With worldwide concerns about obesity and diseases related to it (e.g. diabetes and CVD), there is substantial interest in shifting populations to healthier weights and better health. More precisely, there is interest in reducing body fat since fat – particularly visceral or abdominal fat – may matter more than weight when it comes to health(
Reference Huffman and Barzilai
1
–
Reference Oliveros, Somers and Sochor
3
). Nevertheless, much of the evidence regarding obesity and related diseases focuses on body weight, rather than body fat. In reviewing such evidence, therefore, the present paper will therefore also often use the imprecise term ‘weight’ as opposed to ‘fat’, pointing out when such imprecision might mislead thinking.
One way such imprecision might mislead thinking is in supporting the notions that (i) ‘a calorie is a calorie’Footnote † and (ii) intervening on calories is the best way to address obesity (i.e. the quantitative problem of excess pounds or kilograms on a scale as opposed to the qualitative problem of altered body metabolism). The two calorie notions are largely about balance sheets, essentially considering calories like units of body weight and units of body weight like inverse units of health; according to the logic, obese individuals need only try to consume fewer calories than they burn and they will achieve healthier weights and better health.
Although such logic is intuitive and enticing, reality is not quite so simple and existing evidence challenges calorie-focused notions. A view focused more on food quality, rather than caloric quantity, may help better explain and better address the growing problems of excess weight – or more precisely excess fat – and related conditions. Conversely, messages and initiatives based on the idea of calorie equivalency (that a ‘calorie is a calorie’) and interventions directed at calorie balance sheets may make these problems worse. The present paper reviews various problems with calorie-focused thinking, considers several advantages of ‘more-nuanced thinking’ (that considers calories principally as subordinate concerns to qualitative differences in food) and proposes an alternative path for public health to move forward.
The problem with the idea of calorie equivalency
A calorie is a unit of energy. As related to food energy, calories measure the potential energy a food could release. One calorie of potential energy equals one calorie of potential energy, just as one unit of anything equals another unit of that same anything. To say ‘a calorie is a calorie’ then is tantamount to the identity property in mathematics (A=A). As such, it is irrefutable.
In practice, however, the statement that ‘a calorie is a calorie’ often implies something different from mathematical identity. It implies that any two different foods, which have equivalent amounts of potential energy, will produce identical biological effects with regard to body weight/body fatness when consumed. By this thinking, a calorie’s worth of salmon, olive oil, white rice or vodka would each be equivalent and each expected to have the same implications for body weight and body fatness. Indeed, stating ‘a calorie is a calorie’ suggests that potential energy is the essential concern and that qualitative differences in the substances providing that energy are irrelevant.
But a calorie’s worth of salmon (largely protein) and a calorie’s worth of olive oil (purely fat) have very different biological effects from a calorie’s worth of white rice (refined carbohydrate) or a calorie’s worth of vodka (mostly alcohol) – particularly with regard to body weight/body fatness. Indeed, scientists have recognized differences in the weight-related physiological effects of different calorie sources for more than half a century(
Reference Thomas
4
). Although much early knowledge was based on animal studies, subsequent studies in human subjects have shown that calorie-providing proteins, fats, carbohydrates and alcohol each have substantially different effects on a variety of physiological pathways and hormones relevant to satiety, food consumption, weight maintenance and body composition: for example, different effects on ghrelin (an appetite-stimulating hormone), leptin (an appetite-suppressing hormone), glucagon (a hormone that raises blood sugar) and insulin (a hormone that lowers blood sugar)(
Reference Benedini, Codella and Caumo
5
–
Reference Lomenick, Melguizo and Mitchell
7
).
The aforementioned descriptions of hormone activities are greatly oversimplified and the list of hormones far from exhaustive, but the examples serve to suggest that a given calorie’s worth of salmon, olive oil, white rice or vodka might each behave quite differently in the body and produce different ultimate effects. Indeed, whereas some ‘calories’ (i.e. some amounts of different foods, quantified by their potential energy) induce metabolic pathways and hormones that squelch appetite and promote energy utilization, others stimulate pathways that promote hunger and energy storage. Even controlling for total calorie intake and energy expenditure from physical activity, qualitative differences in calories have different implications for obesity(
Reference Riera-Crichton and Tefft
8
); a calorie’s worth of one food is not the same a calorie’s worth of another(
Reference Riera-Crichton and Tefft
8
–
Reference Westerterp, Wilson and Rolland
14
).
Trying to intervene on calories is implausible and ineffective
It follows from the problematic notion of calorie equivalency that any calorie consumed might be offset by a single calorie expended. Thus individuals wishing to lose weight should simply consume fewer calories than they expend. In other words, individuals should intervene on caloric quantity by consciously trying to ‘eat less’ and ‘move more’ than they otherwise would to establish ‘caloric deficit’ or ‘negative energy balance’(
Reference Guth
15
).
The problem with trying to ‘eat less’ and ‘move more’ to achieve – and more importantly, maintain – caloric deficit or negative energy balance is that it is practically and biologically implausible. Practically, even the most motivated, informed and knowledgeable individuals are unlikely to be able to estimate their actual calorie intake (not just ingested, informed by misleading food labels(
Reference Baer, Gebauer and Novotny
16
,
Reference Urban, Dallal and Robinson
17
), but absorbed(
Reference Hall, Heymsfield and Kemnitz
18
,
Reference Novotny, Gebauer and Baer
19
)) or their actual calorie expenditure (not just in physical activity(
Reference Lee, Kim and Welk
20
) but in variably efficient, silent and constantly fluctuating digestive and metabolic processes(
Reference Feinman and Fine
12
,
Reference Westerterp, Wilson and Rolland
14
,
Reference Hall, Heymsfield and Kemnitz
18
,
Reference Jakubowicz, Barnea and Wainstein
21
)) and do so with sufficient accuracy and precision to maintain any kind of useful real-time calorie balance sheets. Biologically, calorie intake and calorie expenditure are coupled(
Reference Leibel, Rosenbaum and Hirsch
22
–
Reference Ochner, Barrios and Lee
26
). Unless substantial uncoupling occurs, reducing calories consumed will necessarily result in a compensatory drive to reduce calories expended and vice versa(
Reference Ochner, Barrios and Lee
26
–
Reference Hall, Hammond and Rahmandad
31
). For this reason, people who try underconsuming calories become tired (an expenditure compensation) and hungry (an intake compensation), and one reason they often fail to lose weight (or have unimpressive results)(
Reference Sumithran and Proietto
25
,
Reference Ochner, Barrios and Lee
26
,
Reference Nackers, Middleton and Dubyak
32
,
Reference Maclean, Bergouignan and Cornier
33
) may be that resultant hunger, particularly an increased desire for high-calorie foods(
Reference Sumithran and Proietto
25
,
Reference Ochner, Barrios and Lee
26
), drives compensatory overconsumption(
Reference Ochner, Barrios and Lee
26
,
Reference Heymsfield, Harp and Reitman
28
,
Reference Maclean, Bergouignan and Cornier
33
).
Of course, some individuals do succeed at sufficiently uncoupling energy balance (i.e. do expend more calories than they consume) and do lose weight. But saying that these individuals lose weight because they expend more calories than they consume is like saying that students are late for class because they arrive after the bell rings. Both statements are true, but neither is causal. The associations do not explain the ‘why’ (i.e. in the case of expending more calories than consumed, why the uncoupling occurred).
Calorie equivalency and calorie balance sheets cannot explain the ‘why’; why some people succeed in eating less and/or moving more and lose weight while others fail and gain weight. Calorie-focused thinking does not tell us why some people achieve net burning or net storage of calories, or how it is entirely possible to lose weight (as lean mass) and still gain fat (i.e. become more obese). Calorie thinking also cannot account for the dynamic non-linear response of body weight to stable energy imbalances over time(
Reference Shook, Hand and Blair
13
,
Reference Hall, Sacks and Chandramohan
34
,
Reference Hall, Butte and Swinburn
35
). Likewise, calorie thinking does not address why obesity-related metabolic abnormalities(
Reference Shah and Braverman
36
,
Reference Wildman, Muntner and Reynolds
37
) and adverse events of obesity-related diseases(
Reference Coutinho, Goel and Correa de Sa
38
–
Reference Hamer and Stamatakis
40
) may both occur before there is any gain in weight(
Reference Oliveros, Somers and Sochor
3
,
Reference Kramer, Zinman and Retnakaran
41
), why metabolic improvements may occur at stable weight(
Reference Gannon and Nuttall
42
) or why obesity-related adverse events may not decline with weight loss(
43
). Any explanation for obesity should provide insights into these observations.
More-nuanced thinking about obesity and related diseases
To understand another kind of thinking about obesity and related diseases – and why individuals may show metabolic changes associated with being overweight before any detectable weight gain occurs – it is useful to consider body fat. Body fat – particularly visceral or abdominal fat – is a complex tissue that plays critical roles in appetite stimulation, energy expenditure and weight regulation. Normally, when a body’s fat cells are replete (i.e. full with stored fat), they release a hormone called leptin. Leptin stimulates parts of the brain to send additional hormone and nerve signals to the thyroid gland, skeletal muscles, heart, intestines and other fat cells(
Reference Sumithran and Proietto
25
,
Reference Lustig
27
). These signals are to decrease energy intake (i.e. to ‘eat less’) and increase energy expenditure (e.g. to ‘move more’)(
Reference Lustig
27
,
Reference Speakman, Levitsky and Allison
29
).
As individuals start to become obese, however (metabolically speaking, if not yet by weight on a scale), something goes awry with the signalling. Fat-cell repletion is no longer recognized and rather than there being signals to suppress appetite and increase activity as fat stores increase, there are signals to increase energy intake and reduce energy expenditure(
Reference Lustig
27
,
Reference Speakman, Levitsky and Allison
29
,
Reference Ludwig and Friedman
30
,
Reference Richmond, Davey Smith and Ness
44
). In other words, ‘eating more’ and ‘moving less’, thought to be causes of body fattening by calorie-focused thinking, may actually be a result of body fattening(
Reference Lustig
27
,
Reference Speakman, Levitsky and Allison
29
,
Reference Ludwig and Friedman
30
,
Reference Richmond, Davey Smith and Ness
44
).
So if eating more and moving less could be a result of body fattening, what causes bodies to fatten (i.e. to undergo metabolic dysfunction followed by fat gain, and then weight gain) in the first place; that is, what prevents leptin from doing its job of satiating appetite and promoting energy expenditure? The answer is not entirely clear, but one hypothesis implicates concentrated sources of rapidly absorbable carbohydrates in the diet and the hormone insulin.
Insulin is a pancreatic hormone that helps drive ingested nutrients into cells; its release is most brisk and pronounced following the ingestion of rapidly absorbable carbohydrates (as compared with fats, proteins, alcohol and more slowly absorbed carbohydrates(
Reference Raben, Agerholm-Larsen and Flint
6
,
Reference Mann
45
–
Reference Ludwig
48
)). Rapidly absorbable carbohydrates – sugars and refined starches like white rice and foods consisting substantively of white flour – cause blood sugar to rise briskly and insulin levels to respond in kind(
Reference Mann
45
–
Reference Ludwig
48
). The rapid insulin elevations produced by these foods cause correspondingly rapid drops in blood sugar. Food cravings result (to restore fallen fuel levels), particularly appetites for something sweet(
Reference Raben, Agerholm-Larsen and Flint
6
,
Reference Ludwig
48
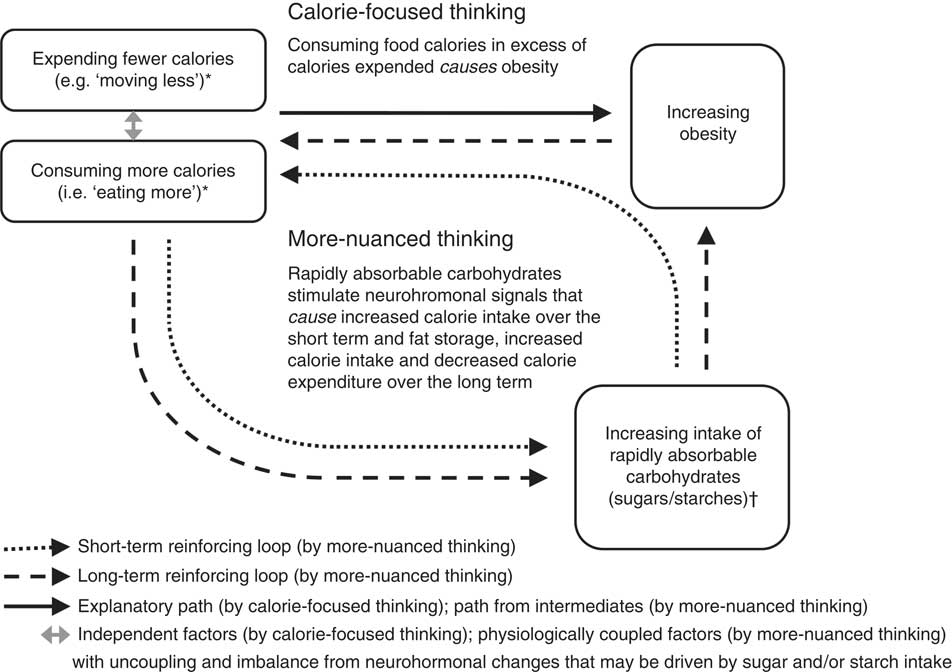
). Thus, in the short term, intake of rapidly absorbable carbohydrates may promote ‘eating more’ in general and create a reinforcing loop for overconsumption of additional rapidly absorbable (sweet) carbohydrates in particular (Fig. 1)(
Reference Lustig
27
,
Reference Ludwig
48
).
Fig. 1 Calorie-focused thinking versus more-nuanced thinking about obesity. Single-headed arrows represent direct associations in presumed causal directions. *‘Expending fewer calories’ includes all energy expenditure, but ‘moving less’ specifically refers to a relatively lower degree of physical inactivity from baseline. ‘Eating more’ refers to relative overeating from baseline. †Over the short term, the intake of rapidly absorbable carbohydrates – through spikes in blood sugar and insulin, and through sweet cravings – promotes a reinforcing loop with ‘eating more’ in general and eating more rapidly absorbable carbohydrates in particular (dotted arrows). Over the long term, neurohormonal alterations, perhaps chiefly through insulin and leptin resistance – leading to and contributed by growing abdominal fat – perpetuate an indirect reinforcing loop with ‘eating more’ (dashed arrows) and also promote ‘moving less’. Decreasing the intake of rapidly absorbed sugars and starches (as found abundantly in processed foods) and increasing the consumption of whole/minimally processed foods may disrupt these loops, overall calorie imbalance, and both the hormonal dysfunction and excess body mass characterizing obesity
Over the long term, overconsumption of rapidly absorbable carbohydrates may promote leptin resistance. Such resistance may occur through microbiota-mediated inflammatory pathways(
Reference Spreadbury
49
) or through other metabolic changes (e.g. chronic insulin elevations)(
Reference Lustig
27
). Regardless, with leptin’s actions largely disabled, the result of high sugar and starch intake is a neurohormonal drive to ‘eat more’ and ‘move less’ (Fig. 1)(
Reference Lustig
27
,
Reference Ludwig
48
,
Reference Spreadbury
49
).
By more-nuanced thinking, then, what counts for obesity and related diseases is not the number of calories in specific foods but rather the concentration and type of carbohydrates these foods contain(
Reference Ludwig and Friedman
30
,
Reference Spreadbury
49
,
Reference Taubes
50
). Total calorie balance is important in both ways of thinking, but whereas calorie-focused thinking directs dietary recommendations towards calorie counts (being primarily quantitative), more-nuanced thinking directs dietary recommendations towards calorie sources (being primarily qualitative); the number of calories consumed and expended are only secondary/intermediate considerations.
Different dietary recommendations by calorie-focused thinking and more-nuanced thinking
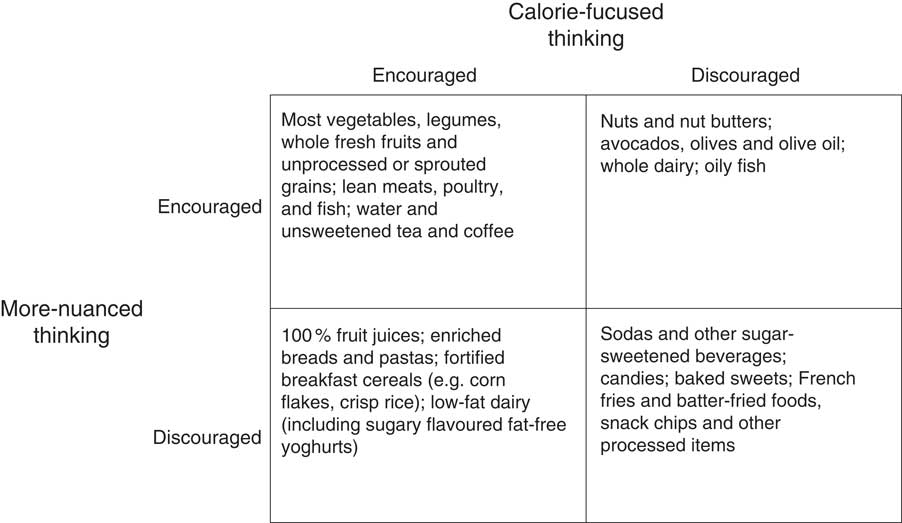
A comparison of selected foods that might be encouraged or discouraged by calorie-focused thinking and a more-nuanced thinking appears in Fig. 2. Concordant cells reveal there is some common ground. For example, both ways of thinking discourage sodas, but whereas more-nuanced thinking discourages sodas based on carbohydrate content and character (i.e. high concentrations of rapidly absorbable sugar), calorie-focused thinking discourages sodas based on the idea of ‘empty calories’. ‘Empty calories’ are foods that contribute energy but few substances thought to be beneficial like vitamins, minerals and fibre. By calorie-focused thinking, ‘empty calories’ waste precious space on the intake side of calorie balance sheets.
Fig. 2 Comparison of selected foods that might be encouraged or discouraged by calorie-focused thinking and more-nuanced thinking. This figure is not comprehensive, is not a description of any specific diet plan, and does not represent the recommendations or guidelines of any particular individual or organization. It does not explicitly address issues relevant to public health nutrition beyond calorie- and carbohydrate-related concerns (e.g. food production, climate change, One Health, etc.). Additionally, categorizations are based on somewhat relative concepts such as how ‘empty’ calories are and how ‘rapidly absorbable’ carbohydrate content is; placement of listed and unlisted items within the construct may be debatable. ‘Encouraged’=okay to eat or even desirable as a focus of one’s diet, particularly as an alternative to foods that are ‘discouraged’; ‘discouraged’=to be avoided or limited in quantity
Figure 2 also demonstrates important discordance between calorie-focused thinking and more-nuanced thinking. For instance, 100 % fruit juices – full of vitamins, minerals and sometimes fibre – are not ‘empty’ and may even be considered healthy and desirable by calorie-focused thinking(
Reference Nicklas, Kleinman and O’Neil
51
). By more-nuanced thinking, however, 100 % fruit juices are just as undesirable as sodas given both are mostly sugar in concentrated liquid form(
Reference Wojcicki and Heyman
52
).
Other discordances in dietary recommendations between calorie-focused thinking and more-nuanced thinking, and perhaps the most important differences, relate to dietary fat. Dietary fat has by far the most calories of any of the energy-providing compounds in food: about 9 kcal/g as compared with roughly 7 kcal/g for alcohol, 4 kcal/g for protein and 4 kcal/g for carbohydrate(
Reference Atwater
53
). Thus, calorie-focused thinking has an inherent bias against dietary fat. This bias leads to public health messages and interventions to decrease the intake of fatty foods or reduce or remove the fat from high-fat foods (often replacing fat with less-calorie-dense – often rapidly absorbable – carbohydrates).
Calorie-focused thinking generally endorses foods that are low in fat and calories, as long as those calories are not ‘empty’. In contrast, more-nuanced thinking has no problems with fat or calories, per se, and places the blame squarely on foods with the most rapidly absorbable carbohydrates (Fig. 2). Clearly these two ways of thinking are very different. A question for public health moving forward is: would food choices that could result from a continued primary focus on calories (calorie-focused thinking – Fig. 2) be best for population weight and health?
Pertinent clinical and population evidence for two different ways of thinking
Consider an experiment in children(
Reference Wansink, Shimizu and Brumberg
54
). Sixth graders with comparable baseline satiety were allowed to eat as much as they wanted of two highly palatable child-friendly snacks: cheese wedges/rounds or potato chips. A quantity of cheese (mostly fat with some protein and negligible carbohydrate) might offer about 50 % more calories than an identical quantity of chips (mostly carbohydrate and fat with negligible protein). By calorie-focused thinking, comparably hungry children should eat more calories of cheese because cheese has more calories. By more-nuanced thinking, comparably hungry children should eat more calories of chips because chips, being rich in rapidly absorbable starch, should tend to promote continued eating (short-term reinforcing loop, Fig. 1)(
Reference Ludwig
48
).
What actually happened in the experiment was that children in the potato chip group consumed over three times more calories than children in the cheese group(
Reference Wansink, Shimizu and Brumberg
54
). While a protein difference between the snacks might certainly have been a factor (with experimental trials suggesting a superior(
Reference Marmonier, Chapelot and Louis-Sylvestre
55
), albeit not always statistically significant(
Reference Vozzo, Wittert and Cocchiaro
56
), satiating power of protein), all foods are inevitable mixes of different components and the point here is that the food with the higher starch content prompted greater consumption. This result is consistent with a meta-analysis showing children have greater energy intake following consumption of the most rapidly absorbable carbohydrates(
Reference Rouhani, Salehi-Abargouei and Azadbakht
57
).
Notably in the experiment described above, the effect of eating more calories in the high-carbohydrate (chips) condition was even more pronounced among overweight and obese children(
Reference Wansink, Shimizu and Brumberg
54
). This result is consistent with another trial showing greater hunger in obese children after a high-carbohydrate meal(
Reference Lomenick, Melguizo and Mitchell
7
) and consistent with the long-term reinforcing loop in Fig. 1.
Although the chips-and-cheese experiment did not assess children’s total caloric intake for the day outside of the single snack episode, it is likely that children consuming cheese ate fewer calories overall for the day, whereas children consuming chips ate more. Such an outcome would be suggested by fifteen of sixteen single-day studies in adults that showed increased hunger, lower satiety or greater calorie intake after consuming rapidly absorbable carbohydrates v. not(
Reference Ludwig
58
). The outcome might also be suggested by two other studies in children in which restaurant fast-food consumption was associated with a net increase in total energy intake for the day(
Reference Powell and Nguyen
59
,
Reference Ebbeling, Sinclair and Pereira
60
) – although only for overweight individuals in one study(
Reference Ebbeling, Sinclair and Pereira
60
), consistent with the long-term reinforcing loop in Fig. 1. Granted, for a given fast-food meal, the studies referenced above cannot distinguish if greater total caloric intake was the result of a greasy burger (per calorie-focused thinking), a refined bun (per more-nuanced thinking) or accompanying French fries (per both ways of thinking). However, substantial evidence now implicates foods that are low in fat (and, thus, relatively low in calories), like potatoes(
Reference Mozaffarian, Hao and Rimm
61
), white rice(
Reference Hu, Pan and Malik
62
) and sugary beverages(
Reference Mozaffarian, Hao and Rimm
61
,
Reference Qi, Chu and Kang
63
–
Reference Bes-Rastrollo, Schulze and Ruiz-Canela
66
), in the development and persistence of obesity and risk for related diseases. Conversely, evidence is mounting to exonerate higher-calorie foods that are rich in fat like nuts(
Reference Mozaffarian, Hao and Rimm
61
,
Reference Tan and Mattes
67
–
Reference Viguiliouk, Kendall and Blanco Mejia
74
), oily fish(
Reference Thorsdottir, Tomasson and Gunnarsdottir
75
) and olive oil(
Reference Estruch, Ros and Salas-Salvado
69
,
Reference Perez-Martinez, Garcia-Rios and Delgado-Lista
76
,
Reference Salas-Salvado, Bullo and Estruch
77
), and even foods high in saturated fat(
Reference Siri-Tarino, Sun and Hu
78
,
Reference Chowdhury, Warnakula and Kunutsor
79
) like dairy products(
Reference Scharf, Demmer and Deboer
80
–
Reference Martinez-Gonzalez, Sayon-Orea and Ruiz-Canela
88
). Indeed, higher-calorie fattier foods and higher-fat diets may produce and sustain as much or more weight loss than calorie-restricted or higher-carbohydrate diets(
Reference Kekwick and Pawan
9
,
Reference Ebbeling, Swain and Feldman
10
,
Reference Hession, Rolland and Kulkarni
89
–
Reference Gow, Ho and Burrows
98
) – particularly among those already having metabolic abnormalities(
Reference Volek, Phinney and Forsythe
93
,
Reference Westman, Yancy and Mavropoulos
94
,
Reference Ebbeling, Leidig and Feldman
99
). Moreover, certain fattier/lower-carbohydrate diets may also be associated with favourable metabolic indicators(
Reference Ebbeling, Swain and Feldman
10
,
Reference Hession, Rolland and Kulkarni
89
,
Reference Hu, Mills and Yao
91
–
Reference Westman, Yancy and Mavropoulos
94
,
Reference Gow, Ho and Burrows
98
–
Reference Bazzano, Hu and Reynolds
109
), reduced adverse health events(
Reference Estruch, Ros and Salas-Salvado
69
,
Reference Corella, Carrasco and Sorli
102
,
Reference Fung, Rexrode and Mantzoros
110
,
Reference Sofi, Macchi and Abbate
111
) and delayed mortality(
Reference Fung, Rexrode and Mantzoros
110
–
Reference Trichopoulou, Orfanos and Norat
113
).
The situation for public health moving forward
Fuelled not exclusively but in no small part by calorie-focused thinking, fats in foods and fattier diets became the enemies of public health campaigns of the 1980s and 1990s. Lower-calorie sugars replaced higher-calorie oils in many foods and people shifted their consumption from fats to carbohydrates (most often, the rapidly absorbable kinds). As in the chips-and-cheese experiment described above, greater refined carbohydrate intake was associated with greater total calorie intake, but now on a population level(
Reference Marantz, Bird and Alderman
114
,
115
). In other words, people did not eat less when lower-calorie foods and diets were advised, they ate more. Obesity rates increased right along with greater consumption(
Reference Marantz, Bird and Alderman
114
,
115
). Diabetes rates increased too(
116
,
117
) and although these findings do not prove causation, they certainly do not support continuing forward under the current logic of calorie-focused thinking, with the food choices it could encourage (Fig. 2) or the tenuous notions that follow from it (Table 1).
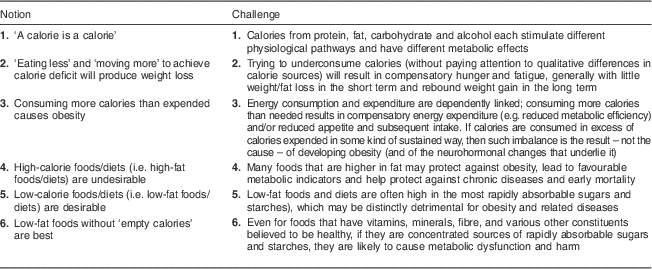
Table 1 Notions derived from calorie-focused thinking and challenges to those notions
Calorie-focused public health initiatives might continue to produce unintended, even ironic, consequences. Initiatives like calorie labelling for example – first for food packages and more recently for restaurant menus and menu boards – are meant to steer both consumer choices and food-industry offerings towards lower-calorie options(
Reference Hodge and White
118
). Despite national enthusiasm for the idea(
119
,
Reference Block and Roberto
120
), whether calorie labelling will have the desired effect seems doubtful(
Reference Swartz, Braxton and Viera
121
–
Reference Sinclair, Cooper and Mansfield
124
). Also in doubt is whether labelling will actually improve population health. There is already suggestion that some labelling may produce effects opposite to those intended(
Reference Downs, Wisdom and Wansink
125
). And there is the distinct possibility that calorie labelling could further move food production and consumption away from healthful high-fat foods (like nuts) and towards sugary and starchy items (like low-fat baked potato chips), promoting further increases in diseases characterized by abdominal fat and metabolic dysfunction.
There are, admittedly, other existing public health initiatives that, at least on the surface, seem more consistent with the logic of ‘more-nuanced thinking’; for instance, proposals to tax and limit sugary beverages(
Reference Wojcicki and Heyman
52
,
Reference Brownell and Frieden
126
,
Reference Brownell, Farley and Willett
127
). Nevertheless, these initiatives are usually framed around the idea of ‘empty calories’, which totally misses the point. Even the Food and Drug Administration’s proposed changes to packaged-food labels – which would newly report the amount of ‘added sugars’ in a product – place even more emphasis on calories than current labels by visually subordinating all other label information and highlighting calories in an enormous bold typeface(
128
).
What existing and planned initiatives seem not to acknowledge is that calories from added sugars and starches are worse than just ‘empty’ (detriment through omission); evidence suggests they are actively harmful (detriment through commission)(
Reference Mozaffarian, Hao and Rimm
61
,
Reference Hu, Pan and Malik
62
,
Reference Schwingshackl and Hoffmann
129
,
Reference Te Morenga, Howatson and Jones
130
). While responses of individual consumers may vary (e.g. due to their personal genetic susceptibility(
Reference Ludwig
48
,
Reference Qi, Chu and Kang
63
) or that of their resident gut microbes(
Reference Turnbaugh, Ridaura and Faith
131
)), there is good reason to believe that rapidly absorbable carbohydrates tend to promote obesity, and diseases commonly associated with it, in general(
Reference Mann
45
,
Reference Ludwig
48
,
Reference Qi, Chu and Kang
63
,
Reference Sorensen, Raben and Stender
132
–
Reference Yang, Zhang and Gregg
137
).
The problem for public health is that continuing to focus on quantifying calories may misdirect thinking on obesity and related diseases and promote destructive messages. For instance, in a 2013 editorial, the president of the Institute of Medicine listed gluttony and sloth as ‘obvious’ ‘deadly sins’ for public health to address(
Reference Fineberg
138
). His argument (which had been made before(
Reference Ramos Salas, Forhan and Sharma
139
)) suggested obesity and related diseases are matters only of personal resolve and self-control; if people just had more motivation and will-power, they could consciously control their calorie balance sheets, eat less, move more and lose weight. It stands to reason that those subscribing to the Institute of Medicine logic might blame an overconsuming, inactive adolescent for growing fat. But would they blame the same overconsuming, inactive adolescent for growing tall?
Just as children do not enter puberty and grow tall because they overeat and sleep more, neither do individuals start to fatten and become obese because they eat too much and move too little. In both cases overconsumption and inactivity are intermediate effects; neurohormonal changes are the cause. The case of pubertal growth represents normal development, but the case of fattening represents decided pathology; pathology that may be modifiable through dietary change. Perhaps if we shifted food production and people’s consumption away from added sugars and refined starches, we could avoid the resultant metabolic dysfunction and corpulence that have come to plague our populations. Instead of futilely promoting messages to ‘eat less’ and ‘move more’(
Reference Shook, Hand and Blair
13
,
Reference Ramos Salas, Forhan and Sharma
139
), perhaps we should do more to promote the consumption of whole/minimally processed foods(
Reference Mozaffarian, Rogoff and Ludwig
140
) – like more of those in the upper row of Fig. 2 – foods that might make ‘eating less’ and moving more’ more possible.
Concluding thoughts
Calorie-focused thinking may have already exacerbated the epidemics of obesity and related diseases. And while there has been much progress in redirecting dietary focus towards actual foods(
Reference Katz and Meller
141
), there is still too much focus on eating ‘too much’(
Reference Guth
15
). Focusing quantitatively, particularly on the calories available from specific foods, fails to recognize the broader metabolic effects of foods themselves. Foods that are highly processed and comprised mostly of rapidly absorbable sugars and starches may be of greatest concern. Such carbohydrates may induce neurohormonal changes that might, in turn, help produce the overeating and inactivity often interpreted as causative for obesity. In other words, unhealthy foods may make double victims of their consumers, who might not only become obese by eating them but also receive harsh criticism for their substantial appetites and apparent laziness that result.
As the saying often attributed to the Albert Einstein goes, ‘not everything that can be counted counts’, and advice to count calories, or to try to change calorie balance sheets by intervening on quantities of undifferentiated foods, seems misdirected. Imagine comparably misdirected advice: for instance, to count fluid ounces, drink less and urinate more – advice that might likewise result in temporary weight loss (but no fat loss) and be uncomfortable, unsustainable, unreasonable and unhelpful; and likewise oppose coupled neurohormonally driven physiology in futility. Yes, calories count, and calorie balance sheets matter, but net intake or expenditure probably results more from qualitative distinctions in the foods we eat than conscious attempts at quantitative control(
Reference Ludwig and Friedman
30
). New public health initiatives and messages focused on encouraging consumption of whole/minimally processed foods would be ideal(
Reference Mozaffarian, Rogoff and Ludwig
140
), especially to counteract industry’s near-exclusive marketing of foods that are highly processed/refined and concentrated sources of the most rapidly absorbable starches and sugars.
Promoting the consumption of whole foods will require careful attention to food systems, cultural traditions, peer influences, food environments, assistance programmes and a host of other issues beyond the scope of the present commentary. But as a guiding principle, the public health community should not be trying to cut calories from available foods(
142
), we should be improving the quality of the foods available that provide our calories. We should be promoting foods that do not prompt, or indeed programme, us to overeat.
Although focusing on refined starch and sugar content might seem like a logical path forward, such narrow focus could lead to unintended consequences, as when public health campaigns demonized fat. For this reason, the recent WHO draft guideline to more strictly limit the intake of all sugars(
143
), the recent proposition in England for a sugar tax(
144
), and the recent proposal in California to place health warning labels on sugary drinks(
Reference Calefati
145
), while all appropriately focused, should be evaluated carefully before wider implementation. Coordination with the food industry will be challenging, but while working towards improving the quality of foods that are produced and working to support the consumption of whole/minimally processed products, at the very least, public health should not continue to promote messages that create and blame victims or that, in all likelihood, continue to exacerbate epidemics of obesity and related diseases.
With worldwide concerns about obesity and diseases related to it (e.g. diabetes and CVD), there is substantial interest in shifting populations to healthier weights and better health. More precisely, there is interest in reducing body fat since fat – particularly visceral or abdominal fat – may matter more than weight when it comes to health( Reference Huffman and Barzilai 1 – Reference Oliveros, Somers and Sochor 3 ). Nevertheless, much of the evidence regarding obesity and related diseases focuses on body weight, rather than body fat. In reviewing such evidence, therefore, the present paper will therefore also often use the imprecise term ‘weight’ as opposed to ‘fat’, pointing out when such imprecision might mislead thinking.
One way such imprecision might mislead thinking is in supporting the notions that (i) ‘a calorie is a calorie’Footnote † and (ii) intervening on calories is the best way to address obesity (i.e. the quantitative problem of excess pounds or kilograms on a scale as opposed to the qualitative problem of altered body metabolism). The two calorie notions are largely about balance sheets, essentially considering calories like units of body weight and units of body weight like inverse units of health; according to the logic, obese individuals need only try to consume fewer calories than they burn and they will achieve healthier weights and better health.
Although such logic is intuitive and enticing, reality is not quite so simple and existing evidence challenges calorie-focused notions. A view focused more on food quality, rather than caloric quantity, may help better explain and better address the growing problems of excess weight – or more precisely excess fat – and related conditions. Conversely, messages and initiatives based on the idea of calorie equivalency (that a ‘calorie is a calorie’) and interventions directed at calorie balance sheets may make these problems worse. The present paper reviews various problems with calorie-focused thinking, considers several advantages of ‘more-nuanced thinking’ (that considers calories principally as subordinate concerns to qualitative differences in food) and proposes an alternative path for public health to move forward.
The problem with the idea of calorie equivalency
A calorie is a unit of energy. As related to food energy, calories measure the potential energy a food could release. One calorie of potential energy equals one calorie of potential energy, just as one unit of anything equals another unit of that same anything. To say ‘a calorie is a calorie’ then is tantamount to the identity property in mathematics (A=A). As such, it is irrefutable.
In practice, however, the statement that ‘a calorie is a calorie’ often implies something different from mathematical identity. It implies that any two different foods, which have equivalent amounts of potential energy, will produce identical biological effects with regard to body weight/body fatness when consumed. By this thinking, a calorie’s worth of salmon, olive oil, white rice or vodka would each be equivalent and each expected to have the same implications for body weight and body fatness. Indeed, stating ‘a calorie is a calorie’ suggests that potential energy is the essential concern and that qualitative differences in the substances providing that energy are irrelevant.
But a calorie’s worth of salmon (largely protein) and a calorie’s worth of olive oil (purely fat) have very different biological effects from a calorie’s worth of white rice (refined carbohydrate) or a calorie’s worth of vodka (mostly alcohol) – particularly with regard to body weight/body fatness. Indeed, scientists have recognized differences in the weight-related physiological effects of different calorie sources for more than half a century( Reference Thomas 4 ). Although much early knowledge was based on animal studies, subsequent studies in human subjects have shown that calorie-providing proteins, fats, carbohydrates and alcohol each have substantially different effects on a variety of physiological pathways and hormones relevant to satiety, food consumption, weight maintenance and body composition: for example, different effects on ghrelin (an appetite-stimulating hormone), leptin (an appetite-suppressing hormone), glucagon (a hormone that raises blood sugar) and insulin (a hormone that lowers blood sugar)( Reference Benedini, Codella and Caumo 5 – Reference Lomenick, Melguizo and Mitchell 7 ).
The aforementioned descriptions of hormone activities are greatly oversimplified and the list of hormones far from exhaustive, but the examples serve to suggest that a given calorie’s worth of salmon, olive oil, white rice or vodka might each behave quite differently in the body and produce different ultimate effects. Indeed, whereas some ‘calories’ (i.e. some amounts of different foods, quantified by their potential energy) induce metabolic pathways and hormones that squelch appetite and promote energy utilization, others stimulate pathways that promote hunger and energy storage. Even controlling for total calorie intake and energy expenditure from physical activity, qualitative differences in calories have different implications for obesity( Reference Riera-Crichton and Tefft 8 ); a calorie’s worth of one food is not the same a calorie’s worth of another( Reference Riera-Crichton and Tefft 8 – Reference Westerterp, Wilson and Rolland 14 ).
Trying to intervene on calories is implausible and ineffective
It follows from the problematic notion of calorie equivalency that any calorie consumed might be offset by a single calorie expended. Thus individuals wishing to lose weight should simply consume fewer calories than they expend. In other words, individuals should intervene on caloric quantity by consciously trying to ‘eat less’ and ‘move more’ than they otherwise would to establish ‘caloric deficit’ or ‘negative energy balance’( Reference Guth 15 ).
The problem with trying to ‘eat less’ and ‘move more’ to achieve – and more importantly, maintain – caloric deficit or negative energy balance is that it is practically and biologically implausible. Practically, even the most motivated, informed and knowledgeable individuals are unlikely to be able to estimate their actual calorie intake (not just ingested, informed by misleading food labels( Reference Baer, Gebauer and Novotny 16 , Reference Urban, Dallal and Robinson 17 ), but absorbed( Reference Hall, Heymsfield and Kemnitz 18 , Reference Novotny, Gebauer and Baer 19 )) or their actual calorie expenditure (not just in physical activity( Reference Lee, Kim and Welk 20 ) but in variably efficient, silent and constantly fluctuating digestive and metabolic processes( Reference Feinman and Fine 12 , Reference Westerterp, Wilson and Rolland 14 , Reference Hall, Heymsfield and Kemnitz 18 , Reference Jakubowicz, Barnea and Wainstein 21 )) and do so with sufficient accuracy and precision to maintain any kind of useful real-time calorie balance sheets. Biologically, calorie intake and calorie expenditure are coupled( Reference Leibel, Rosenbaum and Hirsch 22 – Reference Ochner, Barrios and Lee 26 ). Unless substantial uncoupling occurs, reducing calories consumed will necessarily result in a compensatory drive to reduce calories expended and vice versa( Reference Ochner, Barrios and Lee 26 – Reference Hall, Hammond and Rahmandad 31 ). For this reason, people who try underconsuming calories become tired (an expenditure compensation) and hungry (an intake compensation), and one reason they often fail to lose weight (or have unimpressive results)( Reference Sumithran and Proietto 25 , Reference Ochner, Barrios and Lee 26 , Reference Nackers, Middleton and Dubyak 32 , Reference Maclean, Bergouignan and Cornier 33 ) may be that resultant hunger, particularly an increased desire for high-calorie foods( Reference Sumithran and Proietto 25 , Reference Ochner, Barrios and Lee 26 ), drives compensatory overconsumption( Reference Ochner, Barrios and Lee 26 , Reference Heymsfield, Harp and Reitman 28 , Reference Maclean, Bergouignan and Cornier 33 ).
Of course, some individuals do succeed at sufficiently uncoupling energy balance (i.e. do expend more calories than they consume) and do lose weight. But saying that these individuals lose weight because they expend more calories than they consume is like saying that students are late for class because they arrive after the bell rings. Both statements are true, but neither is causal. The associations do not explain the ‘why’ (i.e. in the case of expending more calories than consumed, why the uncoupling occurred).
Calorie equivalency and calorie balance sheets cannot explain the ‘why’; why some people succeed in eating less and/or moving more and lose weight while others fail and gain weight. Calorie-focused thinking does not tell us why some people achieve net burning or net storage of calories, or how it is entirely possible to lose weight (as lean mass) and still gain fat (i.e. become more obese). Calorie thinking also cannot account for the dynamic non-linear response of body weight to stable energy imbalances over time( Reference Shook, Hand and Blair 13 , Reference Hall, Sacks and Chandramohan 34 , Reference Hall, Butte and Swinburn 35 ). Likewise, calorie thinking does not address why obesity-related metabolic abnormalities( Reference Shah and Braverman 36 , Reference Wildman, Muntner and Reynolds 37 ) and adverse events of obesity-related diseases( Reference Coutinho, Goel and Correa de Sa 38 – Reference Hamer and Stamatakis 40 ) may both occur before there is any gain in weight( Reference Oliveros, Somers and Sochor 3 , Reference Kramer, Zinman and Retnakaran 41 ), why metabolic improvements may occur at stable weight( Reference Gannon and Nuttall 42 ) or why obesity-related adverse events may not decline with weight loss( 43 ). Any explanation for obesity should provide insights into these observations.
More-nuanced thinking about obesity and related diseases
To understand another kind of thinking about obesity and related diseases – and why individuals may show metabolic changes associated with being overweight before any detectable weight gain occurs – it is useful to consider body fat. Body fat – particularly visceral or abdominal fat – is a complex tissue that plays critical roles in appetite stimulation, energy expenditure and weight regulation. Normally, when a body’s fat cells are replete (i.e. full with stored fat), they release a hormone called leptin. Leptin stimulates parts of the brain to send additional hormone and nerve signals to the thyroid gland, skeletal muscles, heart, intestines and other fat cells( Reference Sumithran and Proietto 25 , Reference Lustig 27 ). These signals are to decrease energy intake (i.e. to ‘eat less’) and increase energy expenditure (e.g. to ‘move more’)( Reference Lustig 27 , Reference Speakman, Levitsky and Allison 29 ).
As individuals start to become obese, however (metabolically speaking, if not yet by weight on a scale), something goes awry with the signalling. Fat-cell repletion is no longer recognized and rather than there being signals to suppress appetite and increase activity as fat stores increase, there are signals to increase energy intake and reduce energy expenditure( Reference Lustig 27 , Reference Speakman, Levitsky and Allison 29 , Reference Ludwig and Friedman 30 , Reference Richmond, Davey Smith and Ness 44 ). In other words, ‘eating more’ and ‘moving less’, thought to be causes of body fattening by calorie-focused thinking, may actually be a result of body fattening( Reference Lustig 27 , Reference Speakman, Levitsky and Allison 29 , Reference Ludwig and Friedman 30 , Reference Richmond, Davey Smith and Ness 44 ).
So if eating more and moving less could be a result of body fattening, what causes bodies to fatten (i.e. to undergo metabolic dysfunction followed by fat gain, and then weight gain) in the first place; that is, what prevents leptin from doing its job of satiating appetite and promoting energy expenditure? The answer is not entirely clear, but one hypothesis implicates concentrated sources of rapidly absorbable carbohydrates in the diet and the hormone insulin.
Insulin is a pancreatic hormone that helps drive ingested nutrients into cells; its release is most brisk and pronounced following the ingestion of rapidly absorbable carbohydrates (as compared with fats, proteins, alcohol and more slowly absorbed carbohydrates( Reference Raben, Agerholm-Larsen and Flint 6 , Reference Mann 45 – Reference Ludwig 48 )). Rapidly absorbable carbohydrates – sugars and refined starches like white rice and foods consisting substantively of white flour – cause blood sugar to rise briskly and insulin levels to respond in kind( Reference Mann 45 – Reference Ludwig 48 ). The rapid insulin elevations produced by these foods cause correspondingly rapid drops in blood sugar. Food cravings result (to restore fallen fuel levels), particularly appetites for something sweet( Reference Raben, Agerholm-Larsen and Flint 6 , Reference Ludwig 48 ). Thus, in the short term, intake of rapidly absorbable carbohydrates may promote ‘eating more’ in general and create a reinforcing loop for overconsumption of additional rapidly absorbable (sweet) carbohydrates in particular (Fig. 1)( Reference Lustig 27 , Reference Ludwig 48 ).
Fig. 1 Calorie-focused thinking versus more-nuanced thinking about obesity. Single-headed arrows represent direct associations in presumed causal directions. *‘Expending fewer calories’ includes all energy expenditure, but ‘moving less’ specifically refers to a relatively lower degree of physical inactivity from baseline. ‘Eating more’ refers to relative overeating from baseline. †Over the short term, the intake of rapidly absorbable carbohydrates – through spikes in blood sugar and insulin, and through sweet cravings – promotes a reinforcing loop with ‘eating more’ in general and eating more rapidly absorbable carbohydrates in particular (dotted arrows). Over the long term, neurohormonal alterations, perhaps chiefly through insulin and leptin resistance – leading to and contributed by growing abdominal fat – perpetuate an indirect reinforcing loop with ‘eating more’ (dashed arrows) and also promote ‘moving less’. Decreasing the intake of rapidly absorbed sugars and starches (as found abundantly in processed foods) and increasing the consumption of whole/minimally processed foods may disrupt these loops, overall calorie imbalance, and both the hormonal dysfunction and excess body mass characterizing obesity
Over the long term, overconsumption of rapidly absorbable carbohydrates may promote leptin resistance. Such resistance may occur through microbiota-mediated inflammatory pathways( Reference Spreadbury 49 ) or through other metabolic changes (e.g. chronic insulin elevations)( Reference Lustig 27 ). Regardless, with leptin’s actions largely disabled, the result of high sugar and starch intake is a neurohormonal drive to ‘eat more’ and ‘move less’ (Fig. 1)( Reference Lustig 27 , Reference Ludwig 48 , Reference Spreadbury 49 ).
By more-nuanced thinking, then, what counts for obesity and related diseases is not the number of calories in specific foods but rather the concentration and type of carbohydrates these foods contain( Reference Ludwig and Friedman 30 , Reference Spreadbury 49 , Reference Taubes 50 ). Total calorie balance is important in both ways of thinking, but whereas calorie-focused thinking directs dietary recommendations towards calorie counts (being primarily quantitative), more-nuanced thinking directs dietary recommendations towards calorie sources (being primarily qualitative); the number of calories consumed and expended are only secondary/intermediate considerations.
Different dietary recommendations by calorie-focused thinking and more-nuanced thinking
A comparison of selected foods that might be encouraged or discouraged by calorie-focused thinking and a more-nuanced thinking appears in Fig. 2. Concordant cells reveal there is some common ground. For example, both ways of thinking discourage sodas, but whereas more-nuanced thinking discourages sodas based on carbohydrate content and character (i.e. high concentrations of rapidly absorbable sugar), calorie-focused thinking discourages sodas based on the idea of ‘empty calories’. ‘Empty calories’ are foods that contribute energy but few substances thought to be beneficial like vitamins, minerals and fibre. By calorie-focused thinking, ‘empty calories’ waste precious space on the intake side of calorie balance sheets.
Fig. 2 Comparison of selected foods that might be encouraged or discouraged by calorie-focused thinking and more-nuanced thinking. This figure is not comprehensive, is not a description of any specific diet plan, and does not represent the recommendations or guidelines of any particular individual or organization. It does not explicitly address issues relevant to public health nutrition beyond calorie- and carbohydrate-related concerns (e.g. food production, climate change, One Health, etc.). Additionally, categorizations are based on somewhat relative concepts such as how ‘empty’ calories are and how ‘rapidly absorbable’ carbohydrate content is; placement of listed and unlisted items within the construct may be debatable. ‘Encouraged’=okay to eat or even desirable as a focus of one’s diet, particularly as an alternative to foods that are ‘discouraged’; ‘discouraged’=to be avoided or limited in quantity
Figure 2 also demonstrates important discordance between calorie-focused thinking and more-nuanced thinking. For instance, 100 % fruit juices – full of vitamins, minerals and sometimes fibre – are not ‘empty’ and may even be considered healthy and desirable by calorie-focused thinking( Reference Nicklas, Kleinman and O’Neil 51 ). By more-nuanced thinking, however, 100 % fruit juices are just as undesirable as sodas given both are mostly sugar in concentrated liquid form( Reference Wojcicki and Heyman 52 ).
Other discordances in dietary recommendations between calorie-focused thinking and more-nuanced thinking, and perhaps the most important differences, relate to dietary fat. Dietary fat has by far the most calories of any of the energy-providing compounds in food: about 9 kcal/g as compared with roughly 7 kcal/g for alcohol, 4 kcal/g for protein and 4 kcal/g for carbohydrate( Reference Atwater 53 ). Thus, calorie-focused thinking has an inherent bias against dietary fat. This bias leads to public health messages and interventions to decrease the intake of fatty foods or reduce or remove the fat from high-fat foods (often replacing fat with less-calorie-dense – often rapidly absorbable – carbohydrates).
Calorie-focused thinking generally endorses foods that are low in fat and calories, as long as those calories are not ‘empty’. In contrast, more-nuanced thinking has no problems with fat or calories, per se, and places the blame squarely on foods with the most rapidly absorbable carbohydrates (Fig. 2). Clearly these two ways of thinking are very different. A question for public health moving forward is: would food choices that could result from a continued primary focus on calories (calorie-focused thinking – Fig. 2) be best for population weight and health?
Pertinent clinical and population evidence for two different ways of thinking
Consider an experiment in children( Reference Wansink, Shimizu and Brumberg 54 ). Sixth graders with comparable baseline satiety were allowed to eat as much as they wanted of two highly palatable child-friendly snacks: cheese wedges/rounds or potato chips. A quantity of cheese (mostly fat with some protein and negligible carbohydrate) might offer about 50 % more calories than an identical quantity of chips (mostly carbohydrate and fat with negligible protein). By calorie-focused thinking, comparably hungry children should eat more calories of cheese because cheese has more calories. By more-nuanced thinking, comparably hungry children should eat more calories of chips because chips, being rich in rapidly absorbable starch, should tend to promote continued eating (short-term reinforcing loop, Fig. 1)( Reference Ludwig 48 ).
What actually happened in the experiment was that children in the potato chip group consumed over three times more calories than children in the cheese group( Reference Wansink, Shimizu and Brumberg 54 ). While a protein difference between the snacks might certainly have been a factor (with experimental trials suggesting a superior( Reference Marmonier, Chapelot and Louis-Sylvestre 55 ), albeit not always statistically significant( Reference Vozzo, Wittert and Cocchiaro 56 ), satiating power of protein), all foods are inevitable mixes of different components and the point here is that the food with the higher starch content prompted greater consumption. This result is consistent with a meta-analysis showing children have greater energy intake following consumption of the most rapidly absorbable carbohydrates( Reference Rouhani, Salehi-Abargouei and Azadbakht 57 ).
Notably in the experiment described above, the effect of eating more calories in the high-carbohydrate (chips) condition was even more pronounced among overweight and obese children( Reference Wansink, Shimizu and Brumberg 54 ). This result is consistent with another trial showing greater hunger in obese children after a high-carbohydrate meal( Reference Lomenick, Melguizo and Mitchell 7 ) and consistent with the long-term reinforcing loop in Fig. 1.
Although the chips-and-cheese experiment did not assess children’s total caloric intake for the day outside of the single snack episode, it is likely that children consuming cheese ate fewer calories overall for the day, whereas children consuming chips ate more. Such an outcome would be suggested by fifteen of sixteen single-day studies in adults that showed increased hunger, lower satiety or greater calorie intake after consuming rapidly absorbable carbohydrates v. not( Reference Ludwig 58 ). The outcome might also be suggested by two other studies in children in which restaurant fast-food consumption was associated with a net increase in total energy intake for the day( Reference Powell and Nguyen 59 , Reference Ebbeling, Sinclair and Pereira 60 ) – although only for overweight individuals in one study( Reference Ebbeling, Sinclair and Pereira 60 ), consistent with the long-term reinforcing loop in Fig. 1. Granted, for a given fast-food meal, the studies referenced above cannot distinguish if greater total caloric intake was the result of a greasy burger (per calorie-focused thinking), a refined bun (per more-nuanced thinking) or accompanying French fries (per both ways of thinking). However, substantial evidence now implicates foods that are low in fat (and, thus, relatively low in calories), like potatoes( Reference Mozaffarian, Hao and Rimm 61 ), white rice( Reference Hu, Pan and Malik 62 ) and sugary beverages( Reference Mozaffarian, Hao and Rimm 61 , Reference Qi, Chu and Kang 63 – Reference Bes-Rastrollo, Schulze and Ruiz-Canela 66 ), in the development and persistence of obesity and risk for related diseases. Conversely, evidence is mounting to exonerate higher-calorie foods that are rich in fat like nuts( Reference Mozaffarian, Hao and Rimm 61 , Reference Tan and Mattes 67 – Reference Viguiliouk, Kendall and Blanco Mejia 74 ), oily fish( Reference Thorsdottir, Tomasson and Gunnarsdottir 75 ) and olive oil( Reference Estruch, Ros and Salas-Salvado 69 , Reference Perez-Martinez, Garcia-Rios and Delgado-Lista 76 , Reference Salas-Salvado, Bullo and Estruch 77 ), and even foods high in saturated fat( Reference Siri-Tarino, Sun and Hu 78 , Reference Chowdhury, Warnakula and Kunutsor 79 ) like dairy products( Reference Scharf, Demmer and Deboer 80 – Reference Martinez-Gonzalez, Sayon-Orea and Ruiz-Canela 88 ). Indeed, higher-calorie fattier foods and higher-fat diets may produce and sustain as much or more weight loss than calorie-restricted or higher-carbohydrate diets( Reference Kekwick and Pawan 9 , Reference Ebbeling, Swain and Feldman 10 , Reference Hession, Rolland and Kulkarni 89 – Reference Gow, Ho and Burrows 98 ) – particularly among those already having metabolic abnormalities( Reference Volek, Phinney and Forsythe 93 , Reference Westman, Yancy and Mavropoulos 94 , Reference Ebbeling, Leidig and Feldman 99 ). Moreover, certain fattier/lower-carbohydrate diets may also be associated with favourable metabolic indicators( Reference Ebbeling, Swain and Feldman 10 , Reference Hession, Rolland and Kulkarni 89 , Reference Hu, Mills and Yao 91 – Reference Westman, Yancy and Mavropoulos 94 , Reference Gow, Ho and Burrows 98 – Reference Bazzano, Hu and Reynolds 109 ), reduced adverse health events( Reference Estruch, Ros and Salas-Salvado 69 , Reference Corella, Carrasco and Sorli 102 , Reference Fung, Rexrode and Mantzoros 110 , Reference Sofi, Macchi and Abbate 111 ) and delayed mortality( Reference Fung, Rexrode and Mantzoros 110 – Reference Trichopoulou, Orfanos and Norat 113 ).
The situation for public health moving forward
Fuelled not exclusively but in no small part by calorie-focused thinking, fats in foods and fattier diets became the enemies of public health campaigns of the 1980s and 1990s. Lower-calorie sugars replaced higher-calorie oils in many foods and people shifted their consumption from fats to carbohydrates (most often, the rapidly absorbable kinds). As in the chips-and-cheese experiment described above, greater refined carbohydrate intake was associated with greater total calorie intake, but now on a population level( Reference Marantz, Bird and Alderman 114 , 115 ). In other words, people did not eat less when lower-calorie foods and diets were advised, they ate more. Obesity rates increased right along with greater consumption( Reference Marantz, Bird and Alderman 114 , 115 ). Diabetes rates increased too( 116 , 117 ) and although these findings do not prove causation, they certainly do not support continuing forward under the current logic of calorie-focused thinking, with the food choices it could encourage (Fig. 2) or the tenuous notions that follow from it (Table 1).
Table 1 Notions derived from calorie-focused thinking and challenges to those notions
Calorie-focused public health initiatives might continue to produce unintended, even ironic, consequences. Initiatives like calorie labelling for example – first for food packages and more recently for restaurant menus and menu boards – are meant to steer both consumer choices and food-industry offerings towards lower-calorie options( Reference Hodge and White 118 ). Despite national enthusiasm for the idea( 119 , Reference Block and Roberto 120 ), whether calorie labelling will have the desired effect seems doubtful( Reference Swartz, Braxton and Viera 121 – Reference Sinclair, Cooper and Mansfield 124 ). Also in doubt is whether labelling will actually improve population health. There is already suggestion that some labelling may produce effects opposite to those intended( Reference Downs, Wisdom and Wansink 125 ). And there is the distinct possibility that calorie labelling could further move food production and consumption away from healthful high-fat foods (like nuts) and towards sugary and starchy items (like low-fat baked potato chips), promoting further increases in diseases characterized by abdominal fat and metabolic dysfunction.
There are, admittedly, other existing public health initiatives that, at least on the surface, seem more consistent with the logic of ‘more-nuanced thinking’; for instance, proposals to tax and limit sugary beverages( Reference Wojcicki and Heyman 52 , Reference Brownell and Frieden 126 , Reference Brownell, Farley and Willett 127 ). Nevertheless, these initiatives are usually framed around the idea of ‘empty calories’, which totally misses the point. Even the Food and Drug Administration’s proposed changes to packaged-food labels – which would newly report the amount of ‘added sugars’ in a product – place even more emphasis on calories than current labels by visually subordinating all other label information and highlighting calories in an enormous bold typeface( 128 ).
What existing and planned initiatives seem not to acknowledge is that calories from added sugars and starches are worse than just ‘empty’ (detriment through omission); evidence suggests they are actively harmful (detriment through commission)( Reference Mozaffarian, Hao and Rimm 61 , Reference Hu, Pan and Malik 62 , Reference Schwingshackl and Hoffmann 129 , Reference Te Morenga, Howatson and Jones 130 ). While responses of individual consumers may vary (e.g. due to their personal genetic susceptibility( Reference Ludwig 48 , Reference Qi, Chu and Kang 63 ) or that of their resident gut microbes( Reference Turnbaugh, Ridaura and Faith 131 )), there is good reason to believe that rapidly absorbable carbohydrates tend to promote obesity, and diseases commonly associated with it, in general( Reference Mann 45 , Reference Ludwig 48 , Reference Qi, Chu and Kang 63 , Reference Sorensen, Raben and Stender 132 – Reference Yang, Zhang and Gregg 137 ).
The problem for public health is that continuing to focus on quantifying calories may misdirect thinking on obesity and related diseases and promote destructive messages. For instance, in a 2013 editorial, the president of the Institute of Medicine listed gluttony and sloth as ‘obvious’ ‘deadly sins’ for public health to address( Reference Fineberg 138 ). His argument (which had been made before( Reference Ramos Salas, Forhan and Sharma 139 )) suggested obesity and related diseases are matters only of personal resolve and self-control; if people just had more motivation and will-power, they could consciously control their calorie balance sheets, eat less, move more and lose weight. It stands to reason that those subscribing to the Institute of Medicine logic might blame an overconsuming, inactive adolescent for growing fat. But would they blame the same overconsuming, inactive adolescent for growing tall?
Just as children do not enter puberty and grow tall because they overeat and sleep more, neither do individuals start to fatten and become obese because they eat too much and move too little. In both cases overconsumption and inactivity are intermediate effects; neurohormonal changes are the cause. The case of pubertal growth represents normal development, but the case of fattening represents decided pathology; pathology that may be modifiable through dietary change. Perhaps if we shifted food production and people’s consumption away from added sugars and refined starches, we could avoid the resultant metabolic dysfunction and corpulence that have come to plague our populations. Instead of futilely promoting messages to ‘eat less’ and ‘move more’( Reference Shook, Hand and Blair 13 , Reference Ramos Salas, Forhan and Sharma 139 ), perhaps we should do more to promote the consumption of whole/minimally processed foods( Reference Mozaffarian, Rogoff and Ludwig 140 ) – like more of those in the upper row of Fig. 2 – foods that might make ‘eating less’ and moving more’ more possible.
Concluding thoughts
Calorie-focused thinking may have already exacerbated the epidemics of obesity and related diseases. And while there has been much progress in redirecting dietary focus towards actual foods( Reference Katz and Meller 141 ), there is still too much focus on eating ‘too much’( Reference Guth 15 ). Focusing quantitatively, particularly on the calories available from specific foods, fails to recognize the broader metabolic effects of foods themselves. Foods that are highly processed and comprised mostly of rapidly absorbable sugars and starches may be of greatest concern. Such carbohydrates may induce neurohormonal changes that might, in turn, help produce the overeating and inactivity often interpreted as causative for obesity. In other words, unhealthy foods may make double victims of their consumers, who might not only become obese by eating them but also receive harsh criticism for their substantial appetites and apparent laziness that result.
As the saying often attributed to the Albert Einstein goes, ‘not everything that can be counted counts’, and advice to count calories, or to try to change calorie balance sheets by intervening on quantities of undifferentiated foods, seems misdirected. Imagine comparably misdirected advice: for instance, to count fluid ounces, drink less and urinate more – advice that might likewise result in temporary weight loss (but no fat loss) and be uncomfortable, unsustainable, unreasonable and unhelpful; and likewise oppose coupled neurohormonally driven physiology in futility. Yes, calories count, and calorie balance sheets matter, but net intake or expenditure probably results more from qualitative distinctions in the foods we eat than conscious attempts at quantitative control( Reference Ludwig and Friedman 30 ). New public health initiatives and messages focused on encouraging consumption of whole/minimally processed foods would be ideal( Reference Mozaffarian, Rogoff and Ludwig 140 ), especially to counteract industry’s near-exclusive marketing of foods that are highly processed/refined and concentrated sources of the most rapidly absorbable starches and sugars.
Promoting the consumption of whole foods will require careful attention to food systems, cultural traditions, peer influences, food environments, assistance programmes and a host of other issues beyond the scope of the present commentary. But as a guiding principle, the public health community should not be trying to cut calories from available foods( 142 ), we should be improving the quality of the foods available that provide our calories. We should be promoting foods that do not prompt, or indeed programme, us to overeat.
Although focusing on refined starch and sugar content might seem like a logical path forward, such narrow focus could lead to unintended consequences, as when public health campaigns demonized fat. For this reason, the recent WHO draft guideline to more strictly limit the intake of all sugars( 143 ), the recent proposition in England for a sugar tax( 144 ), and the recent proposal in California to place health warning labels on sugary drinks( Reference Calefati 145 ), while all appropriately focused, should be evaluated carefully before wider implementation. Coordination with the food industry will be challenging, but while working towards improving the quality of foods that are produced and working to support the consumption of whole/minimally processed products, at the very least, public health should not continue to promote messages that create and blame victims or that, in all likelihood, continue to exacerbate epidemics of obesity and related diseases.
Acknowledgements
Acknowledgements: S.C.L. would like to thank Sanjay Basu, MD, PhD, Jennifer L Pomeranz, JD, MPH, Paul R Marantz, MD, MPH and Manisha Sharma, MD for reviewing very early drafts of this manuscript and providing critical comments. Financial support: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors. Conflict of interest: None. Authorship: S.C.L. conducted the primary literature review, conceived the paper, drafted the main arguments, and created Figs 1 and 2. J.J.D. helped revise the text, contributed citations, and drafted Table 1.