Maternal and child micronutrient malnutrition continues to be prevalent in low- and middle-income countries( Reference Black, Allen and Bhutta 1 ). The most frequently reported micronutrient deficiencies worldwide are of vitamin A, Fe, iodine and Zn. These and other micronutrient deficiencies contribute to growth stunting, reduced cognitive function, more severe and increased rates of infection and mortality, and reduced physical capacity( Reference Labadarios and Steyn 2 ). Severe anaemia during pregnancy is estimated to contribute as much as 20 % to maternal mortality( Reference Black, Allen and Bhutta 1 ). Steyn and colleagues reported that vitamin A deficiency and Fe-deficiency anaemia contributed to more than 3500 maternal deaths in 2000 in South Africa( Reference Steyn, Wolmarans and Nel 3 ). During lactation, maternal requirements for vitamins A, B6, C, riboflavin, Zn and iodine are between 40 and 90 % higher than pre-pregnancy, while the requirements for thiamin, niacin, folate, vitamin E and Se are about 25 % higher( Reference Picciano 4 ), placing lactating mothers, especially those consuming diets with low nutrient density, at increased risk for micronutrient deficiencies. Diet diversity in South Africa is low, with those living in tribal areas being worse off( Reference Labadarios, Steyn and Nel 5 ); cereals, bread, roots, tea, sugar, jam and sweets are the most universally consumed foods, none of which are rich sources of micronutrients( Reference Steyn, Nel and Casey 6 , Reference Steyn, Nel and Nantel 7 ). Maize meal and bread are the most commonly consumed staples( Reference Sayed, Bourne and Pattinson 8 ).
Food fortification is a cost-effective nutrition intervention that can improve nutritional status across all age groups. Food fortification legislation was adopted nationally in South Africa in April 2003 and activated 6 months later in October 2003( 9 ). The legislation mandates the fortification of all maize meal and wheat flour with vitamins A, thiamin, riboflavin, niacin, B6 and folic acid and minerals Fe and Zn, as shown in Table 1 ( 9 ). A secondary analysis of adult nutrient intake in South Africa predicted that fortification at the specified levels would be relatively successful in increasing intake of these micronutrients to recommended levels in adults( Reference Steyn, Wolmarans and Nel 3 ). An observational nutrition study of lactating women was being conducted in rural KwaZulu-Natal when the fortification programme began, which provided an opportunity to assess the effectiveness of food fortification in that population. The aim of the present study was therefore to investigate the impact of the South African food fortification programme in a small cohort of lactating women by comparing food records and selected biochemical indicators of nutritional status before and after initiation of the fortification programme.
Table 1 South African food fortification standards
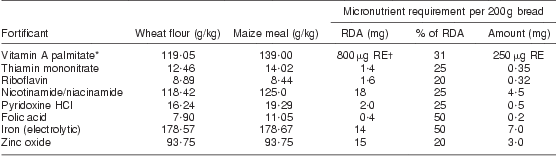
Source: Adapted from Department of Health, Republic of South Africa (2003)( 9 ).
*Palmitate activity: 75 000 μg RE/g.
†μg RE = retinol equivalents = 1 μg retinol = 3·33 IU vitamin A.
Experimental methods
Description of population and data previously reported
The Maternal Nutrition study was conducted from 2002 to 2004 in KwaZulu-Natal, South Africa( Reference Papathakis, Rollins and Chantry 10 ). The study site was largely rural, with a high prevalence of unemployment (54 %)( Reference Hosegood, Vanneste and Timaeus 11 ). Mothers were consecutively enrolled at one clinic in a peri-urban township and at two rural clinics. Mothers participating in an ongoing study investigating the relationship between exclusive breast-feeding and HIV transmission were offered entry into the study. The 142 study participants (ninety-four HIV-infected, forty-eight HIV-uninfected) were enrolled at ∼6 weeks postpartum and had subsequent study visits at ∼14 and ∼24 weeks, and 9 and 12 months postpartum, when blood and dietary records were collected. Multiple micronutrient deficiencies were identified in both HIV-infected and HIV-uninfected mothers, with HIV-infected generally worse off nutritionally( Reference Papathakis, Rollins and Chantry 10 ). Less than 45 % of all mothers were sufficient in vitamin B12, folate or vitamin E, while 25 % had low serum ferritin.
Dietary intake
During the Maternal Nutrition study, a survey of shops with bakeries was undertaken to determine the date when bread production began using fortified wheat flour and when maize meal was available. Shop managers reported that both bread made with fortified wheat flour and packaged fortified maize meal were available near 1 January 2004. It was assumed that families may have a small supply of maize meal at home, so 14 January was arbitrarily selected as the date when fortified maize meal would likely be consumed in the home. In the food record analysis, food composition tables with the new fortification content were used for any 24 h recalls taken after 1 January 2004 for bread and after 14 January 2004 for maize meal.
Zulu-speaking trained nutritionists obtained maternal 24 h recall intake information using open-ended questions during each home visit, up to five times during the 12 months of follow up. To improve the assessment of portion sizes, the nutritionist measured the volume of cups, bowls and plates from which the meals were consumed in the home, and recorded the brand names of staple foods used. A registered dietitian entered the 24 h recall information into the Food Finder® nutrient analysis program, which was developed by the Medical Research Council (Cape Town, South Africa) and contains some indigenous foods. Recipes for the Food Finder database were developed that reflected commonly consumed recipes prepared according to local customs and preferences. With additional computer programming, warnings were set for range limits and consistency checks.
Each woman's average intake was calculated from her multiple 24 h recall records and used to determine the mean intake for the group (n 142). South Africa uses the US Dietary Reference Intake as a standard, so group results were compared with the US Dietary Reference Intake's Estimated Average Requirement (EAR) or Recommended Nutrient Intake (RNI) cut-off point, whichever was the lower of the values, and the Adequate Intake (AI) for Ca to determine the proportion of individuals with inadequate micronutrient intake. Nutrient consumption of the population was considered inadequate if ≥50 % of the sample consumed less than the EAR/RNI( 12 ).
Thirty-four women (referred subsequently as ‘the subsample’) had diet records from both before and after 14 January 2004. Their average nutrient intakes pre-fortification, average maize meal and bread consumption and demographic characteristics were compared with those of all women, excluding the subsample, to determine whether they were different from the larger group. In the subsample, after determining each woman's average intake before and after fortification, average group intakes pre- and post-fortification were compared.
Serum micronutrients
Of the thirty-four women in the subsample, serum micronutrient results were available for thirty-one. Venous blood samples were drawn in the morning for all women, but fasting status was not ascertained. Samples were collected into mineral-free gel separator tubes for minerals and folate. Vials were protected from light, and kept cool until they were centrifuged within 3 h of collection. The serum was separated, frozen and stored at −70°C until tested. Each woman's specific mean concentration pre- and post-fortification was determined for retinol, folate, ferritin and Zn, and was averaged and compared to determine change. The proportions of women marginal and deficient for each of these micronutrients were compared pre-and post-fortification.
Retinol was measured by HPLC (series 200 instrument; Perkin Elmer, Wellesley, MA, USA). Vitamin A deficiency was defined as a plasma retinol concentration <0·35 μmol/l and marginal vitamin A status as <0·70 μmol/l.
Folate in serum was measured by paramagnetic particle, chemiluminescent immunoassay on a Beckman Coulter immunoassay system (Fullerton, CA, USA). Folate deficiency was defined as <6·8 nmol/l and marginal folate status as <14·0 nmol/l( 13 ).
Serum ferritin was measured by immunoassay on a Roche Modular E170 analyser (Basel, Switzerland); Fe deficiency was defined as a serum ferritin concentration of <13 μg/l (laboratory reference).
Zn was measured by inductively coupled plasma mass spectrometry (Elan DRC II instrument; Perkin Elmer). Deficiency was defined as Zn concentration <10·2 μmol/l (non-fasting cut-off)( Reference Hotz and Brown 14 ).
Analysis
The Student's t test for paired samples was used to assess whether the mean intake of each nutrient changed significantly post-fortification and to determine change in serum micronutrient concentrations. The χ 2 test was used to determine the proportion meeting the EAR/RNI for dietary intake and whether the women were deficient/marginal or replete pre- and post-fortification. Linear regression models were run to investigate the potential influence of age, weight, number of previous pregnancies, HIV status and months postpartum as covariates. Interaction between HIV status and other independent variables, such as age, parity and weight, were investigated. A difference of 0·67 sd between groups with 80 % power would be detected with the sample size of thirty-four.
Statistical analysis was performed using the software packages Minitab 16 (2011; Minitab, Inc.) and R version 2·6·1 (R Project for Statistical Computing). Variables not normally distributed were suitably transformed.
Ethics
The original study was approved by the Research Ethics Committee of the Nelson R. Mandela School of Medicine of the University of KwaZulu-Natal, Durban, South Africa and the Human Subjects Committee of the University of California, Davis, USA. All participants provided written informed consent. No incentive or compensation was provided to participants.
Results
Dietary intake
The mean nutrient intake of all 142 women is shown in Table 2. There was an average of 4 food records per woman. On average, women consumed daily 8160 (sd 2181) kJ (1949 (sd 521) kcal), 44·5 (sd 15·0) g protein with 10·3 (sd 9·0) g from animal sources, 52·5 (sd 27·3) g added sugar and 19·6 (sd 7·0) g dietary fibre; energy distribution was 9·3 (sd 1·8) % protein, 65·0 (sd 6·1) % carbohydrate and 25·7 (sd 6·0) % fat. More than 80 % of lactating women did not meet the EAR/RNI for vitamins A, C, D, B6, B12, folate, riboflavin, thiamin and niacin and minerals Zn, iodine and Ca as shown in Table 2. The mean nutrient intake and the proportion of women with dietary intake below the EAR/RNI did not differ by HIV status (data not shown).
Table 2 Mean daily nutrient intakes of breast-feeding women (n 142), KwaZulu-Natal, South Africa, 2002–2004
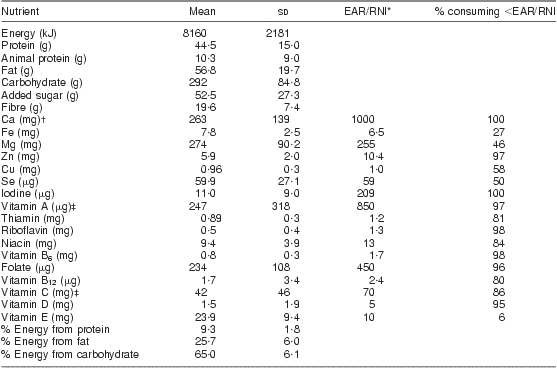
Mean number of 24 h recall food records per woman was 4; ninety-four HIV-infected, forty-eight HIV-uninfected, no difference detected by HIV status.
*Value represents the lower of either the EAR (Estimated Average Requirement) or the RNI (Recommended Nutrient Intake).
†Ca standard uses the Adequate Intake as there is no EAR.
‡Vitamins A and C use the RNI since it is lower than the EAR.
In the fortification subsample, 84 % of women had >3 food records; women averaged 3 food records pre-fortification and 1·5 records post fortification. Two extreme outliers were investigated and determined to be implausible so were removed prior to analysis, one for vitamin A and one for folate. Results from linear regression models indicated no evidence of a difference in the change in nutrient intake by age, weight, number of previous pregnancies, HIV status and months postpartum. HIV by weight, age and months postpartum interactions were also investigated, and none were found. Demographic characteristics of the subsample are described in Table 3 and were no different from the rest of the women. The pre-fortification fortified nutrient intakes and maize meal and bread intakes of the subsample of women were not different from those of the rest of the group, as shown in Table 4.
Table 3 Baseline demographic characteristics of the subsample compared with the remainder of the cohort: breast-feeding women, KwaZulu-Natal, South Africa, 2002–2004
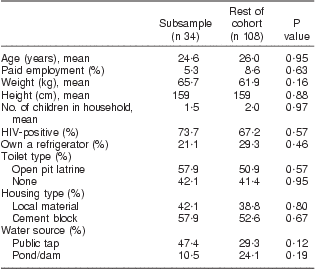
Table 4 Mean pre-fortification daily dietary intakes in the subsample compared with the remainder of the cohort: breast-feeding women, KwaZulu-Natal, South Africa, 2002–2004
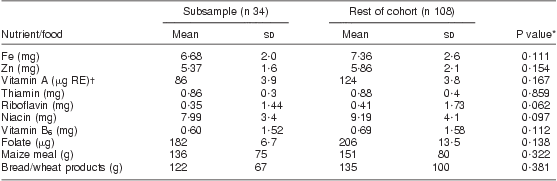
*t Test.
†μg RE = retinol equivalents = 1 μg retinol = 3·33 IU vitamin A.
As shown in Table 5, there was a significant increase in mean dietary intake post-fortification for all nutrients included in the national fortification programme. The median daily intake of fortified maize and of bread or wheat products was 130 g and 120 g, respectively. Even after fortification, more than 70 % of women still did not meet the EAR/RNI for vitamins A, riboflavin and B6 and for the mineral Zn, as shown in Table 6. The EAR/RNI intake was met for niacin and Fe, and nearly met for folate and thiamin, meaning that food fortification was successful in providing appropriate increased intake for the majority of lactating women to meet the EAR/RNI for these nutrients.
Table 5 Mean daily dietary intakes of fortified nutrients before and after fortification: breast-feeding women (n 142), KwaZulu-Natal, South Africa, 2002–2004
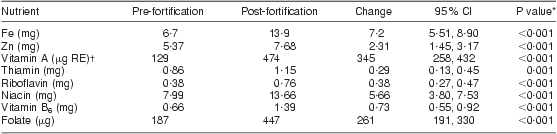
Mean number of food records per woman: 3 pre-fortification, 1·5 post-fortification.
*Paired t test.
†μg RE = retinol equivalents = 1 μg retinol = 3·33 IU vitamin A.
Table 6 Estimated average daily consumption post-fortification and percentage below the EAR/RNI for fortified nutrients: breast-feeding women (n 142), KwaZulu-Natal, South Africa, 2002–2004
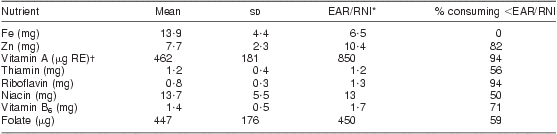
*Value represents the lower of either the EAR (Estimated Average Requirement) or the RNI (Recommended Nutrient Intake). Vitamin A uses the RNI since it is lower than the EAR.
†μg RE = retinol equivalents = 1 μg retinol = 3·33 IU vitamin A.
Serum micronutrients
There was a significant increase among the subsample in serum folate and Zn post-fortification, while there was no significant change in serum ferritin and a reduction in serum retinol, as shown in Table 7. There was no evidence of a difference in the change in nutrient concentration depending on age, weight, number of previous pregnancies, HIV status or time postpartum. HIV by weight, age and months postpartum interactions were also investigated, and none were found.
Table 7 Impact of food fortification: mean plasma nutrient concentration and change post-fortification in the subsample of breast-feeding women (n 34), KwaZulu-Natal, South Africa, 2002–2004
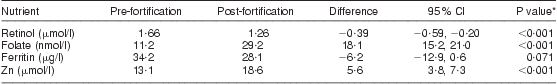
*Paired t test.
There was a significant reduction post-fortification in the proportion of women with marginal/deficient serum folate status (73·5 % pre-fortification v. 3·0 % post-fortification; P < 0·001) and with Zn deficiency (26·5 % pre-fortification v. 5·9 % post-fortification; P < 0·05). For retinol, although there was a significant reduction in mean serum concentration post-fortification, there was no significant change in the proportion of women who were marginal or deficient (6·7 % pre-fortification v. 3·2 % post fortification; P = 0·361), with more than 93 % of women being replete pre- and post-fortification. For ferritin, there was no significant change in the proportion with Fe deficiency post-fortification (16·7 % pre-fortification v. 19·4 % post fortification; P = 0·728).
Discussion
The current publication is the first we are aware of to date to report on the effectiveness of the South African food fortification programme in breast-feeding women using both dietary intake information and biochemical markers of micronutrient status. Lactating women in rural KwaZulu-Natal consumed diets containing adequate energy and macronutrient distribution but poor micronutrient density. The vast majority of women did not meet the EAR/RNI for vitamins A, C, D, B6, B12, folate, riboflavin, thiamin and niacin, and minerals Zn and Ca, before fortification. There were no differences in dietary intake by HIV status although HIV-infected women were worse off nutritionally( Reference Papathakis, Rollins and Chantry 10 ). In the subgroup analysis, the South African food fortification programme successfully increased the intake of all fortified micronutrients; serum concentrations confirmed an increase in both folate and zinc, but with no change in ferritin and a decrease in retinol. In this small group of breast-feeding women, 93 % were replete in folate and Zn post-fortification. In spite of the improved intake of many micronutrients with fortification, the majority of women still did not meet the EAR for the fortified nutrients vitamins A, riboflavin and B6 and the mineral Zn. More than 80 % of breast-feeding women in this rural community had inadequate intake of non-fortified nutrients Ca, vitamins B12, C and D.
Although the present study was conducted on a small sample, it is consistent with other studies which assessed the impact of the South African fortification programme. Duvenage and Schonfeldt found that fortification added significantly to dietary intakes of the fortified nutrients for female caregivers in informal settlements in South Africa, but not to a sufficient degree to eliminate deficiencies( Reference Duvenage and Schonfeldt 15 ). Following national fortification, Sayed et al. reported a significant reduction in the prevalence of neural tube defects( Reference Sayed, Bourne and Pattinson 8 ) and Modjadji et al. observed a significant reduction in folate deficiency but without a significant improvement in Fe status in women of reproductive age in rural Limpopo( Reference Modjadji and Alberts 16 ). A secondary analysis of adult nutrient intake in South Africa predicted that fortification was likely successful in men in urban areas; it predicted an increase to, or above, the RNI for all nutrients except folate. Among urban women, thiamin, folate and Fe intakes were predicted to remain below the RNI, while only Fe was expected to be low among rural women( Reference Steyn, Wolmarans and Nel 3 ).
Even though 100 % of women exceeded the EAR intake for Fe after fortification in the present study, there was no detectable improvement in Fe status and no reduction in Fe deficiency in the subsample. Lack of improvement in Fe status following fortification with electrolytic Fe has been found in efficacy studies( Reference Hurrell, Ranum and de Pee 17 ). The 24 h recall data presented here suggest that the additional intake of Fe may not have a biological impact for several reasons. First, electrolytic Fe, the Fe compound stipulated by the legislation for maize meal and wheat flour in South Africa, has low bioavailability( Reference Hurrell and Egli 18 ). Second, the South African fortification level requirement of 35 ppm of electrolytic Fe for both flour and maize( 9 ) is well below WHO fortification recommendations of 60 ppm for each, given usual consumption of 150–300 g/d( 19 ). If intake of wheat or maize is less than 149 g/d, as is the case in the present subsample, electrolytic Fe is not recommended by WHO( 19 ). Third, although Fe intake increased significantly after the introduction of fortification, Fe absorption may have also been inhibited by the consumption of antinutrients such as phytates and polyphenols found in maize meal and tea, respectively; average consumption of 10·6 mg/d is most likely not sufficient to meet the RNI for lactating women (10–30 mg/d)( 20 ) which considers bioavailability. Fourth, the period of exposure may not have been adequate to improve Fe status. Hallberg et al. estimate that it takes 2 to 3 years after changing the amount of bioavailable Fe in the diet for Fe balance and stores to stabilize( Reference Hallberg, Hulthen and Garby 21 ), while Huang et al. found that using electrolytic Fe as a wheat flour fortificant did not have a significant effect on body Fe stores until 6 months( Reference Huang, Sun and Li 22 ). Finally, women may have consumed non-fortified products. Fortification compliance surveys conducted between February and March 2005 indicated that 65·4 % of all maize meal samples collected from households in KwaZulu-Natal were fortified( Reference Labadarios D, Maunder and Kruger 23 ). This seems unlikely however given the significant serum response for folate and Zn.
Regarding the lack of fortification effect on serum retinol in our subsample, the majority of women were replete pre-fortification, most likely due to the maternal postpartum vitamin A supplementation programme in effect at the time of the study. Pre-fortification measurements were collected in the first few months following delivery while post-fortification measurements were obtained many more months postpartum when the effect of supplementation was likely wearing off. The decrease in retinol following fortification can be explained by the decreasing influence of vitamin A supplementation combined with the continued suboptimal vitamin A intake even after fortification. With the recent change in WHO policy to no longer supplement mothers postpartum with high-dose vitamin A( 24 ), more research will be needed to determine whether the current level of fortification will be adequate to prevent marginal vitamin A status.
Despite these intake improvements in many micronutrients in lactating women, fortification cannot solve all micronutrient deficiencies in South Africa. Nutrient amounts added through fortification must be safe and efficacious for all populations, so may not be adequate for those with increased requirements such as lactating women. Given inadequate dietary intake of all the women, a micronutrient supplement may be warranted during lactation. Some nutrients, such as Fe, may not be provided in high enough amounts or in bioavailable forms to meet the needs of women of childbearing age. Other nutrients, like Ca, are not included in the fortification mix. It is therefore desirable to pursue other complementary options to improve micronutrient intake.
Our study has several limitations. The sample size was limited to women with both food records and serum concentration of several micronutrients; it was therefore small and might not have been able to detect other differences that existed. The women were most likely representative of other rural lactating women living in KwaZulu-Natal, but not of more urban women or women from different regions, so the results may not be generalizable. Dietary assessments are always challenging and the multiple 24 h recall information may not be a fully accurate representation of intake. There were at least three recalls per mother, however, and trained nutritionists obtained the information, so that possible misrepresentation was minimized as best possible. Shop keepers may have erred in their estimation of the time of availability of fortified flour and maize meal and there was a limited period of exposure. Regardless of these limitations, we did see changes in serum values which confirms an effect given the short period of exposure.
Conclusions
Breast-feeding women in rural KwaZulu-Natal demonstrated poor dietary diversity as shown by the majority of them not meeting the EAR/RNI for vitamins A, C, D, B6, B12, folate, riboflavin, thiamin and niacin, and minerals Zn, iodine and Ca. The South African food fortification programme successfully increased the dietary intake of Fe and Zn, vitamins A, thiamin, riboflavin, niacin, B6 and folate in this small group. Even with fortification, the majority of women still did not consume the EAR/RNI for Zn, vitamins A, riboflavin and B6. Serum concentrations confirmed a significant increase in folate and Zn and reduction of deficiency, even in the short time frame (2–5 months) of exposure, although Fe and vitamin A status remained virtually unchanged.
Acknowledgements
The original data collection was supported by the Wellcome Trust (grant 063957); the present analysis was supported by the Global Alliance for Improving Nutrition (GAIN) and California State University Agricultural Research Initiative (grant 49384). There are no conflicts of interest. P.C.P. was responsible for the study design, data collection and drafting the manuscript. K.E.P. contributed to the study design, data analysis and manuscript writing. The authors are grateful to Luz Maria de Regil and James Wirth for their editorial comments on the manuscript, and thank Daniel Thomas for his assistance with data management and statistical analysis.









