Free sugars consumption has been a major issue in public health, primarily because of the possible role of foods high in free sugars in displacing intakes of nutrient-rich foods and thus diluting micronutrients in the diet( Reference Johnson, Appel and Brands 1 – Reference Hess, Latulippe and Ayoob 3 ), the linkage between sugars and dental caries development( Reference Anderson, Curzon and Van Loveren 4 ) and the possible contribution of excessive free sugars intake to the obesity (OB) epidemic( Reference Murphy and Johnson 2 , Reference Te Morenga, Mallard and Mann 5 ). Especially for children and adolescents, ensuring nutrient-dense diets is critical for optimal growth and early prevention of several chronic diseases. Following the worldwide trend revealing a considerable increase in overweight (OW) and OB prevalence during childhood in developed countries( Reference Lobstein and Frelut 6 , Reference Ogden, Carroll and Kit 7 ), recent studies in Greek populations show very high rates of childhood OW and OB and aggravating dietary and lifestyle habits( Reference Farajian, Risvas and Karasouli 8 , Reference Moschonis, Kalliora and Costarelli 9 ). The potential link between excessive intake of free sugars, OB and other health outcomes, including diabetes and CVD risk factor alterations, has been pointed out as an important public health matter, not only in adults but in adolescents as well( Reference Drewnowski and Rehm 10 – Reference Welsh, Sharma and Abramson 13 ).
Studies on the effects of sugar consumption on weight status or gain have shown mixed results. An inverse correlation between total sugar intake and BMI has been reported in children and adults( Reference Ludwig, Peterson and Gortmaker 14 – Reference Song, Wang and Chung 16 ), while in the meta-analyses of Te Morenga et al.( Reference Te Morenga, Mallard and Mann 5 ) the assessment of cohort studies that reported data for the odds of being OW at follow-up in children consuming about one serving of sugar-sweetened beverages (SSB) daily at baseline, compared with children consuming none or very little, showed that those with higher intakes had significantly increased risk of being OW.
Several organizations agree that sugars intake has to be reduced and have issued dietary recommendations for sugars or added sugars that vary in terms of suggested intakes or types of sugars specified( Reference Johnson, Appel and Brands 1 , 17 – 19 ). Perhaps the most commonly used recommendation is the one of the WHO and FAO( 19 ) proposing that ‘free sugars’ (the term preferred by the WHO, and defined as all mono- and disaccharides added to foods by the manufacturer, cook and consumer, plus sugars naturally present in honey, syrup and fruit juice) should constitute <10 % of energy intake. In many studies the investigation of the associations between free sugars and health outcomes has been restricted to the consumption of SSB, although during past years several investigators have suggested to use a holistic dietary approach on disease prevention, giving much attention to pattern analysis( Reference Jacques and Tucker 20 , Reference Trichopoulos and Lagiou 21 ). Thus, instead of looking at individual nutrients or foods, pattern analysis examines the effects of overall dietary choices and nutrient consumption and possibly explains diet–outcome relationships( Reference Kim and Mueller 22 ).
Consequently, the aim of the present cross-sectional study was to estimate the major sources of free sugars in the diet of Greek schoolchildren. Additionally, following the holistic dietary assessment approach of pattern analysis another purpose of the present study was to investigate how the extracted dietary and lifestyle patterns of children were associated with free sugars intake.
Methods
Study sample
Under the context of the GRECO (Greek Childhood Obesity) study, during 2009 a representative number of randomly selected public primary schools (5th and 6th grade primary-school children, 10–12 years old) were invited to participate in the study, according to a stratified sampling procedure by sex and age group, based on the population distribution in ten regions of the country. Detailed information regarding participants’ data collection has been reported elsewhere( Reference Farajian, Risvas and Karasouli 8 , Reference Farajian, Panagiotakos and Risvas 23 ). After checking the completeness of the provided data, from the overall 4786 measured children with signed parental consent forms, a total sample of 3089 children with complete information on free sugars intake was finally included in the analyses of the present study (mean age 10·9 (sd 0·7) years, 53 % girls). The studied sample could be considered as representative of the overall study population (i.e. the 4786 children included for analysis in the GRECO study) regarding age and BMI distributions (P>0·05), as differences were not evident between the present and the overall sample. However, differences were revealed regarding sex (P<0·05) and regional distribution (P<0·01).
Nutrient intake and eating behaviour assessment
Dietary assessment was based on a validated, self-reported, semi-quantitative picture-aid FFQ, consisting of forty-eight food items commonly used in the local Greek cuisine( Reference Bountziouka, Farajian and Risvas 24 , Reference Farajian, Karasouli and Risvas 25 ). All participants were asked about their usual frequency of consumption of the food items with the following response categories: ‘every day’, ‘3–6 times/week’, ‘2 times/week’, ‘1 time/week’, ‘1–2 times/month’ and ‘seldom/never’. The questionnaire included supplementary questions assessing the frequency of breakfast consumption and eating occasions (number of meals and snacks during the day), as well as the frequency of having meals in front of a screen (when watching television (TV)/digital video disks (DVD)/videos and/or using a games console/computer) and the frequency of having meals together with the whole family or at least one family member. Details on the type of foods consumed were also asked (such as whole-wheat bread v. white bread, brown rice v. white, whole-wheat pasta v. white, whole-wheat breakfast cereals v. plain, etc.). To estimate the daily energy and free sugars intakes coming from foods, the Nutrition Data System for Research (NDSR) software version 2011, developed by the Nutrition Coordinating Center at the University of Minnesota (Minneapolis, MN, USA) was used, as well as local food composition tables( Reference Schakel, Sievert and Buzzard 26 ).
Estimation of energy under-reporting
Energy under-reporting was estimated using the Goldberg equation( Reference Black 27 ) according to the ratio of energy intake (EI) to BMR. In particular, Schofield’s age-specific equations were used to estimate BMR from measured weight( Reference Schofield 28 ), while physical activity level was set to 1·55 indicating moderate activity according to mean values of PAQ-C score. The evaluation of individual intake considering long records was based on 28 d as the FFQ used in the present study referred to usual food intake of the past. The within-subject daily variation in EI (CVwE) was set at 23 % as a suitable average value as previously described in detail( Reference Nelson, Black and Morris 29 ), the variation in BMR (CVwB) was set at 8·5 % and the between-subject variation in physical activity (CVtP) was set at 15 % as suitable averages( Reference Black 27 ). According to these, under-reporters of EI were defined as those with EI:BMR<1·09.
Anthropometric measurements
Body weight was measured to the nearest 0·1 kg with the use of a digital scale (Tanita TBF 300). Standing height was measured using a portable stadiometer (Leicester height measure) to the nearest 0·1 cm without shoes. BMI (kg/m2) was calculated by dividing weight by standing height squared. OB and OW among children were calculated using the International Obesity Task Force age- and sex-specific BMI cut-off criteria( Reference Cole, Bellizzi and Flegal 30 ). Waist and hip circumferences were measured to the nearest 0·1 cm with the use of a non-elastic tape (Seca, Germany) and with the participant in standing position. Waist-to-hip and waist-to-height ratios were also calculated. All measurements were performed during morning hours.
Physical activity and sedentary behaviours assessment
Participants were asked to complete the Physical Activity Questionnaire for Older Children (PAQ-C; score 1–5)( Reference Kowalski, Crocker and Faulkner 31 ). In addition, children were also asked to report the average weekday and weekend time (h/d) spent on sedentary activities and more specifically on watching TV/DVD/videos and/or recreational usage of a games console/computer, together defined as screen time. By combining the former two responses, mean time (h/d) spent watching TV/DVD/videos and/or recreational usage of a games console/computer was calculated.
Statistical analysis
Continuous variables that were normally distributed are presented as mean and standard deviation. Normality was evaluated using P–P plots. Associations between categorical variables were tested by the χ 2 test. Student’s t test for independent samples was used to evaluate mean differences between normally distributed variables.
To obtain lifestyle patterns, factor analysis with the principal components method was applied to the food variables( Reference Mardia, Kent and Bibby 32 ). After converting the reported eating frequencies of foods from the semi-quantitative FFQ into servings per day, the food variables used in principal components analysis had continuous distributions representing servings per day of the food item, food group or beverage consumed. The correlation matrix of the food variables used showed that there were several correlation coefficients with absolute value >0·3. The Kaiser–Meyer–Olkin criterion and Bartlett’s test of sphericity were also used to assess the suitability of the data for principal components analysis. An overall Kaiser–Meyer–Olkin criterion close to unity suggests very good inter-correlation of variables and that the data set is suitable for principal components analysis. Moreover, based on the criterion proposed by Kaiser – i.e. the number of components that should be retained is equal to the number of eigenvalues that are greater than one, since these components explain more information than the individual food variables – it was concluded that the first four components should be extracted. Based on the principle that the component scores (loadings) are interpreted similarly to correlation coefficients, and thus higher absolute values indicate that the food variable contributes most to the construction of the component, the food components (patterns) were named according to the foods or food groups that had absolute scores >0·4.
Multiple linear regression analysis was then applied to evaluate the associations between total free sugars intake and dietary patterns derived from the principal components analysis, after adjustment for several potential confounders and testing for collinearity using their first product moments. Finally, multiple logistic regression analysis was applied to evaluate the association of free sugars intake in relation to the likelihood for a child being OW/OB. Results are presented as odds ratios and their corresponding 95 % confidence intervals. All reported P values were based on two-sided tests. Statistical calculations were carried out using the statistical software package PASW Statistics version 18.
Results
Using the Goldberg cut-off, 27·9 % of the children were classified as energy under-reporters. In order to examine the effect of under-reporting in the results of the present study, we compared the percentage of under-reporters and normal reporters exceeding the WHO suggested limit of 10 % of total energy coming from free sugars( 19 ) and found no differences between the different groups. In addition, when examining the main food sources of free sugars, total sugars and energy in the diet of under- and normal-reporting children, no differences were observed in the classification of sources and their contribution to total sugars and energy intake. Therefore, we did not proceed to the exclusion of under-reporters from the data set and continued our analysis with the whole sample.
Among participants, 44·2 % were categorized as having free sugars intake above 10 % of total energy intake. Mean total free sugars intake was 63·5 (sd 46·9) g/d and was significantly higher (P<0·001) among boys (69·7 (sd 51·6) g/d) than girls (58·0 (sd 41·6) g/d); however, the proportion of boys and girls exceeding the recommended cut-off of energy intake coming from free sugars did not differ significantly. The overall mean contribution of free sugars to total energy intake was 11·2 (sd 5·4) %, without any sex differences, and free sugars accounted for 44 % of total sugars. The major food sources of free sugars in the diet of Greek children are shown in Table 1, in addition to the contribution of free sugars to total sugars intake. Anthropometric and lifestyle characteristics of the participating children according to their free sugars intake classification are presented in Table 2. Specifically, children exceeding the cut-off had fewer daily meals and snacks, and also consumed meals in front of a screen (TV, video games console or computer) more often. Mean values of all anthropometric characteristics (i.e. BMI, body weight, waist and hip circumferences, waist-to-height and waist-to-hip ratios) did not differ between the two categories of children, nor did their mean energy intake. Concerning the top ten sources of energy, these are shown graphically in Fig. 1. Among the foods contributing free sugars, sweets, 100 % natural fruit juice, dairy desserts and chocolate milk were also listed in the top ten energy sources of children. Breads (presented as part of the starch group in Fig. 1) contributed 7·6 (sd 7·3) % of total energy intake. Soft drinks and sugared fruit juices when grouped together contributed 2·6 % of total energy, breakfast cereals 2·7 %, biscuits 1·4 %, starchy pies 1·7 % and honey/jam less than 1 %.

Fig. 1 Top ten sources of foods and food groups* contributing to mean total energy intake in a nationwide sample of 3089 children (aged 10–12 years), GRECO (Greek Childhood Obesity) study, 2009. *Starch category includes breads, pasta, rice and potato (excluding breakfast cereals and French fries); cheese category includes full- and low-fat cheese; sweets category includes chocolate, chocolate bars, ice cream and pastries; fast foods category includes burgers, pizza and souvlaki (traditional food with meat); 100 % natural fruit juice category includes freshly squeezed and packaged 100 % fruit juice
Table 1 Consumption of free sugars from foods and food groups and their contribution to total sugars intake in a nationwide sample of 3089 children (aged 10–12 years), GRECO (Greek Childhood Obesity) study, 2009
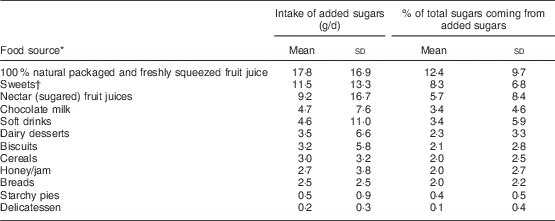
* Sorted by overall contribution of free sugars.
† Sweets category includes chocolate, chocolate bars, ice cream and pastries.
Table 2 Demographic, anthropometric and lifestyle characteristics according to free sugars contribution to total energy intake in a nationwide sample of 3089 children (aged 10–12 years), GRECO (Greek Childhood Obesity) study, 2009

PAQ-C, Physical Activity Questionnaire for Older Children; TV, television.
*The reported P values were calculated using the t test and the χ 2 test.
Based on factor analysis, four components were extracted and studied that explained 50 % of the total variation with regard to the examined variables. The value of the Kaiser–Meyer–Olkin criterion was equal to 0·8 and the P value for Bartlett’s test of sphericity was <0·001, indicating that the dietary variables entered in the analysis were strongly inter-correlated and, therefore, factor analysis could be used for assessing meaningful dietary patterns. The loadings for the four food components (patterns), which represent the correlation of each food item with the corresponding component, are presented in Table 3 (bold font indicates the coefficients with absolute loadings >0·4, which means they are better correlated with the component). Since higher absolute values indicate that the food variable contributes more to the characterization of the component( Reference Mardia, Kent and Bibby 32 ), it could be suggested that the extracted components are characterized as follows: higher consumption of sweets, pizza, souvlaki, burgers, French fries and sugared drinks, more frequently ordering/eating outside home and having meals in front of a screen (component 1); higher consumption of whole fruits, 100 % natural fruit juices, vegetables, legumes and honey/jam (component 2); a pattern loaded heavily on daily number of meals and snacks (eating occasions; component 3); and a pattern characterized mainly by breakfast frequency (component 4).
Table 3 Score coefficients (loadings) derived from factor (principal components) analysis regarding foods or food groups consumed by the children and their lifestyle habits; nationwide sample of 3089 children (aged 10–12 years), GRECO (Greek Childhood Obesity) study, 2009

TV, television; SSB, sugar-sweetened beverages.
Coefficients with absolute loadings >0·4 are indicated in bold font.
* Consumption of home-made and market/packaged 100 % fruit juice.
† Consumption of soft drinks and sugared fruit juices (SSB).
‡ Consumption of chocolate, chocolate bars, ice cream and pastries.
Potential associations between the extracted lifestyle patterns and free sugars intake were explored by multiple linear regression analysis, after controlling for sex, age and energy intake. As shown in Table 4, lifestyle patterns 1 and 2, and children’s age were positively associated with free sugars intake. Conversely, lifestyle patterns 3 and 4 were negatively associated with free sugars intake. Lastly, the evaluation of the association between free sugars intake and OW/OB with the logistic regression analysis showed that free sugars intake was not a significant predictor of OW/OB after adjusting for age, sex, physical activity level and energy intake (Table 5).
Table 4 Multiple linear regression analysis examining the association of lifestyle components with free sugars intake in a nationwide sample of 3089 children (aged 10–12 years), GRECO (Greek Childhood Obesity) study, 2009

β, standardized beta coefficient.
* Lifestyle component 1: higher consumption of sweets, pizza, souvlaki, burgers, French fries and sugared drinks, more frequently ordering/eating outside home and having meals in front of a screen.
† Lifestyle component 2: higher consumption of whole fruits, 100 % natural fruit juices, vegetables, legumes and honey/jam.
‡ Lifestyle component 3: higher daily number of meals and snacks.
§ Lifestyle component 4: higher breakfast frequency.
Table 5 Results from logistic regression analysis to evaluate the main effect of child variables on the likelihood of childhood overweight/obesity in a nationwide sample of 3089 children (aged 10–12 years), GRECO (Greek Childhood Obesity) study, 2009
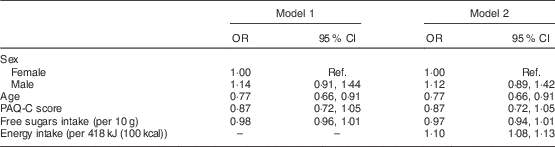
PAQ-C, Physical Activity Questionnaire for Older Children; Ref., referent category.
All odds ratios and their corresponding 95 % confidence intervals were calculated by performing multiple logistic regressions.
Discussion
The present study is the first estimating free sugars intake in a Greek childhood population. Adopting the WHO recommendations( 19 ), it was shown that a large proportion of children exceeded the cut-off of 10 % of energy coming from free sugars. The overall mean contribution of free sugars to total energy intake was 11·2 %, lower than the percentage reported for the child population in the USA (16·2 % for children 6–11 years old and 17 % for adolescents 12–19 years old)( Reference Drewnowski and Rehm 10 ), but similar to other childhood populations( Reference Brisbois, Marsden and Anderson 33 , Reference Colucci, Cesar and Marchioni 34 ). It should be stressed though that it is difficult to compare intakes among different populations since there is no universally accepted definition of the term ‘added sugars’ and a lack of agreement on the foods that contribute to added sugars intake. According to Slining and Popkin( Reference Slining and Popkin 35 ) who examined 15-year trends in intakes and sources of added sugars among US children aged 2–18 years, the intake of added sugars as a percentage of total energy intake declined significantly from 18 % in 1994–1998 to 14 % in 2009–2010, together with the average daily energy intake from added sugars. According to those authors, although the results highlighted a recent decline in intake, they also showed that the average US child still consumes a great amount of energy from added sugars. The main sources of added sugar intake, according to the same report, were SSB, grain-based desserts, candy and other sweet snacks, ready-to-eat cereals, dairy-based desserts, and sweeteners and syrups. Similar findings regarding the major sources of added sugars have been reported for US children, with SSB being the primary source( Reference Drewnowski and Rehm 10 , Reference Huth, Fulgoni and Keast 36 ).
The analysis of the present study revealed a considerably different scenario with 100 % packaged and freshly squeezed fruit juice being the principal source of free sugars, followed by sweets, nectar (sugared) fruit juices, chocolate milk, soft drinks and other foods presented in Table 1. Besides revealing variations in food consumption habits and preferences between childhood populations, these differences also bring to light a serious methodological problem when investigating the association of sugars or added sugars intake with OB or other health outcomes, since there are important differences between studies concerning the inclusion or exclusion of foods that are considered or not sources of added (or free) sugars. The percentage of energy coming from soft drinks and sugared fruit juices in our study was lower than the respective proportions found in other studies where it has been shown that energy intake from sodas and from all SSB account for 5·8 % and 8·5 %, respectively( Reference Reedy and Krebs-Smith 37 ). In further studies and depending on the grouping of the foods prior to the analyses, it has been reported that children and adolescents consume 10–15 % of energy intake from SSB and 100 % fruit juice( Reference Reedy and Krebs-Smith 37 , Reference Wang, Bleich and Gortmaker 38 ).
Irrespective of the finding that added sugars intake seems to have levelled off in some populations, it still remains higher than the recommended upper intake levels( Reference Slining and Popkin 35 , Reference Bray and Popkin 39 ). An array of meta-analyses have shown a significant association between sugar intake (generally focused on sugars from SSB) and OB or diabetes mostly in adults but in children as well( Reference Te Morenga, Mallard and Mann 5 , Reference Bray and Popkin 39 – Reference Hu 41 ). Furthermore, intervention studies in children and adolescents have shown that reducing SSB consumption significantly reduces weight gain and adiposity( Reference Ebbeling, Feldman and Chomitz 42 , Reference de Ruyter, Olthof and Seidell 43 ). However, there are also prospective and cross-sectional studies showing no association or even a negative association between sugars intake and body weight( Reference Ludwig, Peterson and Gortmaker 14 – Reference Song, Wang and Chung 16 ). In the study of Song et al.( Reference Song, Wang and Chung 16 ) it was shown that energy intake rather than total sugar intake was the most significant dietary factor predicting BMI in children and adolescents. The former study highlighted that the determining factor influencing OW and OB rates is ultimately the imbalance between energy intake and energy expenditure rather than the consumption of a specific nutrient per se. In the present study we found no differences in BMI or any other central obesity index between children exceeding or not the cut-off of 10 % of energy coming from free sugars. Furthermore, logistic regression analysis (Table 5) showed that free sugars intake was not a significant predictor of OW/OB, while, as expected, higher energy intake increased the odds for OW/OB. A possible explanation for the lack of association between free sugars and OW/OB could be that the highest proportion of energy intake in the diet of Greek children came from foods and food groups that do not contain free sugars (Fig. 1).
In the present study, 44 % of children exceeded the cut-off of 10 % of energy coming from free sugars when using the recommendation of the WHO, with the primary source of free sugars being 100 % packaged and freshly squeezed fruit juice. Following the American Academy of Pediatrics recommendation, public health authorities in many countries suggest the limitation of fruit juice intake to approximately 237–355 ml/d (8–12 fl oz/d) in children and adolescents 7 to 18 years old, mostly led by the concern that high fruit juice consumption is associated with OW in children( 44 , Reference Dennison, Rockwell and Baker 45 ). Nevertheless, this recommendation is disputed by other researchers mostly because several studies examining the long-term effects of fruit juice consumption find no effect or adverse effect on the body weight of children( Reference Skinner, Carruth and Moran 46 – Reference Newby, Peterson and Berkey 48 ). In addition, it has been stressed that some food sources of added sugars (including 100 % fruit juices) make a major contribution to micronutrient and fibre intakes in the overall diet and that consumers of 100 % fruit juice show better nutrient adequacy and diet quality than non-consumers( Reference Huth, Fulgoni and Keast 36 , Reference O’Neil and Nicklas 49 ).
The free sugars intake was tested in relation to lifestyle patterns of children. Two of the patterns extracted by factor analysis, characterized by higher consumption of sweets, pizza, souvlaki, burgers, French fries and sugared drinks, more frequently ordering/eating outside home and having meals in front of a screen (component 1) and higher consumption of whole fruits, 100 % natural fruit juices, vegetables, legumes and honey/jam (component 2), were positively associated with free sugars intake. Pattern analysis as a method has the advantage of revealing behaviours in populations that could not be assessed with any other traditional dietary assessment method and gives the opportunity to examine the effects of overall dietary choices and lifestyle characteristics on outcomes in a more integrated way( Reference Kim and Mueller 22 ). In the extracted component 1, one of the loadings was SSB consumption which has been independently associated with increased consumption of added sugars( Reference Drewnowski and Rehm 10 , Reference Slining and Popkin 35 , Reference Huth, Fulgoni and Keast 36 ). However, the component reveals a whole unfavourable lifestyle, instead of one food, that promotes free sugars intake and includes regular consumption of sweets and fast foods, eating/ordering outside home and having meals in front of a screen. High fast-food intake, eating out and having meals in front of a screen in adolescents have been associated with unfavourable changes in dietary habits and BMI, and increased consumption of foods high in added sugars( Reference Gebremariam, Bergh and Andersen 50 – Reference Demissie, Eaton and Lowry 52 ). Furthermore, it has been suggested that the negative association between added sugars intake and micronutrient density of diets is more related to patterns of food intakes from which added sugars in the diet are derived, rather than to added sugars intake per se ( Reference Hess, Latulippe and Ayoob 3 ). In the case of component 2, which was also positively associated with free sugars intake, it is mainly characterized by foods that are considered healthy and are generally promoted to be consumed regularly. The association of this particular component with free sugars intake could be mainly attributed to the fact that it is loaded by 100 % fruit juice and honey/jam. It could be hypothesized that both 100 % fruit juice and honey/jam are considered healthy choices by the children and their parents. Previous reports also show that fruit juice is considered to be a ‘healthy’ beverage and most studies of dietary intake patterns associated with an otherwise healthy diet among children find fruit juice as the main beverage parents provide their children( Reference Piernas, Tate and Wang 53 ). Finally, the two patterns characterized mainly by higher daily number of meals and snacks (component 3) and higher breakfast frequency (component 4) showed a negative association with free sugars intake. Previous work in this area has shown that both frequent breakfast consumption and a higher number of meals during the day, besides protecting against childhood OW/OB, are also associated with better diet quality( Reference Deshmukh-Taskar, Nicklas and O’Neil 54 – Reference Nicklas, Baranowski and Cullen 57 ).
The limitations of the present work are mostly due to its cross-sectional nature since cause-and-effect relationships cannot be drawn. The use of international instead of local food composition tables (due to the incompleteness) may have over- or underestimated the energy and free sugars content of some of the foods included in the analyses, although we have used the NDSR software which provides data on free sugars for a large variety of foods. Additionally, we must highlight that research suggests that collecting reliable and accurate dietary data from children and adolescents is difficult since under-reporting appears to be greatest for unhealthy foods or foods perceived to be related to OB, such as those high in added sugars( Reference Slining and Popkin 35 ). In the present study, we identified under-reporting children but decided not to exclude them from the analyses since we did not find differences in the percentage of under-reporters and normal reporters exceeding the limit of 10 % of total energy coming from free sugars and when examining the main sources of free sugars, total sugars and energy in the diet of under- and normal-reporting children, no differences were observed in the classification of food sources. Furthermore, when excluding under-reporters we did not find differences in the associations from the logistic regression analysis evaluating the effect of variables on the likelihood of childhood OW/OB.
Conclusion
The present study reveals that a high proportion of Greek children exceed the current WHO recommendation for free sugars intake. Although it is not clear in the literature what the mechanisms for producing adverse effects on BMI or cardiometabolic factors from increased added sugars intake are, there is a consensus that high free (or added) sugars intake increases the risk for adverse health outcomes through high energy intake. Thus, just like any other nutrient, free sugars should have an upper limit of intake. However, the food sources of free sugars in Greek children were different from those identified in other populations, and more importantly free sugars intake was associated with lifestyle patterns rather than single foods. One of these patterns revealed that frequent ordering out, consumption of fast foods, as well as having meals in front of a screen were associated with increased consumption of foods high in free sugars and consequently higher total free sugars intake. On the other hand, a pattern characterized by consumption of healthy foods (such as whole fruits, 100 % fruit juice, honey, vegetables and legumes) was also related to higher free sugars intake. Therefore, before characterizing foods as ‘good’ or ‘bad’ based only on their concentration of free sugars we have to consider the contribution of every food or dietary pattern to overall diet and health. Any public health initiatives aiming to reduce free sugars intake should focus on promoting the correct or altering the lifestyle habits of the specific childhood population.
Acknowledgements
Financial support: Funding for the study was provided by the General Secretariat of Consumers–Greek Ministry of Development, Hellenic Association of Food and Beverage Companies, Coca-Cola Hellas, Coca-Cola Hellenic Bottling Company, Cereal Partners Hellas, FAGE SA, Unilever Hellas, Nestlé Hellas and Kraft Foods Hellas. This research has also been co-financed by the European Union (European Social Fund – ESF) and Greek national funds through the Operational Program ‘Education and Lifelong Learning’ of the National Strategic Reference Framework (NSRF) – Research Funding Program: ‘Heraclitus II. Investing in knowledge society through the European Social Fund’. All mentioned funders had no role in the design, analysis or writing of this article. Conflict of interest: None. Authorship: A.Z., D.B.P., P.F. and G.R. were responsible for the study design and the supervision of the field study. D.B.P. and P.F. were responsible for the statistical analysis. P.F., D.B.P., G.R. and A.Z. were responsible for the interpretation of the data. All authors carried out data collection or data management, contributed to database preparation and participated in writing the submitted manuscript. All authors read and approved the final manuscript. Ethics of human subject participation: This study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects were approved by the Hellenic Ministry of Education (Department of Primary Education) and the Agricultural University of Athens Research Committee.








