Infant and young child feeding practices are important determinants of nutritional status of children. The WHO recommends exclusive breast-feeding (defined as no other foods or drinks except for medicines and/or nutritional supplements) for the first 6 months of life. Even though the benefits of exclusive breast-feeding are well established, only 39 % of infants less than 6 months old are exclusively breast-fed worldwide( Reference Cai, Wardlaw and Brown 1 ).
The second component of infant and young child feeding is appropriate introduction of complementary foods( 2 ). Timely and adequate introduction of complementary feeding is essential in preventing infant undernutrition( Reference Bhutta, Ahmed and Black 3 ). The WHO recommends introduction of complementary foods from 6 months of age, with continued breast-feeding until the child is 2 years of age( 2 ). A range of nutrient- and energy-dense complementary foods should be given frequently to cover the higher nutritional requirements for growing children. However, as with exclusive breast-feeding, complementary feeding practices are also inadequate worldwide, with complementary foods often introduced too early or too late( 4 ). Even when they are introduced at the right age, they may be nutritionally inadequate and unsafe( 4 ). Suboptimal feeding practices can result in inadequate energy consumption, as well as increased incidence of infant illnesses such as diarrhoea and respiratory infections( Reference Walker, Rudan and Liu 5 ), leading to increased infant mortality and morbidity( Reference Bhutta and Salam 6 ).
Bangladesh is one of the twenty countries where 80 % of the world’s undernourished children live( Reference Bryce, Coitinho and Darnton-Hill 7 ). The prevalence of pre-school children with height-for-age, weight-for-age and weight-for-height Z-scores less than −2·0 is estimated to be 41 %, 36 % and 16 %, respectively( 8 ). This high burden of childhood undernutrition occurs concurrently with a high prevalence of inadequate infant feeding practices in the country. Only 64 % of infants <6 months of age are exclusively breast-fed, even though 100 % are put to the breast at least once during this time( 8 ). Suboptimal feeding practices continue as the child grows older, as complementary feeding is delayed for 33 % of infants 6–9 months of age( 8 ). Furthermore, exclusive breast-feeding prevalence has not improved over the past decade( 8 ). Even for those infants for whom complementary feeding is initiated in a timely manner, the diet may not be nutritionally adequate, with less than half the children between the ages of 6 and 23 months receiving a minimally acceptable diet as defined by the WHO( Reference Kabir, Khanam and Agho 9 ). Among children aged 6–11 months, only 18 % receive a minimally acceptable diet( Reference Kabir, Khanam and Agho 9 ).
Household food security, defined as including both physical and economic access to food that meets people’s dietary needs as well as their food preferences( 10 ), has been identified as an important underlying cause of childhood undernutrition( Reference Black, Allen and Bhutta 11 , Reference Black, Victora and Walker 12 ). However, how household food security impacts infant and young child feeding practices is less well understood.
The relationship between household food security, diet and nutritional status of adults and older children is well established( Reference Kirk, Kuhle and McIsaac 13 – Reference Vozoris and Tarasuk 17 ). Substantial research has also been conducted on identifying the predictors of nutritional status of infants and young children( Reference Black, Allen and Bhutta 11 , Reference Black, Victora and Walker 12 ). And it is no surprise that suboptimal complementary feeding practices are highly predictive of childhood undernutrition( Reference Lassi, Das and Zahid 18 ). However, fewer studies have attempted to assess the relationship between how food secure a household is and the quality of an infant’s diet. This relationship can have implications for infant nutrition intervention programmes, such as whether the appropriate intervention is to identify vulnerable children in a community or improve household food security in general.
Presumably more food-secure households are more likely to have improved infant diet. However, according to the 2011 Bangladesh Demographic and Health Survey, only 31 % of children from the wealthiest, and probably the most food-secure quintile in the country were receiving a minimally acceptable diet( 8 ). The figure was 11 % for children from the lowest quintile( 8 ). Therefore, the aim of the present study was to determine the strength of the association between household food security and infant feeding practices independent of other sociodemographic factors in the context of a food-insecure population in rural Bangladesh.
Methods
Study population and setting
The study setting, population and data collection tools have been described in detail elsewhere( Reference Thomas, Yu and Tirmizi 19 , Reference Yu, Thomas and Owais 20 ). Briefly, data for the study were collected in the context of evaluation of Window of Opportunity, a community-based nutrition and infant and young child feeding programme implemented by CARE in six countries, including Bangladesh, where it was known as Akhoni Shomay. Akhoni Shomay was carried out in Karimganj. A rural sub-district of Kishoreganj with a population of ~320 000, approximately 120 km north of Dhaka, this is one of the most food-insecure regions in Bangladesh.
An integrated set of individual-, household- and community-level interventions was implemented. Women living in a second sub-district, Katiadi, served as controls. To evaluate the programme, a prospective cohort of ~2400 pregnant women (1200 per sub-district) was recruited between January and October 2011. Pregnant women were recruited in their seventh month of gestation, with follow-up of their offspring scheduled to occur at 3, 9, 16 and 24 months of age.
Variable derivation
To evaluate infant diet quality, our main outcome, we used two indicators developed by the WHO. The first, infant dietary diversity, is defined as number of food groups consumed by the infant in the past 24 h( 21 ). Using maternal recall at the 9-months’ follow-up, everything the child ate over the past 24 h was categorized into one of seven food groups. An infant who consumes ≥4 food groups is said to meet WHO’s minimum dietary diversity criteria( 21 ). The second index was whether a child received a minimally acceptable diet in the past 24 h( 21 ). This indicator is derived from the number of food groups as well as the number of meals consumed over a 24 h period, assessed at the 9-months’ follow-up. Furthermore, whether an infant receives a minimally acceptable diet or not is also determined by his or her age and breast-feeding status. A 9-month-old breast-fed infant should receive ≥4 food groups and ≥4 meals over 24 h to be considered as having received a minimally acceptable diet.
Household food security, our main exposure, was assessed via the Food Composition Score (FCS) based on reported maternal diet at the 3-months’ follow-up. Developed by the World Food Programme, FCS is a composite of dietary diversity, frequency with which food is consumed in the household and the nutritional importance of food( Reference Wiesmann, Basset and Benson 22 ). There are seven food groups included in calculating the FCS, which is calculated by multiplying a food group-specific weighting factor with the number of days that food group was consumed over the past 7 d and then summed up. Animal-source protein and dairy products are assigned the largest weighting factor followed by legumes, grains, fruits and vegetables, and sugars and oil.
We also used a scale developed specifically for rural Bangladesh by Frongillo et al.( Reference Frongillo, Chowdhury and Ekstrom 23 ). This questionnaire (called the ‘HHFS questionnaire’ henceforth) grades food insecurity based on frequency of food purchases, cooking and meals consumed, as well management strategies. The HHFS questionnaire was administered at the 3-months’ follow-up. Of the eleven items included in the questionnaire, ten were used to create the HHFS score.
Information on sociodemographic variables, including household socio-economic status, maternal age, literacy and parity, were abstracted from the questionnaire administered at recruitment into the study at gestational age 7 months.
Statistical analysis
Data were imported into the statistical software package SAS version 9·3 for analysis. Median (range) was calculated for continuous variable and frequencies and percentages were calculated for categorical variables. Differences between maternal and infant diet were tested using Fisher’s exact test. Household socio-economic status was assessed through residence characteristics and ownership of assets. Principal component analysis was used to create an asset-based socio-economic status score using methods described by Filmer and Pritchett( Reference Filmer and Pritchett 24 ).
Binary variables for infant dietary diversity and receipt of a minimally acceptable diet were created using WHO cut-offs( 21 ). Quartiles were created for both measures of household food security for analysis. Multivariable logistic regression, adjusted for socio-economic status, maternal age, literacy and parity and infant sex, was used to determine the association between household food security and quality of infant diet. Interactions between both measures of household food security and each of the covariates were assessed. Statistical significance was set at P<0·05.
Results
The 9-months’ follow-up was completed by 2073 out of 2400 mother–child dyads. Based on maternal recall, complementary feeding was initiated at ≤4 months for 7 %, at 5–6 months for 49 % and at ≥7 months for 44 % of infants in our cohort. At the time of the 3-months’ follow-up, all infants were breast-fed and 45·2 % had received nothing but breast milk in the past 24 h( Reference Yu, Thomas and Owais 20 ). At age 9 months, complementary feeding had been initiated for all but three infants in our cohort, with 98 % also continuing to be breast-fed. The most common food groups included in infants’ diet were grains, roots and tubers (93 %), non vitamin A-rich fruits and vegetables (43 %) and flesh foods (33 %). Dairy products, legumes and nuts, eggs and vitamin A-rich fruits and vegetables were consumed by 20 %, 17 %, 11 % and 11 % of infants, respectively.
Although 74 % of infants in the study received at least four meals over a 24 h period, only 16 % of infants in the study met the minimal dietary diversity criteria and hence were categorized as receiving a minimally acceptable diet over a 24 h period. The median maternal FCS assessed at the 3-months’ follow-up was 62 (range: 19·5–112) and the median HHFS score was 40 (range: 23–45). Pearson’s correlation estimate between the two scores was 0·38.
Characteristics of study households stratified by maternal diet and household food security scores are summarized in Tables 1 and 2, respectively. Maternal age, literacy and parity were similar across food security groups. Infant age at complementary feeding initiation and rates of continued breast-feeding were also similar across groups. However, infant diet quality was strongly associated with household food security status. Infants living in more food-secure households (as measured via maternal diet) were more likely to receive a minimally acceptable diet at 9 months, compared with those living in less food-secure households (adjusted OR = 3·0; 95 % CI 2·1, 4·3; Table 3). Results were similar using the HHFS score (Table 3). Household socio-economic status, maternal age and literacy and infant sex were not associated with infant feeding practices.
Table 1 Maternal characteristicsFootnote * and infant feeding practicesFootnote † among 2073 mother–child dyads in 2011–2012 in Kishoreganj, Bangladesh, by maternal Food Consumption Score (FCS)
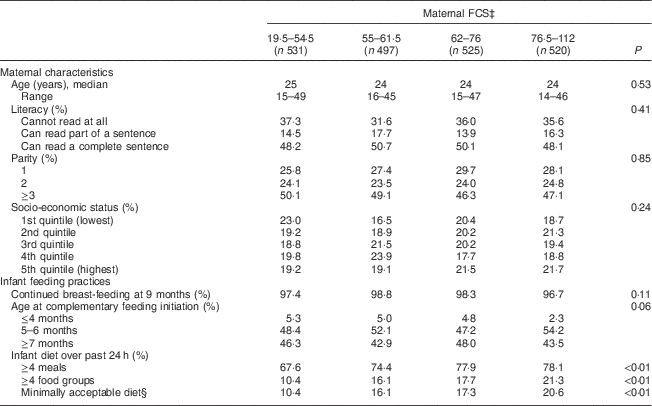
* Assessed at baseline.
† Assessed at infant age 9 months.
‡ Assessed via the World Food Programme method; categories are quartiles.
§ Defined as ≥4 meals and ≥4 food groups.
Table 2 Maternal characteristicsFootnote * and infant feeding practicesFootnote † among 2073 mother–child dyads in 2011–2012 in Kishoreganj, Bangladesh, by household food security (HHFS) score
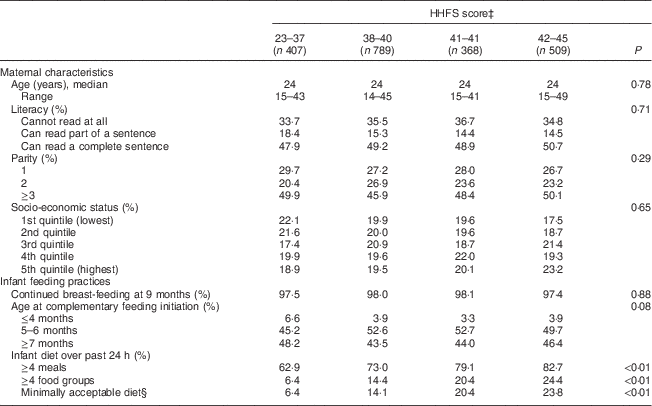
* Assessed at baseline.
† Assessed at infant age 9 months.
‡ Calculated using the eleven-item HHFS questionnaire; categories are quartiles.
§ Defined as ≥4 meals and ≥4 food groups.
Table 3 Association between household food security and infant feeding practices among 2073 mother–child dyads in 2011–2012 in Kishoreganj, Bangladesh
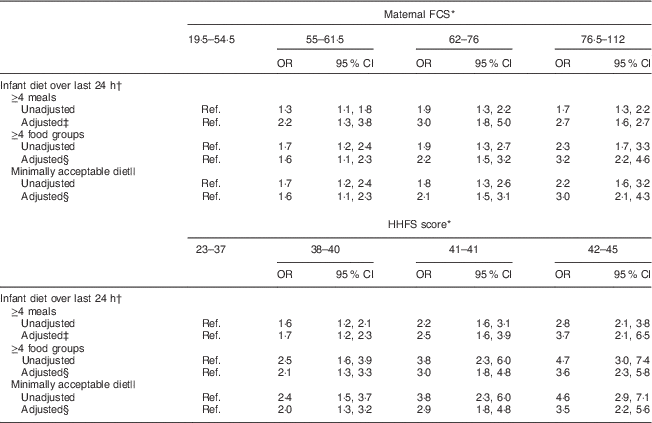
FCS, Food Composition Score; HHFS, household food security; Ref. reference category.
* Assessed at infant age 3 months.
† Assessed at infant age 9 months.
‡ Adjusted for socio-economic status, maternal age, literacy and parity, infant sex, district, time of enrolment and interaction between main exposure and parity.
§ Adjusted for socio-economic status, maternal age, literacy and parity, infant sex, district and time of enrolment.
|| Defined as ≥4 meals and ≥4 food groups.
We tested for interaction between both measures of household food security and each of the covariates. There were no significant interactions for two of the three indicators of infant feeding practices: ≥4 food groups and minimally acceptable diet. The relationship between household food security and ≥4 meals was modified by maternal parity (maternal FCS: P-interaction=0·02; HHFS score: P-interaction=0·048).
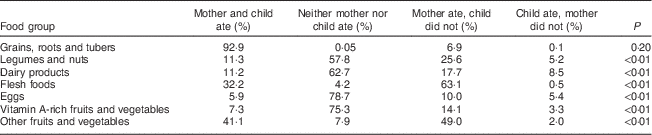
We also examined differences between the maternal and infant diets. Table 4 summarizes the comparison of maternal and infant dietary diversity. The greatest overlap occurred for grains, roots and tubers, which 93 % of mother–child dyads reported consuming over the past 24 h. The highest discrepancy between maternal and infant diets was for flesh foods, with 63 % of mothers reporting consuming meat when their infant did not, followed by non vitamin A-rich fruits and vegetables (49 %) and legumes and nuts (26 %). More than three-quarters of mother–child dyads included in the study reported not consuming eggs (78·7 %) and vitamin A-rich fruits and vegetables (75·3 %). More than half of the respondents also reported not consuming dairy products (62·7 %) and legumes and nuts (57·8 %) for both themselves and their children. We did not observe a difference between maternal and infant dietary diversity by infant sex.
Table 4 Dietary diversity comparison of 2073 mother–child dyads in 2011–2012 in Kishoreganj, Bangladesh (at child age 9 months)
Discussion
Our study assessed the association between household food security and infant feeding practices in a food-insecure population. We found that variations in household food insecurity are significantly associated with infant feeding practices in rural Bangladesh. However, maternal diet is more diverse than infant diet in our population.
To our knowledge, the present study is one of the first to use the FCS as an indicator of household food security. Developed by the World Food Programme, the validity of the FCS as a method for assessing household food security has been established( Reference Wiesmann, Basset and Benson 22 ). Although region- or country-specific measures to assess food security contribute to our understanding of how food security is thought of in different cultures, they make cross-country comparisons difficult to conduct. Using a more objective measure such as the FCS may assist in unbiased analyses. That both methods of assessing household food security are similarly associated with infant feeding practices in the present study makes it likely that the FCS is a valid measure of food security in the context of rural Bangladesh. Additional research is needed to assess whether the validity of the FCS as an indicator of food security holds in other settings.
Our study is also one of the few which looked specifically at how household food security drives infant feeding practices. The study by Saha et al.( Reference Saha, Frongillo and Alam 25 ) was one of the first to assess this association. Their finding that better HHFS score was associated with improved feeding practices for infants aged 6–12 months is similar to ours.
As maternal FCS is a direct measure of maternal diet, we can also conclude that maternal diet is strongly associated with infant diet. This finding is similar to those from another study conducted in Bangladesh as well research conducted in Ethiopia and Vietnam( Reference Nguyen, Avula and Ruel 26 ). However, we also observed discordance between maternal and infant diets. For each of the seven food groups, whenever there was a discrepancy between their diets, it was in favour of the mother, meaning that even when the foods were available in the household they were not being given to the infant. This finding is in contrast to the concept of ‘maternal buffering’ which posits that mothers reduce their own food intake to protect their children from food scarcity( Reference Coates, Frongillo and Rogers 27 ). Rasheed et al.( Reference Rasheed, Haider and Hassan 28 ) observed that in Bangladesh, household knowledge regarding optimal complementary feeding practices differs substantially from international recommendations. Furthermore, cultural norms, such as advice from family members and the importance of feeding young children, also influence complementary feeding practices. Understanding the cultural context that determines infant diet and feeding will be critical in improving infant feeding practices, and thus nutrition, in this setting.
The amount of schooling completed by mothers is highly associated with improved child health, diet and nutritional outcomes, especially in low-income settings( Reference Nguyen, Avula and Ruel 26 , Reference Smith-Greenaway 29 – Reference Meshram, Kodavanti and Chitty 32 ). Therefore, it is surprising that this association does not hold in our study. This finding may be an indication that improved maternal literacy does not translate directly to better child-rearing practices in rural Bangladesh and that interventions for improved child growth and survival in this setting need to be multi-factorial.
A major strength of our study is the large sample size which increases the power of analyses. Furthermore, as this was a prospective cohort study, the main exposure was assessed prior to the outcome. This strengthens our ability to make causal inferences. However, as with any other observational study, this one also has some limitations. First, both the outcome and exposure measures are based on maternal recall of what she and her child ate. However, the time lag between dietary intake and report is comparatively small: 7 d in the case of the mother and only 24 h for the infant. We also could not explore why mothers would not give specific foods to infants. This is an important avenue for future research in identifying barriers to optimal complementary feeding practices in Bangladesh.
Conclusion
The present study adds to the body of evidence on how household food security is related to infant feeding practices in low-income settings. We observed that more food-secure households employ improved infant feeding practices. Our findings imply that interventions aimed at improving infant nutritional status need to focus on both complementary food provision and education.
Acknowledgements
Financial support: This study was supported by grants from CARE USA to Emory University and the International Centre for Diarrheal Disease Research, Bangladesh (icddr,b). The funders had no role in the design, analysis or writing of this article. The authors have no relevant financial relationships to disclose. Conflict of interest: None. Authorship: A.D.S., A.S.G.F. and B.S. designed the cohort study. A.S.G.F. and S.K.D. led field activities and supervised data collection. A.O., D.G.K., P.S.S., B.S. and A.D.S. conceived and planned the data analysis. A.O. analysed the data. A.O. and A.D.S. wrote the manuscript. All authors critically reviewed the manuscript and approved the final version. Ethics of human subject participation: This study was approved by the Research Review Committee and the Ethical Review Committee of icddr,b. At the time of enrolment, informed consent was provided by each participant. Verbal consent, witnessed and formally recorded, was obtained at each follow-up visit.







