Obesity has become a global health burden owing to its complications such as hypertension, type 2 diabetes, dyslipidaemia, coronary artery disease, stroke and cancer( Reference Kumanyika, Obarzanek and Stettler 1 , Reference Barroso, Goday and Ramos 2 ). Overweight and obesity affected over 600 million adults in 2014 worldwide, a rate expected to be about 1·2 billion in 2030( Reference Hosseinpanah, Mirbolouk and Mossadeghkhah 3 ). Commonly BMI is used to determine body fat, but it is not a strong predictor of total body fat and subsequently the risk of CVD. In contrast, waist circumference (WC) as a measure of abdominal obesity (AO) identifies the risk of cardiometabolic disease more precisely than BMI( Reference Han and Lean 4 ). A population-based study in the USA reported that 7·6 % of adults aged ≥20 years had AO and 33·7 % had abnormal waist-to-hip ratio in 2012( Reference Mainous, Tanner and Jo 5 ). The importance of intervention and control of AO as a universal concern is clarified by a study in the National Health and Nutrition Examination Survey (NHANES) framework, which revealed a significant increase in overall AO prevalence from 46·4 % in 1999–2000 to 54·2 % in 2011–2012 within a population consisting of men, women, non-Hispanic whites, non-Hispanic blacks and Mexican Americans( Reference Ford, Maynard and Li 6 ). In the Iranian adult population, AO is more prevalent in women than in men and in older ages in both sexes( Reference Azizi, Salehi and Etemadi 7 ). Based on data from the Tehran Lipid and Glucose Study (TLGS) through phases 1 to 4 (1999 to 2011), AO prevalence increased from 52 to 78 % in men and from 44 to 66 % in women( Reference Barzin, Keihani and Hosseinpanah 8 ). Incidence studies are needed to identify the high-risk individuals for AO. Since AO is a highly relative risk factor for cardiovascular events( Reference Zhang, Rexrode and van Dam 9 , Reference Fan, Li and Zheng 10 ), focus on a cohort study would help clarify of the incidence and risks of AO. We aimed to explore the incidence of AO and its risk factors in an adult Tehranian population in the TLGS over a median 6 years of follow-up.
Methods
Study population
Using the data of participants of the TLGS, a dynamic prospective population-based study, the present study was conducted on a representative sample of the Tehranian population with the aim of determining the prevalence and risk factors of non-communicable diseases. Details of study protocol are available elsewhere( Reference Azizi, Rahmani and Emami 11 , Reference Azizi, Ghanbarian and Momenan 12 ). To summarize, the TLGS has two major components: (i) a cross-sectional prevalence study of non-communicable disease (1999–2001) and associated risk factors; and (ii) prospective follow-up studies at approximately 3-year intervals. About 15005 residents participated in the first examination cycle (1999–2001) and another 3555 residents were first examined at the second examination cycle (2002–2005). Of these 18560, a total of 12808 participants aged ≥20 years at baseline were selected for the current study. Participants with AO (n 5524), BMI below 18·5 kg/m2 (n 322), pregnancy (n 98), corticosteroid consumption (n 245) at baseline and those without any follow-up data (n 1575) were excluded, leaving 5044 participants to be followed up to 2014.
All participants provided written, informed consent. The protocol of the present study, conducted in accordance with principles of the Declaration of Helsinki, was approved by the ethics committee of the Research Institute of Endocrine Sciences of Shahid Beheshti University of Medical Sciences.
Clinical and laboratory measurements
Using a pre-tested questionnaire, a trained interviewer collected information which included baseline demographic information, family history of diabetes, past medical history of CVD, drug use and smoking behaviour. Weight was measured with participants minimally clothed without shoes, using digital scales (Seca 707; Seca Corporation, Hanover, MD, USA; range 0·1–150 kg), and recorded to the nearest 100 g. Height was measured in a standing position without shoes, using a tape meter that was fixed to a wall, while shoulders were in a normal alignment. WC was measured at the umbilical level. Two measurements of systolic blood pressure and diastolic blood pressure were taken using a standardized mercury sphygmomanometer on the right arm, after resting for 15 min in a sitting position; the mean of the two measurements was considered as the participant’s blood pressure. Fasting plasma glucose, HDL-cholesterol and TAG levels were measured by previously reported methods( Reference Azizi, Ghanbarian and Momenan 12 ). BMI was calculated as weight (in kilograms) divided by the square of height (in metres).
Definition of terms
Abdominal obesity was defined as WC≥91 cm for women and WC≥89 cm for men( Reference Delavari, Forouzanfar and Alikhani 13 ). The definition of dysmetabolic state was according to the criteria for metabolic syndrome of the Joint Interim Statement( Reference Alberti, Zimmet and Shaw 14 ), with exclusion of AO, as the presence of any three out of the following four components: (i) fasting plasma glucose≥5·6 mmol/l (100 mg/dl) or 2 h plasma glucose ≥7·8 mmol/l (140 mg/dl) or drug treatment; (ii) fasting TAG≥1·7 mmol/l (150 mg/dl) or drug treatment; (iii) fasting HDL-cholesterol of 1·29 mmol/l (50 mg/dl) in women and 1·03 mmol/l (40 mg/dl) in men or drug treatment; and (iv) raised blood pressure defined as systolic blood pressure≥130 mmHg, diastolic blood pressure ≥85 mmHg or antihypertensive drug treatment. Data on physical activity (PA) were assessed using the Lipid Research Clinics questionnaire in the first phase of the TLGS. Due to the lack of precision of the Lipid Research Clinics questionnaire( Reference Ainsworth, Jacobs and Leon 15 ), the Modifiable Activity Questionnaire, which measures all three types of activity including leisure-time, job and household activities( Reference Kriska, Knowler and LaPorte 16 ), was used in the rest of the follow-up examinations. Since the duration of PA was not accounted for in the Lipid Research Clinics questionnaire, participants who were enrolled in the study from the first phase of the TLGS were considered ‘low PA’ if participating in low and moderate PA and considered ‘high PA’ if participating in vigorous PA for a minimum of 3 d/week. Individuals who entered the study at the second follow-up examination of the TLGS were defined as ‘high PA’ if they achieved a minimum of at least 600 MET-min/week and as ‘low PA’ if they had less than 600 MET-min/week (where MET is metabolic equivalent of task)( 17 ). Education was classified into two groups: below diploma (<12 years education); and diploma or higher (≥12 years education). Marital status was categorized as single (including widowed) and married. A current smoker was defined as a person who smokes cigarettes daily or occasionally. Ex-smokers were considered non-smokers.
Statistical analysis
All analyses were conducted separately for men and women. Categorical variables of the baseline characteristics are expressed as n and %. Comparison of baseline characteristics between men v. women was done using a χ 2 test. In the present study, the exact time of AO incidence was calculated by the interval-censored data method. Interval censoring takes account of the event happening between two time periods. Considering alternative interval-censoring approaches, results were investigated using mid-point censoring( Reference Lindsey and Ryan 18 ). Mid-point censoring was set to the mid-point between the last negative and the most recent positive event time. End points were considered as the time of incident AO and censoring was defined as lost to follow-up or end of the follow-up. Cumulative incidence of AO with 95 % CI was calculated for each sex as the number of new cases of AO over the total number of individuals in that group minus half of the censored population. The person-year method was used to obtain AO incidence rates, which are reported as the number of cases per 1000 person-years. Cox proportional hazard modelling was used to estimate unadjusted and age-adjusted hazard ratios (HR) along with 95 % CI for baseline groups of BMI, PA, dysmetabolic state, educational level, smoking status and marital status. The proportionality assumption was verified by assessing the correlation between the Schoenfield residuals and person-days along with observing log minus log plots (considering different groups as strata variables). All analyses were performed using the statistical software packages IBM SPSS for Windows version 20 and Stata version 12 SE, with a two-tailed P values of 0·05 being considered significant.
Results
In the present study, 5044 participants (37 % male), with mean age of 37·7 (sd 13·5) years, mean BMI of 24·3 (sd 3·1) kg/m2 (23·0 (sd 2·4) and 25·0 (sd 3·2) kg/m2 for men and women, respectively) and mean WC of 79·8 (sd 6·8) cm (80·2 (sd 5·8) and 79·5 (sd 7·4) cm for men and women, respectively) at baseline, were followed for a median of 6 years (25th–75th percentile: 2–10 years). Baseline characteristics of the participants are shown in Table 1. In addition, regarding individual components of dysmetabolic state, 1602 (32·3 %) had high TAG, 3334 (67·1 %) had low HDL-cholesterol, 1043 (20·7 %) had high blood pressure and 674 (13·4 %) had high fasting plasma glucose.
Table 1 Baseline characteristics of study participants. Tehran Lipid and Glucose Study, 1999–2014
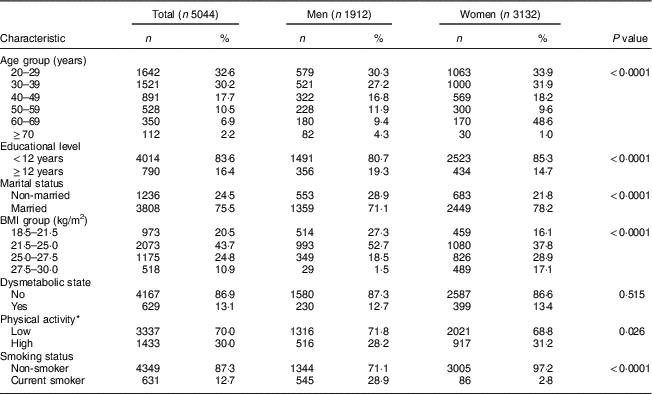
* Physical activity measured by the Lipid Research Clinics questionnaire and the Modifiable Activity Questionnaire.
Men had significantly less PA and a higher frequency of smoking than women at baseline. There was no significant difference between the sexes in prevalence of dysmetabolic state.
During follow-up, 3093 subjects (1720 women) developed AO that led to a cumulative incidence of 76·02 (95 % CI 74·70, 77·32) %. Total cumulative incidence of AO was 70·90 (95 % CI 69·08, 72·69) % for women and 83·59 (95 % CI 81·76, 85·34) % for men.
Incidence rate in the whole population was 96·0 (95 % CI 92·7, 99·5) per 1000 person-years. Corresponding incidence rates among men and women were 138·7 (95 % CI 131·5, 146·3) and 77·1 (95 % CI 73·5, 80·9) per 1000 person-years. The highest incidence rate in women was 146·3 (128·1, 167·0) per 1000 person-years in the 50–59 years age group and in men was 152·6 (138·2, 168·5) per 1000 person-years in the 30–39 years age group. Median survival for men was 4 (sd 2·8) years v. 7 (sd 3·1) years for women.
The association of different risk factors with developing incident AO from clinical and demographic predictors is presented in Table 2 (men) and Table 3 (women).
Table 2 Cumulative incidence, hazard ratios and incidence rate of potential risk factors of abdominal obesity in men. Tehran Lipid and Glucose Study, 1999–2014
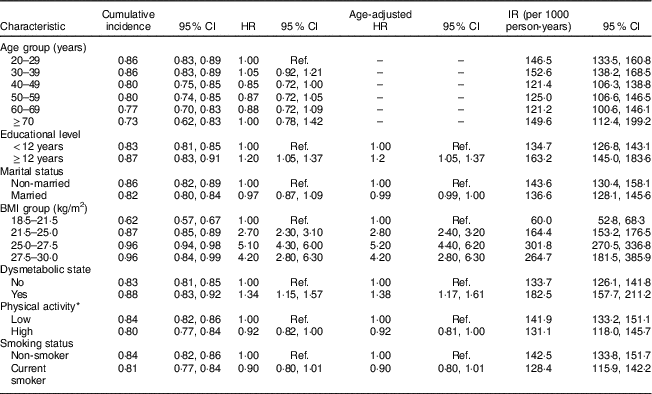
Cumulative incidence = number of incident obesity cases divided by the total number of individuals; HR, hazard ratio; IR, incidence rate = number of incident obesity cases divided by the person-years of follow-up; Ref., reference category.
* Physical activity measured by the Lipid Research Clinics questionnaire and the Modifiable Activity Questionnaire.
Table 3 Cumulative incidence, hazard ratios and incidence rate of potential risk factors of abdominal obesity in women. Tehran Lipid and Glucose Study, 1999–2014
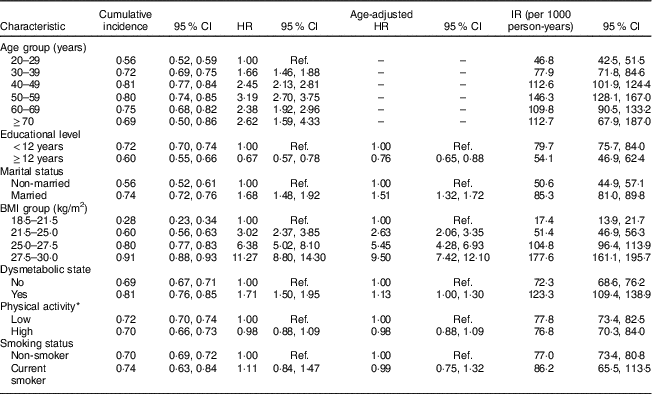
Cumulative incidence = number of incident obesity cases divided by the total number of individuals; HR, hazard ratio; IR, incidence rate = number of incident obesity cases divided by the person-years of follow-up; Ref., reference category.
* Physical activity measured by the Lipid Research Clinics questionnaire and the Modifiable Activity Questionnaire.
Dysmetabolic state and BMI were significantly associated with increasing incident AO in both sexes. Higher educational level in men significantly increased the risk of AO, in contrast to women (HR=1·20; 95 % CI 1·05, 1·37 in men and HR=0·67; 95 % CI 0·57, 0·78 in women). Ageing was significantly associated with AO in women, with the highest HR in the 50–59 years age group (HR=3·19; 95 % CI 2·70, 3·75).
Married status was a significant risk factor of developing AO in women, whereas marital status in men was not significantly associated with AO. Smoking status and PA were not among predictors of AO, in men or women.
Discussion
After a median of 6 years of follow-up, the present study indicated high incidence of AO in an urban adult Tehranian population, which was higher among men than women (83·5 v. 70·9 %). Important risk factors of developing AO at baseline were dysmetabolic state and BMI in both sexes, high educational level and less PA in men, and married status in women. Moreover, men mostly developed AO earlier than women (30–39 v. 50–59 years).
There are limited studies investigating the incidence of AO worldwide, which differ in terms of the mean age of subjects, socio-economic status, WC cut-offs and length of follow-up. Haftenberger et al. reported a high rate of AO within 10 years among adults, by including the data of 15444 men and 17207 women from seven prospective cohort studies in Germany. The rate of progression to AO in participants with low baseline WC (<94 cm in men; <80 cm in women) was reported near to 20 %. Moreover, this rate was higher (50 %) among participants with intermediate WC (94–102 cm in men; 80–88 cm in women) at baseline( Reference Haftenberger, Mensink and Vogt 19 ). Another study from Europe reported age-adjusted incidence of AO in an urban Portuguese population to be 59·7 and 23·8 per 1000 person-years for women and men, respectively( Reference Camoes, Lopes and Oliveira 20 ), which was lower than that of our population. In Iran, as a middle-income country, we found higher incidence of AO as compared with both above-mentioned results from high-income countries. Rising trends of non-communicable diseases including obesity and AO have been reported in low- and middle-income countries during recent years( Reference Reddy 21 ). In fact, industrialization and alterations in occupations and transportation systems cause changes in nutrition pattern( Reference Hosseinpanah, Barzin and Eskandary 22 ). According to studies conducted in China, AO has increased dramatically from 12 % in 1993 to 21 % in 2009 in people with normal BMI, specifically in men. Interestingly, this rising trend was observed in both sexes, rural and urban areas, all ages and education groups( Reference Du, Sun and Yin 23 ). In Iran as a developing country, low PA, sedentary lifestyle, changes in diets and higher energy intake are suggested to play key roles in this alarming rise in incidence of AO( Reference Ghassemi, Harrison and Mohammad 24 , Reference Koohpayehzadeh, Etemad and Abbasi 25 ).
The causes of developing AO are multifactorial, including PA, education, marital status, socio-economic status and smoking( Reference Koh-Banerjee, Chu and Spiegelman 26 ). As a key factor to burn off excess energy, PA is consistently associated with lower risk of AO( Reference Lopez-Sobaler, Rodriguez-Rodriguez and Aranceta-Bartrina 27 , Reference Fam, Amouzegar and Arzhan 28 ). In our study, males with a high level of PA were at lower risk of AO. As our females mostly became abdominally obese during 50–59 years of age, the absence of an association between baseline PA and developing AO could be related to the hormonal changes during menopause, as fat distribution in women results in AO during this period( Reference Poehlman 29 ). Moreover, we found dysmetabolic state to be a risk factor for developing AO. Previous studies revealed that metabolic syndrome components in healthy people can independently predict the incidence of metabolic syndrome itself. Therefore, it could be anticipated that people with an unhealthy metabolic status would be at risk of developing AO as a key component of the metabolic syndrome( Reference Heidari, Hosseinpanah and Mehrabi 30 , Reference Cheung, Wat and Tam 31 ). Moreover, it has been shown that individuals with unhealthy metabolic status without AO are at increased risk of cardiovascular events, compared with non-abdominally obese and healthy metabolic individuals( Reference Keihani, Hosseinpanah and Barzin 32 , Reference van der, Nooyens and van Duijnhoven 33 ).
Moreover, high educational level has been proposed as a protective factor for developing AO mostly in women( Reference Hajian-Tilaki and Heidari 34 ). Likewise, in our study, women with high education levels were at lower risk of AO. Additionally, differences in the incidence of AO between age groups and sexes were depicted in our results by the large proportion of men aged 30–39 years who developed AO; this could be attributed to the high energy density and protein intakes of young men( Reference Bahadoran, Mirmiran and Golzarand 35 ). In contrast, young females did not develop AO, which could be a result of the importance of healthy diets to young women because of their higher educational level and health programmes focusing on women’s health in recent years( Reference Ramezani Tehrani, Bahri and Gholami 36 , Reference Joulaei, Maharlouei and Lankarani 37 ).
Studies have reported diverse kinds of associations of AO with marital status. In a Greek investigation of both men and women, those with married status were at higher risk of AO( Reference Tzotzas, Vlahavas and Papadopoulou 38 ). However, an Iranian study reported this association only for married women, not men( Reference Barzin, Keihani and Hosseinpanah 8 ). Our findings are in agreement with these studies about women, but not men. Interestingly, another report highlights the importance of transition into or out of marriage in cohort studies and the risk of AO( Reference Hosseinpour-Niazi, Mirmiran and Hosseinpanah 39 ). Therefore, since marital status was not tracked in our study, we have to be cautious about interpreting our findings in this regard.
Although both smoking and amount of smoking are reported to be positively associated with developing AO( Reference Saarni, Pietilainen and Kantonen 40 – Reference Kim, Shim and Yoon 42 ), we found no such an association in our study. Several possible reasons for this unexplained finding can be suggested, including behavioural changes in individuals which led to quitting smoking( Reference Azizi, Ghanbarian and Momenan 12 ) and lack of information regarding the dose–response relationship between smoking and development of AO.
Finally, a number of potential shortcomings in our study have to be considered. First, the TLGS is a cohort study conducted on urban Tehranians. Therefore, our results could not be generalized to the whole Iranian population, especially rural areas. Second, we used umbilicus level instead of midpoint for measurement of WC. It has been reported that WC measurement at midpoint is slightly lower than WC measurement at umbilicus and may influence the prevalence of AO. Therefore, the high incidence of AO in our report can, in part, be explained by this measurement( Reference Bosy-Westphal, Booke and Blocker 43 , Reference Mason and Katzmarzyk 44 ). Third, some important risk factors for incidence of AO such as dietary habits and socio-economic status were not taken into account. In addition, we measured all possible risk factors at a single time point at the beginning of the study; however, similar to us, most epidemiological studies in this field have provided single-point measurements for possible risk factors. The present study has its own strengths as well; to the best of our knowledge, it is one of the few population-based studies with an intermediate length of follow-up that evaluates the sex-stratified incidence of AO and its risk factors. We also used measured data for anthropometric parameters, rather than self-reported data.
Future studies on the current topic are required to investigate the effect over longer follow-ups, tracking changes of risk factors by repeated measurements and including the impact of nutrition, socio-economic and menopausal status on the incidence of AO. The evidence from the present study implies that in a large metropolitan city of a developing country, over a median follow-up of 6 years, the incidence of AO is high especially among young men, which requires that current health priorities and strategies should target this age group to provide effective prevention. Moreover, we recommend more focus on screening the general population for dysmetabolic state as an outstanding risk factor for developing AO in both sexes.
Acknowledgements
Acknowledgements: The authors express their appreciation to participants of District 13, Tehran, for their enthusiastic support in this study. Financial support: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors. Conflict of interest: None. Authorship: M.B., M.V., F.A. and F.H. designed the study. M.B., S.S. and Z.P. collected and analysed the data and drafted the manuscript. F.H. and M.V. critically reviewed the paper and edited the manuscript. F.H. supervised and F.A. advised throughout the study. Ethics of human subject participation: The protocol of the present study, conducted in accordance with principles of the Declaration of Helsinki, was approved by the ethics committee of the Research Institute of Endocrine Sciences of Shahid Beheshti University of Medical Sciences. All participants provided written, informed consent.





