Fundamental, clinical and epidemiological research has demonstrated the potential role of long-chain (LC) n-3 PUFA from the diet in the prevention of several diseases, in particular CVDReference Kris-Etherton, Harris and Appel(1–Reference Psota, Gebauer and Kris-Etherton6). Fish and other seafood are the richest natural dietary source of these LC n-3 PUFA, in particular EPA and DHA. The knowledge about the beneficial effects of LC n-3 PUFA has led in many countries to the formulation of dietary recommendations to achieve an adequate intake. In Belgium, the country under consideration in the present paper, such recommendations have been in place since 2003(7).
Recent research shows that the current LC n-3 PUFA intake is inadequate in different subgroups of the Belgian population compared with the Belgian recommendation, which is 0·3 % of the total energy intakeReference Sioen, Pynaert, Matthys, De Backer, Van Camp and De Henauw(8–Reference Sioen, Huybrechts, Verbeke, Van Camp and De Henauw10). Similar findings have been reported for other countries, e.g. GermanyReference Bauch, Lindtner, Mensink and Niemann(11), the UKReference Sontrop and Campbell(12) and the USAReference Deckelbaum and Akabas(13, Reference Gebauer, Psota, Harris and Kris-Etherton14). Based on these results, it is reported that a modest increase in LC n-3 PUFA intake would have important and beneficial public health outcomesReference Gebauer, Psota, Harris and Kris-Etherton(14). Increased fish consumption is suggested as a possible strategy to increase LC n-3 PUFA intakes in order to bridge the gap between current intakes and recommendations.
However, at the same time, fish and other seafood are a source of persistent chemical contaminants that accumulate in the marine environment. Non-carcinogenic (e.g. methylmercury (MeHg)) and carcinogenic (e.g. dioxins and polychlorinated biphenyl ethers (PCB)) contaminants accumulate in the marine food chain by bioaccumulation and biomagnificationReference Burreau, Zebuhr, Broman and Ishaq(15). As a result, increased fish consumption aimed to achieve an adequate LC n-3 PUFA intake may simultaneously increase the intake of contaminants to levels of toxicological concern. Chronic exposure to Hg affects the central nervous system(16) and exposure to dioxin-like compounds causes dermal toxicity, immunotoxicity, carcinogenicity, reproductive and developmental toxicity, and disruption of endocrine functions(17). On the other hand, consumers decreasing their fish intake in order to avoid contaminant exposure may be incurring an inadequate intake of LC n-3 PUFAReference Cohen, Bellinger, Connor, Kris-Etherton, Lawrence, Savitz, Shaywitz, Teutsch and Gray(18).
The present study investigated whether the recommended intake of LC n-3 PUFA can be reached by fish consumption only, without exceeding the provisional tolerable weekly intake (PTWI) of MeHg and the tolerable weekly intake (TWI) of dioxin-like compounds. The rationale for focusing on MeHg was that fish is the most important dietary source of Hg in the human food chain. The selection of dioxin-like compounds was motivated by the fact that fish has a higher concentration of dioxin-like compounds than other food items. Since dioxin-like compounds are lipophilic, their concentration in fish is highly related to the fat content of the fishReference Sioen, Van Camp, Verdonck, Van Thuyne, Willems and De Henauw(19). Most of the previously published quantitative analyses of the benefits and risks of fish consumption are limited as they were restricted to salmonReference Foran, Good, Carpenter, Hamilton, Knuth and Schwager(20–Reference Huang, Hites, Foran, Hamilton, Knuth, Schwager and Carpenter22) or restricted to Hg as considered contaminantReference Cohen, Bellinger, Connor, Kris-Etherton, Lawrence, Savitz, Shaywitz, Teutsch and Gray(18, Reference Ponce, Bartell, Wong, LaFlamme, Carrington, Lee, Patrick, Faustman and Bolger23, Reference Levenson and Axelrad24). In the present study, a quantitative assessment was performed to calculate the simultaneous intake of LC n-3 PUFA and multiple contaminants. Moreover, a combination of fish consumption and margarine enriched with LC n-3 PUFA was examined. An analysis of the ensuing health risk was performed and fish consumption recommendations were formulated by balancing the associated risks and benefits to maximize public health.
Materials and methods
The quantitative assessment was performed on the basis of hypothetical scenario analyses. The elaboration and implementation of the different scenarios are presented in Fig. 1. Three consumption scenarios were built starting from the current Belgian fish consumption pattern (based on the seven most consumed species). This consumption pattern was artificially changed in two ways to end up with three consumption scenarios: (i) the current consumption pattern: (ii) increasing the consumption of fatty fish up to 50 % of the total fish consumed; and (iii) replacing all lean fish species by fatty fish species. Next, three sub-scenarios were added per consumption scenario: (i) consuming fish only once a week; (ii) consuming fish twice a week; and (iii) consuming fish three times a week.

Fig. 1 Scheme of the elaboration and implementation of the different scenarios
Nutrient and contaminant data
The nutrient and contaminant concentrations used in the present study originated from two extensive, newly compiled databases containing published data on nutrient and contaminant concentrations in different fish species relevant for Belgian consumptionReference Sioen, Van Camp, Verdonck, Van Thuyne, Willems and De Henauw(19, Reference Sioen, De Henauw, Verdonck, Van Thuyne and Van Camp25). The sum of EPA and DHA concentrations (EPA + DHA; expressed in mg/g fish) was considered as one aggregate nutrient (LC n-3 PUFA). In addition, the following contaminants were included: MeHg (expressed in ng/g fish), dioxin-like PCB (dlPCB; congeners 77, 81, 126, 169, 105, 114, 118, 123, 156, 157, 167, 189; expressed in pg WHO-TEQ/g fish), dioxins plus furans (referred to below as PCDD/F, i.e. the sum of seven polychlorinated dibenzo-p-dioxin (PCDD) congeners and ten polychlorinated dibenzofuran (PCDF) congeners; expressed in pg WHO-TEQ/g fish) and total dioxin-like compounds (referred below to as total TEQ (totTEQ), i.e. the sum of all dioxin-like compounds = 12 dlPCB congeners + 17 PCDD/F congeners; expressed in pg WHO-TEQ/g fish).
Considering the concentrations of dioxin-like compounds in salmon and herring, contaminant concentrations measured in Baltic salmon and herring were excluded from the analyses; they risk having totTEQ concentrations above European Union (EU) limits because the Baltic Sea has been contaminated for many years by dioxin-like compounds from emissions of paper and metal industry plants and waste incineration plantsReference Kiviranta, Vartiainen, Parmanne, Hallikainen and Koistinen(26–Reference Gallani and Boix28). The European Commission set a maximum allowable concentration in edible parts of fish of 4 pg WHO-TEQ/g fresh weight for PCDD/F and 8 pg WHO-TEQ/g fresh weight for totTEQ (except eel may contain up to 12 pg WHO-TEQ/g fresh weight)(29). Only Finland and Sweden had an exemption order until the end of 2006 to place fish from the Baltic region with concentrations above this limit on the domestic market, but they were not allowed to export itReference Kiviranta, Vartiainen, Parmanne, Hallikainen and Koistinen(26, Reference Roots and Zitko27). The presence of Baltic fish on the Belgian market is, therefore, considered negligible.
Table 1 shows the median, the 5th and the 95th percentile of the species-specific ratio of the EPA + DHA concentration to the MeHg or totTEQ concentration: the higher the ratio, the higher the nutrient concentration relative to the contaminant concentration. The data illustrate that for some species the distribution of the ratio is very wide and skewed to the right, e.g. (EPA + DHA):totTEQ for tuna and salmon.
Table 1 Contribution of the seven different fish species (%) to the total fish consumption for the three consumption scenarios, as well as the concentration ratios of (EPA + DHA) to methylmercury (MeHg) or total dioxin-like compounds (totTEQ)

P5, 5th percentile; P95, 95th percentile.
Consumption and body weight data
The current fish species consumption pattern on which the scenarios were built took into account the seven most consumed fish species, determined through the pan-European SEAFOODplus consumer survey(30). Table 1 shows that currently 65 % of the total fish consumption in Belgium is composed of lean fish species (≤5 % fat), with cod as the most important species. Salmon is the most consumed fatty fish (>5 % fat). From this first consumption pattern, two scenarios with an altered fatty/lean species share were constructed to end up with three different consumption patterns: (i) the current consumption pattern; (ii) increasing the contribution of fatty fish (>5 % fat) consumption to 50 % of the total fish consumption; and (iii) replacing all lean fish species (≤5 % fat) by fatty fish. The contribution of the different species to the total fish consumption in the altered patterns was calculated proportionally to their contribution in the current pattern (Table 1).
For the intake assessment, a hypothetical population sample of 600 individuals was used (300 men, 300 women), equally divided over four different age classes (30–39 years; 40–49 years; 50–59 years; 60–69 years). Normal body weight distributions were applied per gender and age interval, based on available data for the Belgian population (BIRNH studyReference Kornitzer and Dramaix(31, Reference De Backer32); Table 2). The number of 600 individuals was sufficient to lead to a good convergence of the intake results (results not shown here).
Table 2 Mean (sd) of the applied body weight distributions (based on representative Belgian data*)
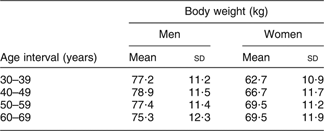
*From references 31 and 32.
Simulation model and probabilistic methodology
The following simulation model, combining species-specific fish consumption data with nutrient and contaminant concentration data, was used for the intake assessment:
where Yi is the average daily intake of individual i per kg body weight (BW); Xv ,i ,t is the amount (g) of fish species v consumed by individual i (with BWi) at day t (t = 1,…,T); and Cv is the concentration of a specific nutrient/contaminant in fish species v. A probabilistic approach was applied for the simulations, taking into account the variability of the consumption, body weight and concentration data. For consumption, the variability was taken into account in a non-parametric way, i.e. by using the data as such. For the body weight and concentration data, the variability was taken into account in a parametric way, i.e. by using species- and compound-specific probability distributions fitted to the available concentration data, using the @Risk software package version 4·5 (Pallisade Corporation, Newfield, NY, USA). Details about this latter procedure have been given previouslyReference Sioen, Van Camp, Verdonck, Van Thuyne, Willems and De Henauw(19, Reference Sioen, De Henauw, Verdonck, Van Thuyne and Van Camp25).
To execute the simulations, a software module called ProbIntakeUG was applied (developed at Ghent University, Belgium). ProbIntakeUG is applicable in the freely available software program R® (R Foundation for Statistical Computing, Vienna, Austria)(33). The simulation procedure in this program for each individual worked as follows: each single consumption data point was multiplied with a concentration data point. This multiplication was conducted for all consumed fish species and for all different compounds. Next, the assessed intakes per compound were enumerated and this sum was divided by the number of consumption days considered and by the individual’s body weight. Finally, this procedure was repeated for all individuals. For the purpose of optimizing integration of the inter-species variability in the nutrient and contaminant concentrations during the intake assessment, it was assumed that consumers kept this consumption pattern for a whole year (52 weeks) to finally calculate the average daily intake over a long-term period.
Evaluation of nutrient and contaminant intakes
To evaluate population intakes of EPA plus DHA, an ad hoc reference value for EPA + DHA of 681 mg/d or 9·7 mg/kg BW per d was calculated starting from the existing Belgian recommendation equal to 0·3 % of the total energy intake(7) and assuming a mean body weight of 70 kg and a mean energy intake of 8·56 MJ/d (2046 kcal/d), the latter based on the data of the most recent Belgian Food Consumption Survey (BFCS; 3245 individuals older than 15 years: 1623 women, 1622 men)(34). Dividing the EPA + DHA reference value by the body weight was relevant in this study in order to express the reference values for nutrients and contaminants on the same scale. For MeHg, a PTWI of 1·6 μg/kg BW per week (0·228 μg/kg BW per d) is proposed(16) and for dioxin-like compounds, the EU proposes 14 pg WHO-TEQ/kg BW per week (2 pg WHO-TEQ/kg BW per d)Reference Gallani and Boix(28).
To visualize the results, plots were created showing the intake of MeHg and totTEQ respectively, divided by their (P)TWI (expressed per day, i.e. tolerable daily intake (TDI)), in relation to the intake of EPA + DHA divided by the reference value (9·7 mg/kg BW per d). Consequently, the limit value for being at risk due to a too high contaminant intake or inadequate EPA + DHA intake is ‘1’ on both axes. Extra reference lines were added on the plots: (i) at half of the TDI for totTEQ, to take into account that the human diet contains other sources of dioxin-like compounds; and (ii) at half of the reference value for EPA + DHA, since the Belgian recommendation for EPA + DHA is high compared with other countries (see Discussion below). By adding these reference lines, different zones are obtained describing whether or not a sufficient amount of fish is consumed to meet the recommendation for EPA + DHA, with or without exceeding the contaminant TDI.
Inclusion of long-chain n-3 PUFA-enriched margarine
Currently, margarine enriched with EPA and DHA is commonly available on the Belgian market and is therefore also considered in the present study. The EPA and DHA concentration in enriched margarine varies a lot depending on the brand, but it varies also in time. The concentrations used here are obtained from the nutritional information mentioned on the product labels. A first brand available in Belgium claimed that their EPA + DHA-enriched margarine contains 5 mg EPA + DHA/g margarine. A second brand indicated that the enriched margarine contains 7·5 mg EPA + DHA/g. A third manufacturer stated that its enriched variant of margarine contains 0·9 mg DHA/g. Belgian dietitians assessed that one slice of bread with a regular layer of margarine contains 5 g margarine(35). Assuming a daily consumption of 4 to 7 slices of bread leads to a consumption of 20–35 g margarine daily and 100–262·5 mg EPA + DHA daily (using the two versions of margarine richest in EPA + DHA). The results of the most recent BFCS(34) indicated that currently the mean daily consumption of culinary fats and margarines is 21·2 g with an interquartile range of 6·0–28·6 g. In the scenario analyses executed, it was assumed that all consumers would use the average daily amount of enriched margarine containing 7·5 mg EPA + DHA/g margarine.
Results
Fish as only source of EPA and DHA
Table 3 and Figs 2 and 3 show the intake assessment results for the different scenarios and sub-scenarios. The results indicate that increasing the contribution of fatty fish will reduce the intake of MeHg. This could already be concluded based on comparison of the ratio (EPA + DHA):MeHg between lean and fatty fish species (Table 1). In contrast to MeHg, the intake of totTEQ increases when replacing lean by fatty fishes. This was expected given the lipophilic character of these contaminants. Simultaneously, increasing the contribution of fatty fish increases the intake of EPA + DHA. Some lean species also have a relative high (EPA + DHA): totTEQ ratio compared with other species, e.g. cod and pollock (Table 1), but the absolute concentration of EPA + DHA in these species is so low that an unrealistically large amount of these species would have to be eaten to achieve the recommended EPA + DHA intake.
Table 3 Mean intake of different compounds for the three different fish consumption patterns and three different scenarios of consumption frequency

MeHg, methylmercury; iPCB, seven indicator polychlorinated biphenyl ethers; dlPCB, dioxin-like PCB; PCDD/F, dioxins plus furans; totTEQ, total dioxin-like compounds; BW, body weight.

Fig. 2 Methylmercury (MeHg) intake divided by the tolerable daily intake (TDI; 228 ng/kg body weight (BW) per d) in relation to the intake of EPA plus DHA divided by the recommendation (9·7 mg/kg BW per d) for three different fish consumption patterns (+, current consumption pattern; ▵, 50 % lean and 50 % fatty fish; □, only fatty fish) and three different scenarios of consumption frequency (one, two or three times a portion of 150 g fish per week); note logarithmic scales

Fig. 3 Intake of total dioxin-like compounds (totTEQ) divided by the tolerable daily intake (TDI; 2 pg WHO-TEQ/kg body weight (BW) per d) in relation to the intake of EPA plus DHA divided by the recommendation (9·7 mg/kg BW per d) for three different fish consumption patterns (○, current consumption pattern; ▵, 50 % lean and 50 % fatty fish; □, only fatty fish) and three different scenarios of consumption frequency (one, two or three times a portion of 150 g fish per week); note logarithmic scales
Figures 2 and 3 provide scatter plots of EPA + DHA v. MeHg (Fig. 2) or totTEQ (Fig. 3) based on the results of the different consumption scenarios. Considering the EPA + DHA intake, the results show that only a fish consumption pattern consisting of 50 % lean fish and 50 % fatty fish with a minimum consumption frequency of three times a week, or a fish consumption pattern consisting only of fatty species with a frequency of minimum twice a week, will lead to an adequate intake of EPA + DHA in respectively 48·0 % and 92·5 % of the population when using the Belgian recommendation and not taking into account other sources of these fatty acids. Figure 2 shows that none of the considered consumption scenarios will lead to the health-based guidance value for MeHg being exceeded, indicating that the Hg contamination of fish available on the Belgian market is not an issue of major concern. In contrast, Fig. 3 shows that when consuming a portion of fatty fish three times a week, the intake of dioxin-like compounds will approach the TDI and a certain proportion of the population (8·5 %) will exceed this value. Knowing that the human diet also contains other important sources of totTEQ, an intake of three portions fatty fish per week may be of toxicological concern. Therefore, consuming fatty fish more than twice a week is not recommended.
Enriched margarine as extra dietary source of EPA and DHA
Assuming that all consumers use 21·2 g enriched margarine containing 7·5 mg EPA + DHA/g margarine daily will lead to a mean daily intake of 159 mg EPA + DHA, being 23·3 % of the Belgian recommendation (681 mg/d). In Fig. 4, scatter plots are shown for the different fish consumption scenarios with and without adding enriched margarine as a source of LC n-3 PUFA, neglecting the contribution of margarine consumption to the intake of contaminants since no recent contamination data for margarine were available. Consuming enriched margarine will help to increase the EPA + DHA intake. Nevertheless, the contribution is rather limited and margarine as the only source of LC n-3 PUFA would not be sufficient to reach the recommendation. A consumption scenario of 150 g lean fish and 150 g fatty fish per week combined with a daily consumption of LC n-3 PUFA-enriched margarine leads to an EPA + DHA intake close to the recommendation with a mean totTEQ intake below half of the TDI.

Fig. 4 Intake of total dioxin-like compounds (totTEQ) divided by the tolerable daily intake (TDI; 2 pg WHO-TEQ/kg body weight (BW) per d) in relation to the intake of EPA plus DHA divided by the recommendation (rec; 9·7 mg/kg BW per d) for three different fish consumption patterns (current consumption pattern, 50 % lean and 50 % fatty fish, only fatty fish) and three different scenarios of consumption frequency (one, two or three times a portion of 150 g fish per week), with (▵) and without (○) taking long-chain n-3 PUFA-enriched margarine into account; note logarithmic scales
Discussion
The present results showed that the Belgian recommendation for EPA plus DHA can be reached through regular fish consumption, more specifically: (i) a combination of lean and fatty fish (on average 50 %) a minimum of three times a week; or (ii) fatty fish a minimum of twice a week. A consumption of fatty fish three times a week, however, leads to an intake of totTEQ close to the health-based guidance value, which is of potential toxicological concern because other food items, mainly of animal origin, also contribute to the daily totTEQ intake. Recent research assessed totTEQ intake via the total diet in three age groups on the basis of data from the Flemish Environment and Health study. The median estimated intakes were 2·24, 2·09 and 1·74 pg TEQ/kg BW per d in respectively adolescents (14–15 years), mothers (18–44 years) and adults (50–65 years). It was found that seafood was the most important contributor, accounting for 25·0, 29·4 and 43·3 % in the group of adolescents, mothers and adults, respectively. The other main contributors were, in order of importance, added fats, dairy products, and meat and meat products(36). MeHg contamination does not seem to be an issue of toxicological concern, even in scenarios with elevated fish consumption frequencies. Hence, the consumption limits for fish determined in the present study are driven by the presence of dioxin-like contaminants, which was also concluded by Foran et al.Reference Foran, Good, Carpenter, Hamilton, Knuth and Schwager(20) when performing an analysis of the risks and benefits related to salmon consumption.
Belgian adults currently do not consume seafood regularly(30). The results of the most recent BFCS(34) indicated a mean daily intake of 24 g seafood. Almost 70 % of the population consumed less than 210 g seafood/week (30 g/d). Many obstacles at the consumer level exist to prevent people form consuming fish twice a week. Low perceived convenience, high price perception, and low liking of fish taste act as major barriers to increasing the consumption of fish in BelgiumReference Olsen, Scholderer, Brunsø and Verbeke(37). Therefore, it was worth investigating the role of EPA + DHA-enriched food items. The results showed that regular fish consumption (twice a week), including fatty fish species, in combination with regular consumption of EPA + DHA-enriched margarine can be advised to safely increase the LC n-3 PUFA intake.
Apart from margarines, LC n-3 PUFA-enriched eggs are available on the Belgian market. A first brand stated that an enriched egg contains 110 mg EPA + DHA. The second reported a concentration of 125 mg DHA/egg. The mean weight of a normal egg is assumed to be 60 g(35). On the basis of the most recent BFCS(34), it is known that Belgian adults consume on average 10·0 g egg/d, i.e. one egg a week(34). Assuming that all eggs consumers would eat are EPA + DHA-enriched eggs (110 mg EPA + DHA/egg), this would lead to an average daily intake of 18·3 mg EPA + DHA, being 2·7 % of the recommendation. To reach the recommendation of 681 mg EPA + DHA/d, consumers should eat six eggs a day, increasing the cholesterol intake to 1483·2 mg/d (412 mg cholesterol/egg), whereas the Belgian recommendation states to reduce cholesterol intake to a maximum of 300 mg/d(7). This indicates that the contribution of EPA + DHA-enriched eggs to the total intake is low, due to the rather low concentration of EPA + DHA in eggs and their limited consumption. Enriched eggs can help to increase the LC n-3 PUFA intake, but they cannot be advised as the only or major source to achieve the EPA plus DHA recommendation. Nevertheless, we must admit that the use of eggs in prepared food items such as cakes and pastries are not taken into consideration in this calculation, which leads to an underestimation.
According to literature, another option to increase the intake of EPA + DHA is through supplementation with DHA-rich micro-algae or fish oil. The use of such supplements as an alternative for fish will have disadvantages owing to the lack of other nutrients like protein and vitamin D, and minerals such as I and Se, that are abundantly present in fish. Moreover, fish is low in saturated fat and cholesterol and, therefore, regular replacement of meat and meat products by fish can help to reduce the intake of saturated fatReference Sioen, Pynaert, Matthys, De Backer, Van Camp and De Henauw(8, 9). Besides supplements, efforts are being made to enrich the EPA + DHA concentration of food items produced from terrestrial animals through adapted animal feeds and to develop a new generation of genetically modified plants with a modified fatty acid profileReference Gebauer, Psota, Harris and Kris-Etherton(14, Reference De Henauw, Van Camp, Sturtewagen, Matthys, Bilau, Warnants, Raes, Van Oeckel and De Smet38, Reference Kinney39). Nevertheless, the availability of these food items containing EPA and DHA is still limited and their potential to increase the LC n-3 PUFA intake is still debated and most likely not sufficientReference De Henauw, Van Camp, Sturtewagen, Matthys, Bilau, Warnants, Raes, Van Oeckel and De Smet(38).
It is of interest to note that the EPA and DHA recommendation as formulated by the Belgian Health Council(7) (0·3 % of the total energy intake, estimated to be equal to 681 mg/d) seems to be high compared with other international recommendations. In France, the recommendation for EPA and DHA is 0·2 % of the total energy intake, with a minimum of 0·05 % contributed by DHAReference Legrand, Bourre, Descomps, Durand and Renaud(40), estimated to be equal to 500 mg/d for French men and 400 mg/d for French women. In Germany, a daily intake of 350 mg LC n-3 PUFA is recommendedReference Bauch, Lindtner, Mensink and Niemann(11). In the UK, LC n-3 PUFA intake of minimal 450 mg/d is recommended(41). In the USA, the American Heart Association (AHA) formulated a dietary recommendation of 500 mg EPA + DHA daily for CVD risk reduction. For patients with documented CHD, the AHA recommends 1 g EPA + DHA/dReference Gebauer, Psota, Harris and Kris-Etherton(14, Reference Kris-Etherton, Harris and Appel42). Application of such a lower recommendation (e.g. the French recommendation) for EPA + DHA would lead to the conclusion that (i) consumption of fish twice a week, varying between lean and fatty species (Figs 2 and 3), and (ii) combination of fish once a week with regular use of LC n-3 PUFA-enriched margarine (Fig. 4), would be sufficient to reach the EPA + DHA intake recommendation.
With regard to the risk–benefit analysis executed in the present study, it should be emphasized that the cut-offs used for the evaluation of human health benefits and risks were determined taking into consideration different endpoints. Nevertheless, we attempted to describe the situation as accurately as possible by a simultaneous intake assessment of nutrients and contaminants. At this moment, no common metric exists to evaluate the benefits as well as the risks in one single step. Attempts have been undertaken to combine both assessments in terms of quality- or disability-adjusted life years (QALY or DALY)Reference Cohen, Bellinger, Connor, Kris-Etherton, Lawrence, Savitz, Shaywitz, Teutsch and Gray(18, Reference Ponce, Bartell, Wong, LaFlamme, Carrington, Lee, Patrick, Faustman and Bolger23, Reference van Kreijl and Knaap43), but many uncertainties remain to be solved before a broad application of this procedure becomes possible. The largest uncertainties are associated with the dose–response relationshipsReference Cohen, Bellinger, Connor, Kris-Etherton, Lawrence, Savitz, Shaywitz, Teutsch and Gray(18). Moreover, the QALY investigations related to fish consumption did not take into account dioxin-like contaminants, but focused on MeHg onlyReference Cohen, Bellinger, Connor, Kris-Etherton, Lawrence, Savitz, Shaywitz, Teutsch and Gray(18, Reference Ponce, Bartell, Wong, LaFlamme, Carrington, Lee, Patrick, Faustman and Bolger23).
The present study focused on the contaminants for which abundant concentration data are publicly available. Of course, other contaminants are also present in fish, e.g. As, Pb and polybrominated diphenyl ethers. The rationale for focusing on MeHg was that fish is the most important dietary source of Hg in the human food chain. In the marine environment, inorganic Hg is to a high extent transformed to MeHg, which further accumulates in the marine food chain and is very toxic to manReference Plessi, Bertelli and Monzani(44, Reference Storelli, Giacominelli-Stuffler, Storelli and Marcotrigiano45). The selection of dioxin-like compounds was motivated by the fact that fish has a higher concentration of dioxin-like compounds than other food items. Studies from Belgium and other European countries indicated fish as an important dietary source of dioxin-like compoundsReference Vrijens, De Henauw, Dewettinck, Talloen, Goeyens, De Backer and Willems(46–Reference Kiviranta, Ovaskainen and Vartiainen51).
In conclusion, the present study showed that the Belgian EPA plus DHA recommendation can be reached through regular consumption of fish, more specifically through a combination of lean and fatty fish (on average 50 % of each) a minimum of three times a week or through consuming fatty fish twice a week. Consuming fatty fish more than twice a week, however, leads to totTEQ intake close to the TWI, which is of potential toxicological concern. In contrast, MeHg contamination does not seem to be an issue of toxicological concern in Belgium, even for heavy fish consumers. On the basis of these conclusions, clear dietary advice about fish consumption can be given to the Belgian population, in order to increase their LC n-3 PUFA intake without raising major toxicological concerns.
Acknowledgements
None of the authors had any conflict of interest. The Belgian Science Policy (SPSDII-project CP/02/56) and the Institute for the Promotion of Innovation through Science and Technology in Flanders (IWT-Vlaanderen, Brussels, Belgium) are acknowledged for financial support. S.D.H., J.V.C. and J.W. wrote the project proposal of the study. I.S. conducted the scenario analyses and wrote the article. F.V. provided statistical advice and helped in the interpretation of the results. All authors helped in interpreting the results and writing the manuscript. Nicky Van Thuyne and Peter Vanrolleghem (BIOMATH, Ghent University, Belgium) are acknowledged for support in the development of ProbIntakeUG.









