70, respectively; Table 3). In the USA, maternal T1D was associated with earlier introduction of cow's milk (HR = 1·31; 95 % CI 1·06, 1·62) and non-maternal T1D family history was associated with later introduction of cow's milk (HR = 0·80; 95 % CI 0·69, 0·93; Model 1); however, these associations were attenuated after adjusting for clinical factors (Model 2, Table 3). In Germany and Finland no significant association between the presence of diabetes in the family and introduction of cow's milk was observed.
Introduction of gluten occurred significantly later in children with a mother with T1D as well as in children with a father and/or sibling with T1D compared with infants without diabetes in the family (median age 6 months, IQR 5–7 months v. median age 5 months, IQR 4·5–6 months), both in the univariate and multivariate analyses (Fig. 1(d) and Table 2). Country-specific analysis revealed that this association could only be observed in Germany (Table 3).
Discussion
Our study confirms previous findings of an association between shorter exclusive breast-feeding duration and maternal T1D or GDM that can mainly be explained by demographic and clinical confounding variables( Reference Hummel, Winkler and Schoen 5 – Reference Sorkio, Cuthbertson and Bärlund 8 ). Due to the shorter exclusive breast-feeding duration, offspring of mothers with diabetes were exposed to cow's milk earlier compared with infants without diabetes in the family. Our observations are not consistent with previous studies that reported no effect of maternal T1D on exclusive breast-feeding duration( Reference Webster, Moore and McMullan 9 , Reference Stage, Norgard and Damm 10 ). It is likely that the inconsistencies between the above-mentioned studies( Reference Hummel, Winkler and Schoen 5 – Reference Stage, Norgard and Damm 10 ) are resulting from different strategies in the assessment of breast-feeding habits, a large variation in numbers of children included in the studies and different infant feeding cultures. The TEDDY study provides the possibility to fill these gaps: the TEDDY study is a multinational, epidemiological study following prospectively an adequate number of children with a family history of diabetes. Within the TEDDY study, an extensive amount of dietary, sociodemographic and clinical data are collected according to a harmonized study protocol, enabling between-country comparisons. Furthermore, due to the prospective study design and the frequent data collection, recall bias in questionnaires addressing infant diet is minimized.
Although exclusive breast-feeding duration in infants without diabetes in the family was shorter than recommended by the WHO, the duration of exclusive breast-feeding was even shorter in infants of mothers with T1D and GDM. Our finding that this association is attenuated after adjusting for clinical factors is consistent with previous findings from studies in offspring of mothers with diabetes( Reference Sparud-Lundin, Wennergren and Elfvin 7 , Reference Sorkio, Cuthbertson and Bärlund 8 ). In contrast, age at end of any breast-feeding was not associated with maternal diabetes when analysing data of the total cohort. We further identified that the association between diabetes exposure in the mother and shorter exclusive breast-feeding duration differs strongly between countries. Sweden showed the strongest associations that remained significant after adjusting for sociodemographic and clinical factors. This finding does not confirm results from a recently published Swedish study claiming that factors that are associated with maternal diabetes, such as problems with establishing breast-feeding early postpartum due to the higher degree of maternal and neonatal complications, affect breast-feeding duration( Reference Sparud-Lundin, Wennergren and Elfvin 7 ). The inconsistency between the two studies may be due to different analytical approaches.
In the USA the associations between infant diet and maternal diabetes were attenuated after adjusting for clinical factors, indicating that in the USA maternal and neonatal complications that are associated with maternal diabetes mainly affect breast-feeding behaviour. The country-specific differences in the effect of maternal diabetes on exclusive breast-feeding behaviour were most apparent between Finland and Sweden, both countries located in the northern part of Europe and following comparable numbers of TEDDY children. Compared with Sweden, diabetes in the family was not associated with breast-feeding duration in Finland. This finding suggests that country-specific guidelines affect breast-feeding behaviour in mothers with diabetes. In our study, the definition of exclusive breast-feeding included mother's own breast milk or banked breast milk. Compared with the other countries, in Finland banked breast milk is given more commonly in hospitals to newborn infants of mothers who are not able to successfully breast-feed their infants( Reference Erkkola, Salmenhaara and Kronberg-Kippilä 18 ). In contrast, in Sweden offspring of mothers with diabetes are given more commonly infant formula during the first days of life to avoid hypoglycaemia (C Andrén Aronsson, personal communication, March 2012). We hypothesize that these different neonatal feeding guidelines/practices may be the cause for the observed country-specific differences. We did further observe that ignoring infant formula supplementation during the first week of life when defining age at end of exclusive breast-feeding did not change the reported association of earlier end of exclusive breast-feeding and maternal T1D or GDM (data not shown). This observation strengthens the hypothesis that supplementation of infant formula during the first days of life results in the earlier end of exclusive breast-feeding in offspring of Swedish mothers with diabetes.
In contrast to previous studies, the current study furthers our knowledge on infant feeding patterns by evaluating infants with a mother without diabetes but another first-degree relative with T1D and comparing them to infants without a diabetes history in the family. We observed that in infants with a father and/or sibling with T1D, end of breast-feeding and first exposure to cow's milk and gluten-containing cereals occurred later compared with infants without diabetes in the family. These associations remained significant after adjusting for confounders, indicating that the observed infant feeding patterns are independent of sociodemographic and clinical factors. All families who are participating in TEDDY were informed about the increased T1D risk in their offspring, which is tenfold higher in offspring with a first-degree relative with T1D. Families who are aware of the increased diabetes risk in their offspring are known to modify their behaviour, including feeding patterns, with the aim to prevent disease( Reference Baughcum, Johnson and Carmichael 19 ), although families did not receive any specific recommendations on infant diet by the TEDDY study. Furthermore, in none of the participating TEDDY countries are families with diabetes given specific infant feeding recommendations by health-care providers. Among the countries participating in TEDDY, Finland, Germany and parts of the USA have been participating in dietary intervention trials to prevent islet autoimmunity and T1D in high-risk children by delaying introduction of cow's milk( Reference Akerblom, Krischer and Virtanen 20 ) or gluten( Reference Hummel, Pflüger and Hummel 21 ). Families with the presence of T1D in the family may have known about these intervention strategies and implemented them in infant diet. We hypothesize that due to the difficulties in breast-feeding that are encountered by mothers with T1D, a longer breast-feeding duration and later exposure to cow's milk could only be observed in infants with a father or sibling with T1D. The fact that first exposure to gluten-containing cereals, a feeding pattern which, compared to cow's milk introduction, is not associated with successful breast-feeding, was delayed in offspring of mothers with T1D as well as in infants with a father or sibling with T1D further strengthens this hypothesis.
The association between delayed gluten introduction in German offspring with a first-degree relative with T1D is probably due to the fact that the BABYDIET study, a dietary intervention study applying delayed gluten exposure, was performed only in Germany where newborns with T1D in a first-degree relative were recruited between 2001 and 2004( Reference Hummel, Pflüger and Hummel 21 ).
In conclusion, our results show that diabetes in the family influences the duration of exclusive breast-feeding, age at introduction of cow's milk and age at introduction of gluten-containing cereals. The finding that neonatal complications are strongly affecting exclusive breast-feeding behaviour in offspring of mothers with diabetes needs to be considered when developing strategies to improve breast-feeding behaviour in mothers with diabetes and strategies to prevent disease.
These associations, however, clearly differ by country, indicating that country-specific recommendations on infant feeding and guidelines on neonatal care in offspring of mothers with diabetes strongly influence infant feeding patterns. This may also explain inconsistencies in findings between previous studies in this field.
Acknowledgements
Sources of funding: The TEDDY study is funded by grant numbers DK 63829, 63861, 63821, 63865, 63863, 63836, 63790 and UC4DK095300 and contract number HHSN267200700014C from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), the National Institute of Allergy and Infectious Diseases (NIAID), the National Institute of Child Health and Human Development (NICHD), the National Institute of Environmental Health Sciences (NIEHS), the Juvenile Diabetes Research Foundation (JDRF) and the Centers for Disease Control and Prevention (CDC). The NIDDK, NIAID, NICHD, NIEHS, JDRF and CDC had no role in the design, analysis or writing of this article. Conflicts of interest: There are no conflicts of interest. Authors’ contributions: S.H. designed the study, interpreted data and wrote the manuscript. K.V. and W.M. performed analysis of data and contributed to interpretation of data. C.A.A., N.F. and P.G. contributed to acquisition of data and provided input to interpretation of data. U.U. and J.Y. provided input to the analysis and interpretation of data and revised the article critically. J.M.N. and S.M.V. provided major input to the study design and analysis and interpretation of data, contributed to writing and revised the article critically. J.M.N and S.M.V. are sharing last authorship. Acknowledgements: The authors express their gratitude to the children and parents who participated, and wish to thank the TEDDY staff for excellent collaboration over the years.
Appendix
The Teddy Study Group
Colorado Clinical Center
Marian Rewers, MD, PhD, Principal Investigator1,4,6,10,11; Katherine Barriga12; Kimberly Bautista12; Judith Baxter9,12,15; George Eisenbarth, MD, PhD; Nicole Frank2; Patricia Gesualdo2,6,12,14,15; Michelle Hoffman12,13,14; Lisa Ide; Rachel Karban12; Edwin Liu, MD13; Jill Norris, PhD2,12; Kathleen Waugh7,12,15; Adela Samper-Imaz; Andrea Steck, MD (University of Colorado, Anschutz Medical Campus, Barbara Davis Center for Childhood Diabetes).
Georgia/Florida Clinical Center
Jin-Xiong She, PhD, Principal Investigatora,1,3,4,11; Desmond Schatz, MDb,4,5,7,8; Diane Hopkinsa,12; Leigh Steeda,6,12,13,14,15; Jamie Thomasb,12; Katherine Silvisa,2; Michael Haller, MDb,14; Meena Shankarb,2; Kim Englisha; Richard McIndoe, PhDa; Haitao Liu, MDc; John Nechtmanc; Joshua Williamsa; Gabriela Foghisa; Stephen W. Anderson, MDd (aGeorgia Health Sciences University; bUniversity of Florida; cJinfiniti Biosciences LLC, Augusta, GA; dPediatric Endocrine Associates, Atlanta, GA).
Germany Clinical Center
Anette G. Ziegler, MD, Principal Investigatora,1,3,4,11; Alexandra Achenbach, PhDb,12; Heike Boerschmanna,14; Ezio Bonifacio, PhDb,5; Melanie Bunka; Johannes Förscha; Lydia Hennebergera,2,12; Michael Hummel, MDa,13; Sandra Hummel, PhDa,2; Gesa Joslowskic,2; Mathilde Kersting, PhDc,2; Annette Knopffa,7; Nadja Kochera; Sibylle Koletzko, MDd,13; Stephanie Krausea; Claudia Matzkea; Astrid Mittermeiera; Claudia Peplowb,12; Maren Pflügera,6; Claudia Rammingera; Elisabeth Straussa; Sargol Rash-Sura; Roswith Roth, PhDa,9; Julia Schenkela; Joanna Stocka; Katja Voita; Christiane Winkler, PhDa,2,12,15; Anja Woscha (aForschergruppe Diabetes eV at Helmholtz Zentrum München; bCenter for Regenerative Therapies, TU Dresden; cDr von Hauner Children's Hospital, Department of Gastroenterology, Ludwig Maximillians University Munich; dResearch Institute for Child Nutrition, Dortmund).
Finland Clinical Center
Olli G. Simell, MD, PhD, Principal Investigatora,b,1,4,11,13; Heikki Hyöty, MD, PhDc,d,6; Jorma Ilonen, MD, PhDa,e,3; Mikael Knip, MD, PhDc,d; Maria Lönnrot, MD, PhDc,d,6; Elina Mäntymäakia,b; Juha Mykkänen, PhDa,b; Kirsti Näntö-Salonen, MD, PhDa,b,12; Tiina Niininenc,d; Mia Nyblomc,d; Anne Riikonenc,d,2; Minna Romoa,b; Barbara Simella,b,12,15; Tuula Simell, PhDa,b,9,12; Ville Simella,b,13; Maija Sjöberga,b,12,14; Aino Steniusf,g; Eeva Varjonena,b; Riitta Veijola, MD, PhDf,g; Suvi M. Virtanen, MD, PhDc,d,h,2 (aUniversity of Turku; bTurku University Hospital; cUniversity of Tampere; dTampere University Hospital; eUniversity of Kuopio; fUniversity of Oulu; gOulu University Hospital; hNational Institute for Health and Welfare, Helsinki).
Sweden Clinical Center
Åke Lernmark, PhD, Principal Investigator1,3,4,8,10,11,15; Daniel Agardh, MD, PhD13; Peter Almgren; Eva Andersson; Carin Andrén Aronsson2,13; Maria Ask; Ulla-Marie Karlsson; Corrado Cilio, MD, PhD5; Jenny Bremer; Emilie Ericson-Hallström; Thomas Gard; Joanna Gerardsson; Gertie Hansson12,14; Monica Hansen; Susanne Hyberg; Rasmus Håkansson; Fredrik Johansen; Linda Jonsson; Helena Larsson, MD, PhD14; Barbro Lernmark, PhD9,12; Maria Markan; Theodosia Massadakis; Jessica Melin; Maria Månsson-Martinez; Anita Nilsson; Kobra Rahmati; Monica Sedig Järvirova; Sara Sibthorpe; Birgitta Sjöberg; Anna Skogberg; Carina Törn, PhD3,15; Anne Wallin; Åsa Wimar; Sofie Åberg (Lund University).
Washington Clinical Center
William A. Hagopian, MD, PhD, Principal Investigator1,3,4,5,6,7,11,13,14; Xiang Yan, MD; Michael Killian6,7,12,13; Claire Cowen Crouch12,14,15; Kristen M. Hay2; Stephen Ayres; Carissa Adams; Brandi Bratrude; David Coughlin; Greer Fowler; Czarina Franco; Carla Hammar; Diana Heaney; Patrick Marcus; Arlene Meyer; Denise Mulenga; Elizabeth Scott; Jennifer Skidmore; Joshua Stabbert; Viktoria Stepitova; Nancy Williams (Pacific Northwest Diabetes Research Institute).
Pennsylvania Satellite Center
Dorothy Becker, MD; Margaret Franciscus12; MaryEllen Dalmagro-Elias2; Ashi Daftary, MD (Children's Hospital of Pittsburgh, University of Pittsburgh Medical Center).
Data Coordinating Center
Jeffrey P. Krischer, PhD, Principal Investigator1,4,5,10,11; Michael Abbondondolo; Lori Ballard3,9,14,15; Rasheedah Brown12,15; Brant Burkhardt, PhD5,6; David Cuthbertson; Christopher Eberhard; Steven Fiske; Veena Gowda; David Hadley, PhD; Hye-Seung Lee, PhD3,6,13,15; Shu Liu; Kristian Lynch, PhD9; Jamie Malloy; Cristina McCarthy12,15; Wendy McLeod2,5,6,13,15; Laura Smith, PhD9; Susan Smith12,15; Ulla Uusitalo, PhD2,15; Kendra Vehik, PhD4,5,9,14,15; Earnest Washington; Jimin Yang, PhD, RD2,15 (University of South Florida).
Project Scientist
Beena Akolkar, PhD1,3,4,5,7,10,11 (National Institute of Diabetes and Digestive and Kidney Diseases).
Other contributors
Kasia Bourcier, PhDa,5; Thomas Briese, PhDb,6,15; Henry Erlich, PhDc,3; Suzanne Bennett Johnson, PhDd,9,12; Steve Oberste, PhDe,6 (aNational Institute of Allergy and Infectious Diseases; bColumbia University; cChildren's Hospital Oakland Research Institute; dFlorida State University; eCenters for Disease Control and Prevention).
Committees
1Ancillary Studies; 2Diet; 3Genetics; 4Human Subjects/Publicity/Publications; 5Immune Markers; 6Infectious Agents; 7Laboratory Implementation; 8Maternal Studies; 9Psychosocial; 10Quality Assurance; 11Steering; 12Study Coordinators; 13Celiac Disease; 14Clinical Implementation; 15Quality Assurance Subcommittee on Data Quality.







