Worldwide, more than 10 % of infant deaths secondary to congenital anomalies are caused by nervous system anomalies( Reference Rosano, Botto and Botting 1 ). Neural tube defects (NTD) are the most common major congenital anomalies of the central nervous system, constituting an important public health problem in terms of mortality, morbidity, social cost and human suffering( 2 ). The incidence of NTD ranges from 0·5 to 14 per 1000 live births( Reference Herrmann and Obeid 3 ).
The findings obtained from randomized trials by the British Medical Research Council in 1991( 4 ) and by the Hungarian National Institute of Hygiene (a WHO Collaborating Centre for the Community Control of Hereditary Diseases) in 1992( Reference Czeizel and Dudas 5 ) about the effect of periconceptional folic acid supplementation on NTD prevention were revolutionary and remain consensual to the present day( 6 ). Furthermore, folic acid seems to prevent other congenital anomalies (cleft lip( Reference Wilcox, Lie and Solvoll 7 , Reference Kelly, O’Dowd and Reulbach 8 ) and cardiovascular malformations), but the results are not consistent( 2 , Reference Czeizel and Dudas 5 , Reference De-Regil, Fernandez-Gaxiola and Dowswell 9 , Reference Rozendaal, van Essen and Te Meerman 10 ). The relationships between maternal folate levels and other pregnancy outcomes, such as placental abruption, placental weight and gestational age, also remain inconclusive( Reference Charles, Ness and Campbell 11 – Reference Lassi, Salam and Haider 14 ).
Curiously, in two observational studies, folic acid supplementation did not show any influence on trends of incidence of NTD( 15 , Reference Botto, Lisi and Robert-Gnansia 16 ), probably because most women started supplementation after the target period( Reference Pinto, Barros and dos Santos Silva 17 ). In order to prevent NTD, and considering that the neural tube closes by day 28( 18 ) when most women do not know they are pregnant( 2 ), even in planned pregnancies, folic acid supplementation should start before conception( 6 , 19 ). However, estimates indicate that 41 % pregnancies worldwide are unintended( Reference Singh, Sedgh and Hussain 20 ).
Regarding these promising results provided by the randomized trials, the water-soluble nature of folic acid and the unawareness of adverse effects (apart from neurological complications in people with vitamin B12 deficiency( 6 )), combined with the difficulty of global supplementation in the desired period, fortification of foods with this vitamin has been considered safe( Reference Butterworth and Tamura 21 ). This motivated some countries to opt for folic acid fortification of cereal products, namely flour. Nowadays, fortification of flour with folic acid is mandatory in forty-seven countries, in the continents of America and Oceania, in the Middle East and a few in Africa( 22 , 23 ). The effectiveness of mandatory folic acid fortification has been studied in some countries and a significant decline in prevalence of some NTD was observed; for instance, after mandatory folic acid fortification in the USA and Canada, the occurrence of some NTD declined by 28 %( Reference Williams, Rasmussen and Flores 24 ) and 46 %( Reference De Wals, Tairou and Van Allen 25 ), respectively. Nevertheless, part of this decline can be explained by better prenatal screening and the termination of affected pregnancies( Reference Kondo, Kamihira and Ozawa 26 ). Moreover, in a recent review, the authors defended that women of childbearing age may not yet be adequately targeted, while the general population may be over-fortified with folic acid( Reference Osterhues, Ali and Michels 27 ). Uncertainties remain over the minimum effective folate intake and status required for NTD prevention, and the safe upper folate level( Reference Osterhues, Ali and Michels 27 ). Voluntary fortification is permitted in most European countries, but none has mandatory fortification( 15 ). Recently the EU introduced new rules to regulate voluntary food fortification( 15 ).
After mandatory folic acid fortification, many questions arose regarding individual liberty (to choose fortified or non-fortified foods) and about feasible monitoring of potential hazards of the fortification( 15 ). The possible carcinogenicity of excessive intake of folic acid has been suggested( 2 , 28 ), supported by biologically plausible mechanisms: folate is essential in biological methylation reactions and nucleotide synthesis and impairment of these processes is thought to be involved in cancer development( 15 ). Some researchers suggested that a link could exist between high folic acid intake and cancer incidence( Reference Cole, Baron and Sandler 29 , Reference Ebbing, Bonaa and Nygard 30 ), but this is not consensual( 15 , Reference Kennedy, Stern and Moretti 31 , Reference Vollset, Clarke and Lewington 32 ).
Recently, a new concern has arisen regarding the potential adverse effects of universal maternal supplementation on adverse pregnancy outcomes, early or later in life, both in the mother and child( Reference Fekete, Berti and Trovato 13 , 33 ). Studies have associated maternal folic acid supplementation with increased birth weight( Reference Charles, Ness and Campbell 11 – Reference Lassi, Salam and Haider 14 ), insulin resistance in children( Reference Yajnik, Deshpande and Jackson 34 ) and asthma in children( Reference Burdge and Lillycrop 35 , Reference Whitrow, Moore and Rumbold 36 ).
Overall, current evidence suggests that the relationship between maternal folic acid supplementation and pregnancy outcomes displays a U-shaped association, with adverse effects at both low and high dosages. Official health institutions should deliver clear and evidence-based messages regarding folic acid intake in order to maximize health gains and to minimize adverse effects.
The present study aimed to summarize the recommendations on folate intake and folic acid supplementation and fortification, in the periconceptional period, aimed at NTD risk reduction, provided by official health organizations in different countries worldwide and WHO. This work could constitute a first step of a global discussion about the divergences between recommendations, in order to improve them.
Methods
Data about folate (provided by diet) and folic acid (provided by supplements or fortified foods) recommendations in the periconceptional period were obtained from national health official websites across forty-six countries worldwide and from the WHO website. Eligibility criteria for country selection, defined by the research team, were the following: EU countries (Austria, Belgium, Bulgaria, Croatia, Cyprus, Czech Republic, Denmark, Estonia, Finland, France, Germany, Greece, Hungary, Ireland, Italy, Latvia, Lithuania, Luxembourg, Malta, Netherlands, Poland, Portugal, Romania, Slovakia, Slovenia, Spain, Sweden and UK) and some European countries not belonging to the EU (Iceland, Liechtenstein, Norway, Switzerland and Turkey) that were mentioned on the European Commission website, on the web page entitled Public Health – Trustworthy websites on ‘Population groups’ ( 37 ); BRICS countries (Brazil, Russia, India, China and South Africa); Group of Eight (G8) countries (that include some EU and BRICS countries already stated; they are Canada, France, Germany, Italy, Japan, Russia, UK and USA); Asian Tiger/Asian Dragon countries (Hong Kong, Singapore, South Korea and Taiwan); and Australia.
Developed countries and emerging economies (according to the UN, International Monetary Fund, Organisation for Economic Co-operation and Development, and World Bank) in well-defined groups were selected because of the easiest access to data.
The keywords used for searching in each country’s websites were the following: folic acid, folate, pregnancy and nutrition. These terms and the information found in the countries’ websites were translated on Google® Translate, from English to the official languages and vice versa. Ministry of Health websites were chosen for their national coverage of the recommendations. However, for some countries we considered scientific organizations’ websites when mentioned or linked with the Ministry of Health website.
Data extracted from each website covered the following topics: (i) amount of recommended folate intake for women of childbearing age and pregnant women (measured in folate or dietary folate equivalents (DFE)); (ii) recommendation of a healthy diet and folate-rich foods; and (iii) folic acid supplementation for NTD risk reduction (recommended dosage, tolerable upper intake level (UL), dosage for high risk of NTD, when to start and to finish the supplementation). Data about mandatory folic acid fortification were obtained at the European Surveillance of Congenital Anomalies (EUROCAT) website( 23 ). The names of the official entities, the types of information (for health professionals or the general public) and the website addresses were also registered for further validation. Only nine countries (25·0 %) had all desired information available (Australia, Austria, Denmark, Estonia, Finland, Germany, Switzerland, Canada and USA).
Websites were chosen instead of collecting data from scientific articles or sending emails to each national health representative asking for information, because the found reviews( Reference Botto, Lisi and Robert-Gnansia 16 , 38 ) did not include all the desired countries nor all information. We also intended to find which information is available through the World Wide Web, where many people (from health professionals to the general public) consult and follow health recommendations( Reference Theodosiou and Green 39 – Reference Houston and Allison 41 ). Information on mandatory fortification was collected only to complete this overview, since we intended to summarize information that could be searched by people and used to influence their behaviours. Mandatory fortification is a governmental decision that potentially affects the whole population.
Emails were sent for all forty-six selected countries in order to obtain data validation or to obtain missing data (data that could be on the website, but had not yet been found). In the validation process, the information was confirmed, corrected and/or completed. Data without official validation were equally considered for results and analysis, after confirmation from the websites on at least three different days.
Data were collected from May until October 2013, and updated in December 2014.
Results
From the forty-six countries initially considered, ten (21·7 %) were excluded – six in the EU (Croatia, Cyprus, Czech Republic, Greek, Lithuania and Slovakia), two European countries not belonging to the EU (Liechtenstein and Turkey), one BRICS country (India) and one Asian Tiger/Asian Dragon country (South Korea) – on the basis of data being unavailable or not found. Thus a final sample of thirty-six countries was obtained: twenty-two in the EU (Austria, Belgium, Bulgaria, Denmark, Estonia, Finland, France, Germany, Hungary, Ireland, Italy, Latvia, Luxembourg, Malta, Netherlands, Poland, Portugal, Romania, Slovenia, Spain, Sweden and UK), three European countries not belonging to the EU (Iceland, Norway and Switzerland), four BRICS countries (Brazil, Russia, China and South Africa), three more G8 countries (the other five were already stated in EU and BRICS; Canada, Japan and USA), three Asian Tiger/Asian Dragon countries (Hong Kong, Singapore and Taiwan) and Australia; see Fig. 1.
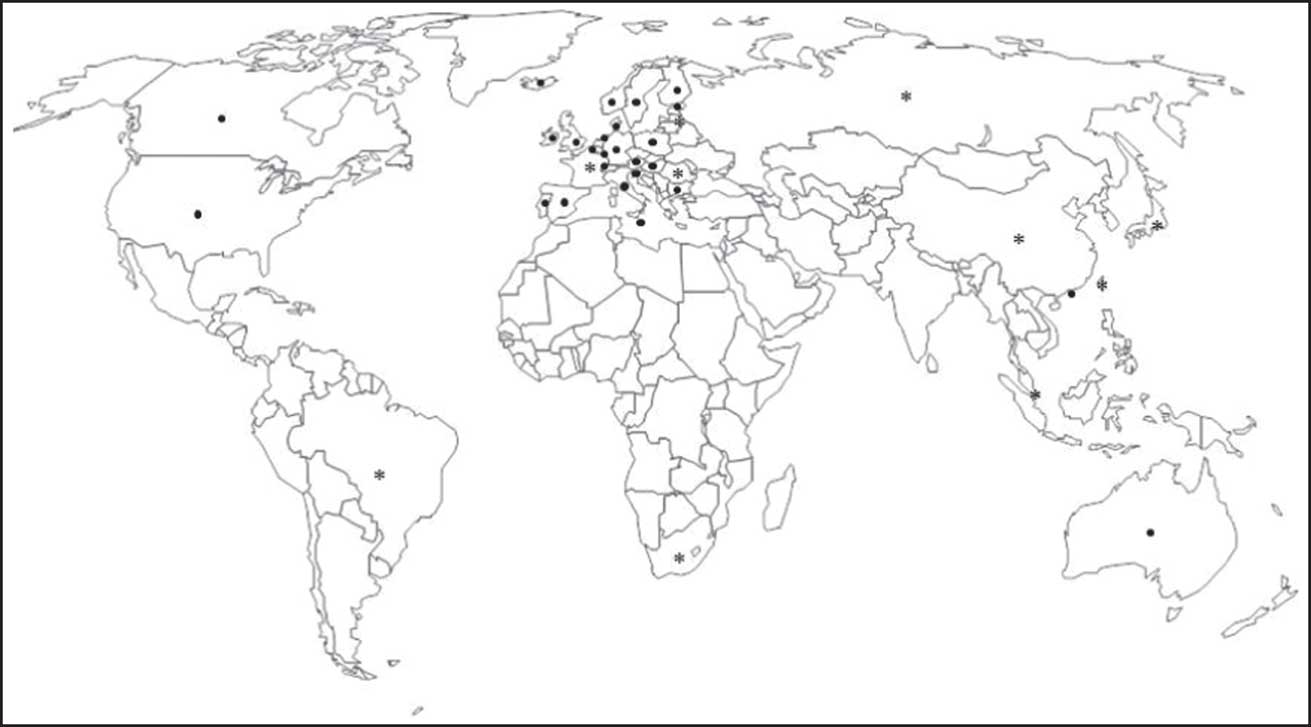
Fig. 1 World map showing the thirty-six countries in which recommendations for folate and folic acid intake in the periconceptional period were analysed (●, country with official validation; ∗, country without official validation)
From the thirty-six countries included, twenty-six countries validated the information by email (72·2 %) and ten did not (Brazil, China, France, Japan, Latvia, Romania, Russia, Singapore, South Africa and Taiwan). From the other ten countries without data, only Liechtenstein validated the (missing) information. The WHO did not validate data.
The recommendations for folate and folic acid intake in the periconceptional period are synthesized in Table 1.
Table 1 Folate and folic acid recommendations for women in the periconceptional period from official websites of national health organizations of some countries worldwide (n 36) and WHO

NTD, neural tube defects; UL, tolerable upper intake level; DFE, dietary folate equivalents; D-A-CH, Deutschland–Austria–Confoederatio Helvetica (reference values for nutrient intake by German Nutrition Society, Austrian Nutrition Society, Swiss Society for Nutrition Research and Swiss Society for Nutrition). – means that no information was available.
* Without official validation.
† ‘Unhealthy diet means having less than 400 µg of folate.’
‡ ‘A varied diet may be enough’ (France)/‘Keep up your folate status by eating a healthy diet’ (Singapore)/‘A balanced diet is assumed to be enough to achieve the recommendations’ (Taiwan).
§ ‘For women with a high intake of folate-rich foods, such as vegetarians and vegans eating large quantities of pulses and vegetables, folate intake from food may happen to be enough. For them there is no need for extra folic acid supplementation’.
A healthy diet and/or folate-rich food recommendations were issued by all countries (100·0 %), but the WHO website only mentioned it for women with high risk of NTD. Interestingly, five countries (13·9 %; Finland, France, Sweden, Singapore and Taiwan) considered that a healthy diet containing adequate amounts of folate may be enough, with no need for supplementation. Three countries (Hong Kong, Slovenia and Sweden) published e-leaflets for the general public with complete and detailed information about healthy diet during pregnancy, consumption of folate-rich foods, cooking methods and quantities. Eight more countries (Austria, Denmark, Estonia, France, Iceland, Luxembourg, Taiwan and Poland) also published e-leaflets with information for pregnant women including healthy diet. Some others mentioned it directly on the website.
Concerning recommended folate intake (and estimated average requirements) for women of childbearing age and pregnant women, respectively, we identified three main different recommendations: (i) Dietary Reference Intakes from the US Institute of Medicine (IOM; followed by 22·2 % of countries), namely 400 µg/d and 600 µg/d (320 µg/d and 520 µg/d); (ii) Nordic Nutrition Recommendations from the Nordic Council of Ministers (NNR; followed by 16·7 % of countries), namely 400 µg/d and 500 µg/d (200 µg/d); and (iii) reference values for the nutrient intake from the dominant states of the German language (Deutschland–Austria–Confoederatio Helvetica (D-A-CH); followed by 8·3 % of countries), namely 300 µg/d and 550 µg/d (220 µg/d and 420 µg/d). WHO adopted the folate recommendations from the IOM( 28 , 42 ). About half of the countries (52·8 %) did not have any references regarding recommended folate intake through diet. For Switzerland, different values were available in the two sources consulted (D-A-CH and those referred by the Swiss Federal Office of Public Health). Slovenia followed D-A-CH recommendations, with high values only after the first trimester. This appears more practicable as physiologically women have higher energetic needs in the second and third trimesters( 43 ), notwithstanding the role of folate after the first trimester is not completely established. It is also apparent that NNR presented the smallest increase from preconception to pregnancy, possibly more attainable. The highest values from the IOM seem less achievable than those from D-A-CH or NNR.
Regarding folic acid supplementation for NTD prevention, the majority (n 33, 91·7 %) of the analysed countries have some information on their national health official websites about this topic. Most countries (80·6 %) recommend a folic acid supplement of 400 µg/d, two countries state that the dosage should be recommended by a health-care provider (without mentioning any dosage) and one country recommends 5 mg/d. Concerning the time for initiating the supplementation, 33·3 % of countries state at least 4 weeks before conception, as settled by D-A-CH recommendations; 27·8 % of countries state that ‘supplementation should begin when planning pregnancy’ or ‘when there is a chance to become pregnant/capable of becoming pregnant’, as stressed by the IOM recommendations; 13·9 % of countries mention at least 12 weeks before pregnancy; two countries refer to 4–8 weeks or 8–12 weeks before conception; two advocate to start when contraception is stopped; and one country recommends at least 8 weeks before stopping contraception. Most countries (75·0 %) consider that supplementation should be maintained until the end of the first trimester (12 weeks) or almost (10 or 8–12 weeks), but three countries (Canada, China and USA) recommend supplementation until the end of pregnancy.
On the subject of the UL, 44·4 % of countries and two entities (D-A-CH and IOM) state 1 mg/d and two countries appeal for not exceeding the recommendations without mentioning the UL. Finally, the dosages recommended for women with high risk of NTD are 5 mg/d (adopted by 27·8 % of countries and by WHO recommendations) or 4 mg/d (considered by 13·9 % of countries). Some countries suggest these dosages should be discussed with a physician (8·3 % of countries and D-A-CH recommendations) and two countries refer to only ‘higher dosages’.
Although the observed recommendations differ between countries, the recommendation for folic acid of 400 µg/d before conception until the first trimester of pregnancy is common for most countries (69·4 %) and WHO.
Countries partially out of this common recommendation are those that recommended 5 mg/d (Brazil) or supplementation throughout pregnancy (Canada, China and USA) or that did not show the dosage, referring to check with a health-care provider on the need for taking supplements and to ensure an adequate folate status (Singapore). This resembles an attempt to personalize the intervention.
Some countries had incomplete information about supplementation as follows: dosage was missing in Portugal; time for initiation was absent in Romania; the end time was missing in South Africa, Portugal and Romania; and supplementation was not mentioned at all in Russia, Slovenia and Taiwan.
Taiwan’s website referred that the best method to ensure sufficient folate for fetal development is to consume foods high in folate on a frequent basis, giving examples. This perspective contrasted with that of countries with a focus mainly on supplementation, for example Netherlands and USA.
From the analysed countries, five had mandatory fortification (Australia, Brazil, Canada, South Africa and USA). All of these also advised folic acid supplementation.
Given the diversity of the recommendations for folate from international entities (from D-A-CH, NNR, IOM and WHO), we opted to build a diagram overlapping different recommendations with gestational age, periconceptional period and neurulation period, as shown in Fig. 2.
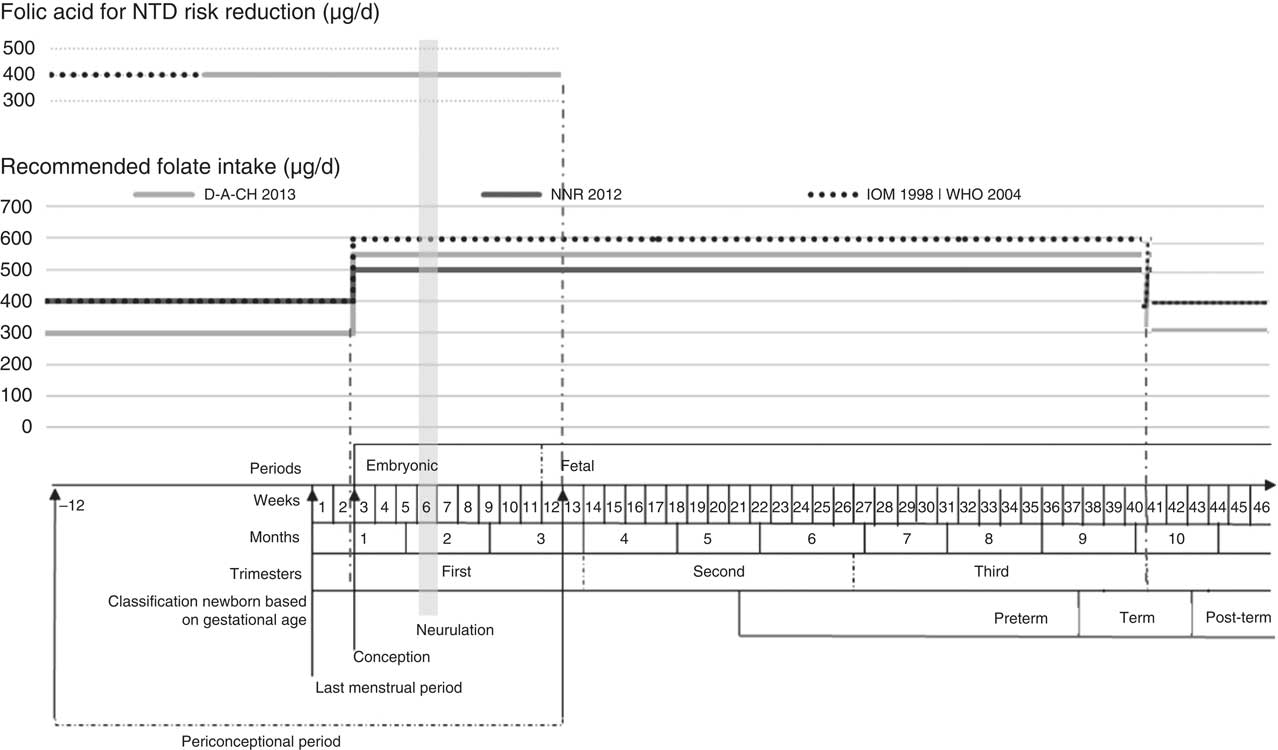
Fig. 2 Recommended folate intake and recommended folic acid supplementation for prevention of NTD during the periconceptional period (NTD, neural tube defects; D-A-CH, Deutschland–Austria–Confoederatio Helvetica (reference values for nutrient intake by the German Nutrition Society, Austrian Nutrition Society, Swiss Society for Nutrition Research and Swiss Society for Nutrition); NNR, Nordic Nutrition Recommendations (by Denmark, Finland, Iceland, Norway and Sweden); IOM, Institute of Medicine, Dietary Reference Intakes; WHO, Vitamin and Mineral Requirements in Human Nutrition ( 42 )). Definition of periconceptional period adapted from Steegers-Theunissen et al.( Reference Steegers-Theunissen, Twigt and Pestinger 44 )
Discussion
The association between adequate maternal folate levels during the periconceptional period and the reduction in congenital anomalies, especially NTD, is well established. However, achieving an adequate folate status in the neurulation period is a challenge worldwide, according to prior studies( 2 , 6 , Reference Steegers-Theunissen, Twigt and Pestinger 44 ). To fill this gap, the WHO and many national health organizations have been developing recommendations related to folic acid supplementation and a folate-rich diet. Some countries have also implemented mandatory folic acid fortification.
In public health it is indispensable to communicate widespread clear and unique messages among health professionals and the general public, once the general public is becoming more interested in a topic, to judge the information provided by health professionals.
The question of folate adequacy seeking NTD prevention is very interesting, because the critical period for that adequacy is the first six gestational weeks( 2 ) when the majority of women do not know they are pregnant, even in planned pregnancies (exceptions would be medically assisted pregnancies). Some authors advocate that, ideally, a correct diet would supply adequate intakes, but this is not the reality for all( Reference Pinto, Barros and dos Santos Silva 17 ). Supplementation could be a solution for this deficiency, but it is not always effective because women are often unaware of their pregnancies during the target period( 15 , Reference Botto, Lisi and Robert-Gnansia 16 ). Food fortification with folic acid appears a better solution, providing adequate intakes. So, recommendations should have a clear message regarding at least two of the three possibilities for an adequate folate intake: diet, fortification and supplements. Mandatory fortification is restricted to countries that opt for this measure. Others may have voluntary fortification and recent EU legislation was created in order to regulate this kind of fortification( 15 ).
Nowadays, the World Wide Web is available for the majority of the population in many countries worldwide. This is an excellent vehicle for giving messages to health professionals and the rest of the population. For this reason, this source of information was inspected. Initially, it was intended to obtain information for forty-six countries, but for ten of them it was impossible to reach any information on official websites. For the other thirty-six countries diverse information was obtained. Notwithstanding that differences should exist among countries regarding different dietary patterns and different disease prevalences, probably there is not a clear rational justification for the disparities observed between countries.
Folate intake recommendations differ between international entities: D-A-CH, NNR, Scientific Committee on Food, IOM( 2 , Reference Botto, Lisi and Robert-Gnansia 16 , 38 , 45 , 46 ) and WHO( 28 , 42 ). A huge amount of work should be done regarding dietary advice. Public health efforts should incorporate practical advice on storage and cooking to increase folate intake, helping to optimize folate status( Reference McKillop, Pentieva and Daly 47 ), as well as recommendations on dietary pattern( Reference Sotres-Alvarez, Siega-Riz and Herring 48 ). A prudent dietary pattern, even in the era of fortification, may decrease the risk of NTD and some heart defects in non-users of folic acid( Reference Sotres-Alvarez, Siega-Riz and Herring 48 ). In addition to folate, other micronutrients may decrease the risk of NTD occurrence( Reference Chandler, Hobbs and Mosley 49 ) and once again promoting a healthy diet has several benefits. Furthermore, the practice of a Western diet by mothers increases the risk of offspring with a cleft lip or cleft palate approximately twofold( Reference Vujkovic, Ocke and van der Spek 50 ). A high preconceptional intake of nutrients predominantly present in fruits and vegetables reduces the risk of offspring affected by orofacial cleft( Reference Krapels, van Rooij and Ocke 51 ). Fast food may increase the risk of NTD( Reference Sumiyoshi 52 ).
With the present study we drew a roadmap of the recommendations about folate and folic acid intake before and during pregnancy in several countries worldwide (developed countries and emerging economies) and we also included WHO recommendations because it is plausible that many other countries consider them. The recommendations differ between countries, although the majority (66·7 %) recommend folic acid supplements of 400 µg/d, in the periconceptional period, combined with a folate-rich diet. A small number of countries emphasize the importance of a healthy diet naturally rich in folate with no need for folic acid supplementation. According to some authors( Reference Sotres-Alvarez, Siega-Riz and Herring 48 ), this advice should be the main in all recommendations for NTD prevention( Reference McKillop, Pentieva and Daly 47 ), namely because a healthy diet also supplies other micronutrients that may decrease the risk of NTD occurrence( Reference Chandler, Hobbs and Mosley 49 ). At the other end of the spectrum, some countries advise supplementation and have mandatory folic acid fortification.
It seems to be consensual that women at high risk of NTD benefit from higher dosages (4–5 mg/d) of folic acid supplementation( 4 , Reference Kondo, Kamihira and Ozawa 26 , Reference Kennedy and Koren 53 , Reference Talaulikar and Arulkumaran 54 ). However, not all cases of NTD are preventable by increasing the folic acid intake( Reference Heseker, Mason and Selhub 55 ). Several other lifestyle and environmental factors as well as genetic variations may have an influence, possibly by affecting one-carbon metabolism and thus epigenetic events( Reference Osterhues, Ali and Michels 27 ). Known risk factors for NTD are genetic (about 60 %( 2 )), chronic alcohol intake( Reference Talaulikar and Arulkumaran 54 ), smoking( Reference Kennedy and Koren 53 ), use of certain drugs (e.g. carbamazepine, phenobarbital, methotrexate)( Reference Kennedy and Koren 53 , Reference Talaulikar and Arulkumaran 54 ), some pathologies (e.g. epilepsy( Reference Talaulikar and Arulkumaran 54 ), malabsorption disorders( Reference Kennedy and Koren 53 , Reference Talaulikar and Arulkumaran 54 ), obesity( Reference Kondo, Kamihira and Ozawa 26 , Reference Kennedy and Koren 53 , Reference Talaulikar and Arulkumaran 54 ), pre-gestational diabetes( Reference Kondo, Kamihira and Ozawa 26 , Reference Kennedy and Koren 53 , Reference Talaulikar and Arulkumaran 54 )) and personal or family history of NTD( Reference Talaulikar and Arulkumaran 54 ).
The current study found that the designations mentioned as the adequate period for supplementation differ between countries. It can be considered that the more adequate are the ones that include the general designation ‘before conception’ and that refer to ‘planning plus chance of becoming pregnant’. The designation ‘reproductive age’ is completely broad and if supplementation is suggested these women could maintain supplementation for large periods of time, unnecessarily.
Apart from recommendations about supplementation, some countries have introduced fortification and others not. Surprisingly, one of the countries with mandatory fortification (Brazil) recommends supplementation with high doses of folic acid (5 mg/d) for all women and not only for those at high risk of NTD. But no evidence sustains the recommendation of such doses for all pregnant women( 15 , 45 ). Moreover, nowadays the scientific community is concerned about potential adverse effects of folic acid fortification and supplementation( 2 , 15 , 28 – Reference Vollset, Clarke and Lewington 32 ) and adverse pregnancy outcomes, early or later in life, both in the mother and child, such as increased birth weight( Reference Charles, Ness and Campbell 11 – Reference Lassi, Salam and Haider 14 ), insulin resistance in children( Reference Yajnik, Deshpande and Jackson 34 ) and asthma in children( Reference Burdge and Lillycrop 35 , Reference Whitrow, Moore and Rumbold 36 ).
Although carcinogenicity induced by high doses of folic acid is not completely established, this issue should continue to be studied, since it constitutes a legitimate public health concern and needs careful monitoring( Reference Kim 56 ). It is known that through exposure to mandatory food fortification and vitamin supplement use, a large number of people have an unprecedented high folic acid intake( Reference Troen, Mitchell and Sorensen 57 ) and that the capacity of the body to convert folic acid to 5-methyltetrahydrofolate is limited( Reference Kelly, McPartlin and Goggins 58 ). An inverse association was also found between the presence of unmetabolized folic acid in plasma and natural killer cell cytotoxicity, but further studies on immune function and health are needed( Reference Troen, Mitchell and Sorensen 57 ). Given the possibility that excessive folic acid exposure may relate to cancer risk, monitoring the long-term effect should be warranted( Reference Kim 56 , Reference Bailey, Mills and Yetley 59 ) especially considering that the vast majority of the population is not at risk of NTD( Reference Bailey, Mills and Yetley 59 ).
In some countries, an effort to personalize intervention was observed. Some delegated to the physician or the health provider the decision for supplementation and/or dosage. In Singapore the importance of folate status, to be achieved by diet, was mentioned.
It is not easy to know the real intake of folic acid as supplements in different countries, because many scientific studies limit this kind of information, providing only the proportion of supplemented women without reference to the dose( Reference Pinto, Barros and dos Santos Silva 17 , Reference Lunet, Rodrigues and Correia 60 ). In order to measure accurately the real impact of such recommendations, systematic health registries are imperative. For instance, regarding congenital anomalies, it would be necessary to compile information regarding miscarriages, medical abortions, stillbirths and neonates with congenital information, information not always easy to aggregate. This is a critical issue in the comparison among countries with different levels of accuracy in their registries.
Strengths and weaknesses
As advantages of the present study we can point out its novelty, considering that all information was searched from official national health websites, a source of information that is widely used and accessible to health professionals and the general public. The previous studies found in the literature included fewer countries and did not analyse data available on the Internet( Reference Botto, Lisi and Robert-Gnansia 16 , 38 ). Most data were officially validated, by email, ensuring a better accuracy. The present study allowed clustering of recommendations to check similarities and disparities within and between countries, and missing information. Countries that do not disseminate clear messages were also identified.
The study is limited by the lack of information about excluded countries, other countries worldwide and by having some countries without official validation, within which we cannot assure that other recommendations are emanated. The methodology underlying each recommendation was not analysed, nor was compliance or the impact of the recommendations in each country.
Public health implications
The role of folate and folic acid during pregnancy being unquestionable, the present study provides a huge amount of structured information about worldwide folate and folic acid recommendations in the periconceptional period and can form a basis for future discussions on these issues. Many questions arise at the end of the study, as follows. What are the reasons behind the different recommendations? Is there any association between recommendations and the epidemiology of NTD in the country? How can we optimize the recommendations, taking into account different sources (healthy diet, fortified foods and supplements)? How can we deal with unplanned pregnancies, other than to recommend supplementation during the entire childbearing age? Are fortified foods really a good solution when the entire population is considered (potential adverse effects)? How to reduce adverse effects in the mother, child and overall population? Is supplementation needed when women have adequate folate status before pregnancy (and considering adverse effects)? Will the future hold a place for personalized recommendations, namely based on blood analyses of folate status (serum and erythrocyte)?
Conclusions
We conclude that the recommendation more frequently used worldwide, from analysed countries and the WHO, about folic acid supplementation in the periconceptional period is 400 µg/d. The recommendation for folate intake is in the range of 300–400 µg/d for women of childbearing age and 500–600 µg/d for pregnant women.
In some countries there are clear indications for the use of higher dosages among women with higher risk of congenital anomalies. The recommendation of a healthy diet, naturally rich in folate, is unanimous. However, big disparities were seen for the recommendations between some countries: some recommend supplementation and a healthy diet plus mandatory fortification; in contrast, some recommend only a healthy diet.
Accurate and ideally evidence-based recommendations are needed regarding folate and folic acid intake during the periconceptional period.
Acknowledgements
Acknowledgements: The authors are grateful to the Ministries of Health of the following twenty-seven countries that validated data available in the websites: Australia, Austria, Belgium, Bulgaria, Canada, Denmark, Estonia, Finland, Germany, Hong Kong, Hungary, Iceland, Ireland, Italy, Liechtenstein, Luxembourg, Malta, Netherlands, Norway, Poland, Portugal, Slovenia, Spain, Sweden, Switzerland, UK and USA. The authors are grateful to Yu-shuo Kuo who helped with research on Asian websites; and to Jessica Sheppard who helped with English revision. Financial support: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors. The views expressed in this article are the authors’ own and do not reflect an official position of any institution. Conflict of interest: None. Authorship: E.P. and S.G. were responsible for designing the study. S.G. was responsible for data collection, the contacts with national entities and the statistical analyses. S.G. wrote the manuscript jointly with E.P. and C.L. All three authors contributed to the interpretation of the findings and approved the contents of this manuscript.






