Adverse childhood experiences (ACE) have been strongly linked to a number of chronic diseases, including CVD, cancer and type 2 diabetes, in adulthood and premature death(Reference Felitti, Anda and Nordenberg1–Reference Anda, Dong and Brown5). ACE refers a range of traumatic experiences such as abuse and household dysfunction that have occurred prior to the age of 18 years, which pose a substantial risk for the subsequent development of negative health outcomes. ACE is common in the United States, where it was estimated that more than half (61·55 %) of adults are exposed to at least one ACE, and more than one in five adults (24·64 %) have experienced three or more ACE(Reference Merrick, Ford and Ports6).
In recent decades, increasing attention has focused on elucidating the mechanisms that underlie the associations between ACE and chronic diseases in adulthood. During early childhood, the brain undergoes its most rapid period of growth and development and is a highly sensitive stage for the detrimental effects of ACE(7). Without adequate protective factors (e.g. secure attachment and key buffering relationships with caregivers), ACE and other types of childhood adversity, such as poverty, food insecurity, poor educational opportunities and community violence, could lead to a type of stress response known as toxic stress(Reference Bucci, Marques and Oh8,Reference Shonkoff and Garner9) . Toxic stress causes long-term changes in brain architecture and organ systems. For example, toxic stress generated upon exposure to ACE could lead to biological modifications, such as alterations in hormonal and immune system factors, which may ‘rewire’ the brain so as to make individuals more vulnerable to subsequent stressors(Reference Miller, Chen and Parker10). Altered patterns of brain development among children exposed to ACE might contribute to the onset of chronic diseases, in part, by increasing the tendency to engage in high-risk behaviours(Reference Shonkoff and Garner9). As a matter of fact, ACE is reported to be associated with higher levels of substance use, poor mental health, overeating and obesity(Reference Mersky, Topitzes and Reynolds11,Reference Duffy, McLaughlin and Green12) .
Suboptimal diet is a leading preventable risk factor for chronic diseases and has been linked to nearly half of all estimated annual mortality from CVD and diabetes in the United States(Reference Micha, Shulkin and Penalvo13). Regardless of continuous efforts to promote fruit and vegetable intake in the United States, inadequate consumption of these persists. Previous studies have reported that 76 % of adults did not meet fruit intake recommendation (1·5–2·0 cups equivalent of fruits daily), and 87 % did not meet vegetable intake recommendation (2·0–3·0 cups of vegetables daily)(14,Reference Lee-Kwan, Moore and Blanck15) .
Limited cross-sectional research has explored the association between ACE and dietary behaviour. Given the high prevalence of chronic diseases and ACE, a better understanding of these potential relationships, particularly fruit and vegetable intake, has a potential to contribute to public health by highlighting novel targets for intervention(Reference Merrick, Ford and Ports6). Understanding the factors that place groups at a greater risk for poor outcomes could inform the development of effective prevention and intervention strategies, and would ensure that resources are used efficiently to address the needs of vulnerable groups. To address the issue, the present study aimed to extend the literature on ACE and unhealthy behaviour by focusing on low fruit and vegetable intake among adults based on recent population-based data. The study tested the following hypotheses: there is a graded relationship between the number of ACE and low fruit and vegetable intake among adults. In addition, we conducted an exploratory analysis to examine whether any individual types of ACE are associated with low fruit and vegetable intake. Although literature suggests that ACE types seldom occur in isolation, the impacts of individual ACE types were examined since there is no previous literature addressing the relationships between ACE and dietary pattern(Reference Anda, Dong and Brown5).
Methods
Data sources
This cross-sectional study used data from the 2017 Nevada Behavioral Risk Factor Surveillance System (BRFSS). BRFSS is a combined landline and cell-phone health survey that uses a random digit dialling technique(16,17) . This telephone survey collects health information among non-institutionalised adults aged ≥18 years. Trained interviewers administered standardised national survey questions, optional questions and state-added questions, including ACE module questions(16,17) . In 2017, 3764 Nevadans participated in the BRFSS. Of these, 2939 participants had complete data for all ACE questions.
Measures
The independent variables of interest were measures of ACE reported by the BRFSS ACE module, which consists of eleven questions adopted from the original ACE study and assessed the occurrence of eight different areas of abuse and household dysfunction prior to the age of 18(Reference Felitti, Anda and Nordenberg1). Measures of abuse included physical abuse (one item), sexual abuse (three items) and psychological/emotional abuse (one item). Measures of household dysfunction included living with an adult who was depressed, mentally ill or suicidal (one item), living with anyone who abused substances (two items), living with someone who had been sentenced to serve time in a prison, jail or other correctional facility (one item), having parents who were separated/divorced (one item), and living in a home where adults or parents physically harmed each other (one item)(Reference Felitti, Anda and Nordenberg1). Items about mental illness, drinking problem, drug use, family incarceration and parental divorce/separation asked whether or not the respondent had been exposed to the respective ACE. The remaining items allowed respondents to report the frequency of occurrence of a specific ACE (i.e. never, once or more than once). For the current study, all items were further dichotomised to indicate whether or not the experience had ever happened. Because three items measured facets of sexual abuse and two items measured parts of living with a substance abuser, they were each combined into a single measure of sexual abuse and a single measure of living with a substance abuser, respectively, resulting in a total of eight ACE measures(Reference Frankenberger, Clements-Nolle and Yang18). To assess the cumulative exposure to ACE, the counts of ACE types for each participant were categorised into the following groups: no ACE, 1 ACE, 2 ACE, or ≥3 ACE(Reference Frankenberger, Clements-Nolle and Yang18).
Our dependent variable was the frequency of fruit and vegetable intake per day. Fruit and vegetable consumption frequencies were measured by six questions in the BRFSS core module: (i) ‘How often did you drink 100 % fruit juice such as apple or orange juice? Do not include fruit-flavoured drinks or fruit juices you added sugar to.’ (ii) ‘During the past month, how often did you eat fruit? Do not include juices. You can tell me times per day, per week or per month.’ (iii) ‘How often did you eat a green leafy or lettuce salad, with or without other vegetables?’ (iv) ‘How often did you eat any kind of fried potatoes, including french-fries, home fries or hash browns?’ (v) ‘How often did you eat any other types of potatoes, such as baked, boiled, mashed potatoes or potato salad?’ (vi) ‘Not including lettuce salads and potatoes, how often did you eat other vegetables?’ We limited our analyses to fruits and excluded 100 % fruit juice, because fruit juice is associated with an increased risk of type 2 diabetes and greater weight gain(Reference Muraki, Imamura and Manson19,Reference Mozaffarian, Hao and Rimm20) . French-fries and potato consumption was also excluded because three different cohorts of health professionals observed an increased risk for obesity when consuming more potatoes, and a decreased risk for obesity when consuming whole fruits and non-starchy vegetables(Reference Mozaffarian, Hao and Rimm20). Responses for fruits, dark green vegetables and other vegetables were converted into values representing consumption per day. The daily consumption values were summed to create a total daily fruit and vegetable consumption variable. Low fruit and vegetable consumption was defined as consuming <2 fruits and/or vegetables daily(21).
Several variables were included as potential confounders in the analyses. Gender was dichotomised into male or female. Age groups were categorised into three groups: 18–34, 35–64, and ≥65. Race included white, black, Hispanic or other. Due to small proportions of Asians, American Indians or Alaskan Natives, and Pacific Islanders, these race categories were combined to other. Marital status was dichotomised into single or married. Educational attainment was described as: high school or less, some college and college graduate. BMI (kg/m2) was calculated from self-reported weight and height, and was categorised into three groups: <25, 25–29·9, ≥30·0 kg/m2. Poor mental health was assessed by the following BRFSS question: ‘Now thinking about your mental health, which includes stress, depression and problems with emotions, for how many days during the past 30 d was your mental health not good?’ Participants who reported fourteen or greater poor mental health days in the past 30 days were defined as having poor mental health. Previous studies indicated that health risk behaviours often co-occur together in a population(Reference Noble, Paul and Turon22). Three health risk behaviours (heavy drinking, smoking and physical inactivity) that are well established to be related to dietary quality were chosen as covariates. Participants were categorised as a heavy drinker if they reported more than fourteen drinks per week for males and more than seven drinks per week for females. As for cigarette smoking, participants were asked whether or not they currently smoked every day, some days or not at all. Based on this question, participants who reported they did not currently smoke were defined as non-smokers. All others were defined as current smokers. Leisure-time physical inactivity was dichotomised based on participants’ response when asked if they exercised any, in addition to physical activity performed as part of their employment within the last 30 d.
Statistical analyses
All analyses were completed using a statistical software (SAS, version 9.4; SAS Institute Inc.). To account for BRFSS sampling methodology, our analyses used survey sampling weights. In descriptive analyses, we reported frequencies and proportions for categorical variables, and means and standard deviations for continuous variables. The weighted χ 2 test was used to assess the relationship between low fruit and vegetable intake and gender, age, race/ethnicity, marital status, education, smoking status, BMI, poor mental health, heavy drinking, leisure-time physical inactivity, individual ACE types and overall ACE scores. Weighted multiple logistic regression models were used to estimate OR, adjusted OR (AOR) and 95 % CI for the examination of associations between ACE and low fruit and vegetable intake. We conducted two sets of multiple logistic regression analyses controlling for selected covariates. The first set assessed the relationship between cumulative ACE scores and low fruit and vegetable intake, and the second set examined the correlation between each of the eight ACE types and low fruit and vegetable intake. We used bivariate analyses to detect statistical differences between variables and considered α = 0·05 as the level of significance.
Results
Table 1 shows the characteristics of all respondents. Slightly over half of respondents were female (55·5 %), white (53·0 %), single (52·1 %) and aged between 35 and 64 years (50·9 %). 46·1 % of respondents obtained a high school education or less, and 40·1 % of them were categorised into BMI 25–29·9 kg/m2. The prevalence of three health risk behaviours was 6·2 % for heavy drinkers, 29 % of leisure-time physical inactivity and 17 % for current smokers. Approximately one-third of respondents experienced parental divorce/separation (33·6 %), verbal abuse (33·0 %) and living with a substance abuser (31·3 %). Nearly two-thirds (65·5 %) of all respondents had experienced at least one ACE, and 28·3 % of all respondents were exposed to ≥3 ACE. 47·2 % of respondents reported low fruit and vegetable intake.
Table 1 Sample characteristics, adverse childhood experiences (ACE) and low fruit and vegetable intake, Nevada Behavioral Risk Factor Surveillance System 2017, adults ≥18 years (n 2939)
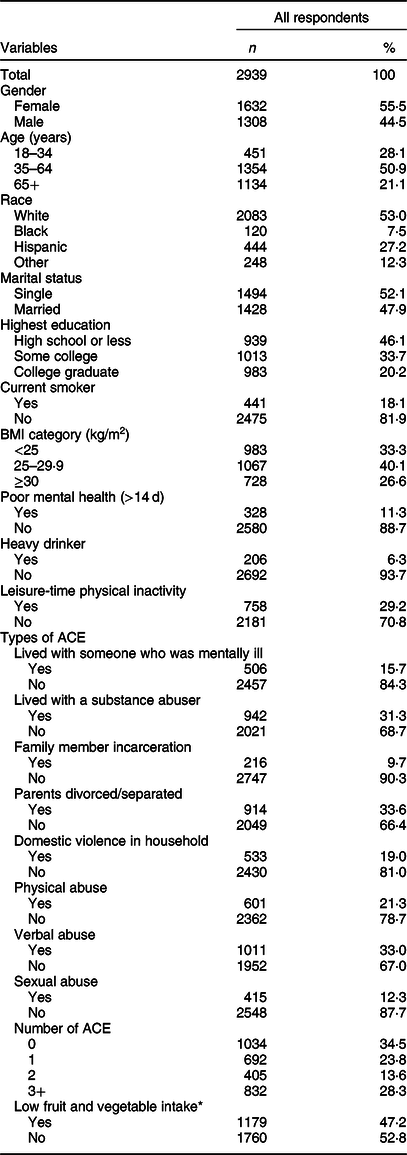
* Consumed fruits, dark green vegetables and other vegetables less than twice a day.
Table 2 presents the weighted prevalence and AOR of low fruit and vegetable intake by covariates and ACE score. Participants who consumed less fruits and vegetables were more likely to be male (55·0 v. 39·2 %), single (52·8 v. 40·8 %), have high school or less education (high school or less = 51·7 %, some college = 49·2 %, college graduate = 33·8 %), current smokers (59·1 v. 44·7 %), have a BMI ≥ 30·0 kg/m2 (BMI ≥30·0 kg/m2 = 52·4 %; BMI 25·0 to <30·0 kg/m2 = 47·7 %; BMI <25 kg/m2 = 42·5 %), have poor mental health status (60·7 % v. 45·3 %) and did not engage in leisure-time physical activity (59·6 v. 42·2 %). The number of ACE did not reach statistical significance based on a P-value of 0·71.
Table 2 Weighted prevalence of low fruit and vegetable consumption and adjusted OR for the association between adverse childhood experiences (ACE) and fruit and vegetable intake, Nevada Behavioral Risk Factor Surveillance System 2017, adults ≥18 years (n 2939)
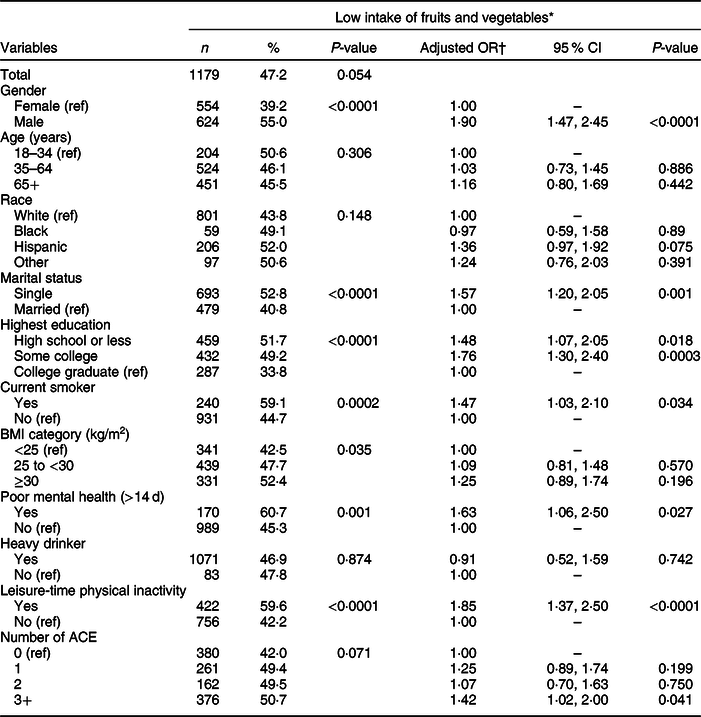
* Consumed fruits, dark green vegetables and other vegetables less than twice a day.
† Weighted logistic model includes ACE, gender, age, race, marital status, education, BMI, smoking status, mental health status, alcohol use and leisure-time physical inactivity.
Table 3 shows the weighted prevalence of low fruit and vegetable intake as well as AOR for each of the ACE types. Participants with a low intake of fruits and vegetables were more likely to report parental divorce/separation (52·5 v. 44·6 %) and physical abuse (54·9 v. 45·2 %). In the adjusted model, the odds of low fruit and vegetable intake were significantly higher among the participants who reported parental divorce/separation compared to those who did not experience this particular type of ACE (AOR 1·50, 95 % CI 1·13, 1·98).
Table 3 Weighted prevalence and adjusted OR of low fruit and vegetable intake by each type of adverse childhood experiences (ACE), Nevada Behavioral Risk Factor Surveillance System 2017, adults ≥18 years (n 2939)
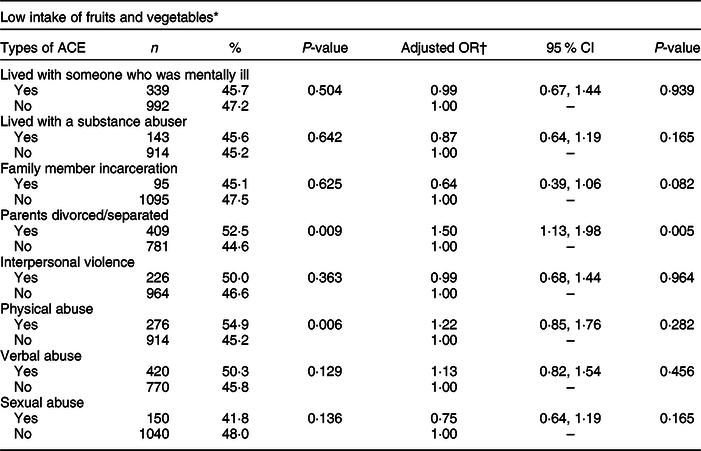
* Consumed fruits, dark green vegetables and other vegetables less than twice a day.
† Weighted logistic model includes ACE, gender, age, race, marital status, education, BMI, smoking status, mental health status, alcohol use and leisure-time physical inactivity.
Discussion
ACE has been linked to risky health behaviours, chronic health conditions, low life potential and early death(Reference Felitti, Anda and Nordenberg1–Reference Anda, Dong and Brown5,Reference Williamson, Thompson and Anda23,Reference Metzler, Merrick and Klevens24) . Using a population-based study design, the study aimed to examine the relationships between ACE and low intake of fruits and vegetables in adulthood. Our study extends the literature by commenting on the impact of ACE on dietary behaviour in adulthood, which is a potential intermediate outcome for common chronic diseases. With approximately three in every five adults experiencing at least one ACE as a child, the prevalence of ACE in our study is comparable to that of previous population-based studies(Reference Merrick, Ford and Ports6). Overall, our findings establish that the odds of low fruit and vegetable intake were significantly higher among those who experienced three or more types of ACE. However, contrary to our study hypotheses, we did not find a graded relationship between the number of ACE and low intake of fruits and vegetables. When individual ACE types were examined, exposure to parental divorce/separation was the only ACE type associated with a low intake of fruits and vegetables.
Few previous studies corroborate the relationships between ACE and dietary behaviour in adulthood found in this study. Russel et al.(Reference Russell, Hughes and Bellis25) conducted a cross-sectional study among adults in England and found that adults with unhappy and violent childhood were at a higher risk for low daily fruit and vegetable intake (AOR 2·67, 95 % CI 2·15, 3·06) compared to those with happy and non-violent childhoods. The OR of low fruit and vegetable intake reported in that study was significantly higher than one found in our study. This may be due, in part, to the different measures used in our study v. a previous study, in which self-assessment of childhood experience was gauged by asking two 10-point-scale questions. Another study conducted among college students has found that higher ACE scores were associated with poorer lifestyle habits, including lower fruit and vegetable intake(Reference Windle, Haardörfer and Getachew26). One of the potential explanations for these observed associations between ACE and fruit and vegetable intake could be that adults with multiple types of ACE are exposed to toxic stress, leading them to use food as a coping mechanism(Reference Wingenfeld, Kuehl and Boeker27,Reference Schrepf, Markon and Lutgendorf28) . People who are exposed to stress tend to have an increased drive to eat highly palatable, comfort food (i.e. high calorie-dense foods with a high content of sugar and fat) while decreasing their consumption of healthy foods, including vegetables and fruits(Reference Ulrich-Lai, Fulton and Wilson29,Reference Groesz, McCoy and Carl30) .
Another highlight of this study is that exposure to parental divorce/separation is a significant predictor of low intake of fruits and vegetables when individual ACE types were examined. Exposure to parental divorce/separation in childhood has been linked previously to poorer physical and mental health in adulthood and to stress biomarkers such as cortisol and C-reactive protein(Reference Lacey, Kumari and McMunn31,Reference Kraft and Luecken32) . A population-based study in Greece reported that parental divorce is significantly associated with overweight among children(Reference Yannakoulia, Papanikolaou and Hatzopoulou33). Because parental divorce/separation entails significant alterations in socioeconomical status and family environment surrounding a child, this particular types of ACE may have a consistent and lasting impact on dietary behaviour(Reference Mauskopf, O’Leary and Banihashemi34).
Several theories consistent with our findings connect ACE with the dietary behaviour in adulthood(Reference Miller, Chen and Parker10,Reference Vinther, Conklin and Wareham35) . There is increasing evidence suggesting that exposure to toxic stress during childhood might cause altered brain development leading to an array of negative consequences. These include impacts on the plastic processes of brain development, emotional regulation, cognitive response, memory and learning, and autonomic, endocrine and immune systems(Reference Miller, Chen and Parker10). Exposure to ACE during critical development period has a long-term effect on CNS areas associated with cognitive function, such as decision-making and motivated behaviour(Reference Lovallo36). Early stress may lead one to develop poor self-regulation skills, creating a proclivity for unhealthy lifestyle choices, including unhealthy diet(Reference Miller, Chen and Parker10).
The study also identified that both sociodemographic factors and health behaviours play significant roles in relationships between ACE and low fruit and vegetable intake. In terms of sociodemographic factors, being male, single and having less than college education had an increased risk for low fruit and vegetable intake. The odds of consuming low fruits and vegetables were also higher among those who were current smokers, had poor mental health status and did not engage in leisure-time physical activity. Findings are consistent with earlier works demonstrating that gender, marital status, education level as well as smoking status, mental health status and physical activity level are significant predictors of fruit and vegetable consumption(Reference Lee-Kwan, Moore and Blanck15,Reference Vinther, Conklin and Wareham35,Reference Grosso, Micek and Godos37,Reference Saghafian, Malmir and Saneei38) . Future analyses are needed to explore the potential interactions between these variables. In addition, further investigation is needed to determine if other components of dietary behaviour, such as the consumption of high-fat, high-calorie diet, could impact the strength of association between fruit and vegetable consumption among adults with ACE.
There are some strengths and limitations to our study that should be kept in mind when interpreting our findings. The primary strength of this study is a population-based study design with a large sample size. This is one of the first studies, to our knowledge, to examine the relationships between cumulative ACE scores and fruit and vegetable intake. In addition, the study further explored the impact of individual ACE measures on fruit and vegetable intake in adulthood. The results of this study should be interpreted in light of several considerations. It is known that a number of ACE cluster together among subgroups of populations, and an examination of individual ACE might overestimate the effect by the presence of other ACE(Reference Dong, Giles and Felitti4,Reference Brown, Rienks and McCrae39) . This study is limited by the cross-sectional design, and as such, it precludes making an analysis of the temporal relationship between ACE and low fruit and vegetable intake. Second, we relied on retrospective self-reports of ACE and fruit and vegetable intake, which may be affected by recall or other reporting bias. Longitudinal studies of child abuse demonstrate that adults may underreport incidences of child abuse when reporting on their experience as an adult(Reference Femina, Yeager and Lewis40,Reference Williams41) . The accuracy of dietary surveys relies on respondents’ memory, and respondents may adjust their reported consumption patterns to simplify the interview process as well as to impress the interviewer(Reference Roark and Niederhauser42,Reference Woodside, Young and McKinley43) . We asked how many times per day, week or month the participants consumed fruits and vegetables, which did not account for serving size, nor were any questions asked about other food groups. Socioeconomic status is known to affect fruit and vegetable intake among adults. However, income level was not included in the analysis due to a large missing value. Instead, education level was included in the analysis. Lastly, the measurement of ACE omits other early-life experiences such as childhood socioeconomic status, food insecurity, emotional and physical neglect, which may also have a lasting impact on dietary behaviour in adulthood.
Conclusion
Despite certain limitations, findings of this study are noteworthy. The study suggests that the cumulative effects of experiencing multiple ACE and a specific ACE, notably exposure to parental divorce/separation, deteriorate fruit and vegetable intake in adulthood. The growing evidence connecting ACE scores with high-risk behaviour and the relatively high prevalence of ACE in the United States highlight the need for targeted public health interventions and policies to prevent child abuse and household dysfunction among high-risk populations. In addition, it is equally important to promote fruit and vegetable intake among the populations experiencing ACE through appropriate strategies. Future research should attempt to examine how ACE translates into dietary behaviour longitudinally and explore ways to promote healthy eating among those with ACE.
Acknowledgements
Acknowledgements: None. Financial support: This research received no specific grant from any funding agency, commercial or not-for-profit sectors. Conflict of interest: None. Authorship: M.H. developed research questions, performed data analysis and wrote the manuscript. W.Y. supervised the study including data collection, study design and manuscript drafts. Both authors read and approved the final manuscript. Ethics of human subject participation: This study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects were approved by the Institutional Review Board of the University of Nevada, Reno. Verbal informed consent was obtained from all subjects, and verbal consent was witnessed and formally recorded.






