Gestational diabetes mellitus (GDM) is defined as any degree of glucose intolerance with onset or first recognition during pregnancy( 1 ). GDM is associated with an increased risk of perinatal complications( Reference Yogev, Xenakis and Langer 2 – Reference Marchetti, Carrozzino and Fraticelli 4 ) and in the long term, women with GDM( Reference Kim, Newton and Knopp 5 ) and their offspring are at elevated risk of developing obesity and type 2 diabetes mellitus( Reference Feig, Corcoy and Jensen 6 ). Given the rise in GDM incidence over the past decades in China( Reference Zhang, Dong and Zhang 7 , Reference Zhu, Fan and Yang 8 ), preventing GDM has become a public health priority.
Previous studies have reported that excessive gestational weight gain (GWG) may result in maternal fat deposition, which may decrease insulin sensitivity. It is biologically plausible that GWG is positively associated with GDM( 9 ). Previously, most related studies have focused on the association between total GWG and GDM, but the result could not be factually reflected due to clinical intervention after GDM diagnosis, and even reverse causation might be obtained( Reference Nohr, Vaeth and Baker 10 ). Recently, studies have examined the association between GWG prior to the GDM screening test and the risk of GDM, but the conclusions are not consistent. Several case–control studies have shown that GWG prior to the GDM screening test, especially gestational weight gain in the first trimester (GWG-F), was associated with the risk of GDM, while an association between gestational weight gain in the second trimester (GWG-S) and GDM was not observed( Reference Morisset, Tchernof and Dube 11 – Reference Brunner, Stecher and Ziebarth 13 ). One cohort study found that greater early GWG was associated with increased risk of GDM and elevated weight gain in mid-pregnancy increased the risk of GDM only among pregnant women with greater weight gain in the first trimester( Reference Liu, Ao and Yang 14 ). Several studies have also examined the association between GWG prior to the GDM screening test and the risk of GDM in women with different pre-pregnancy BMI, but the reported data were controversial. One study found that the rate of gestational weight gain in the first trimester (RGWG-F) was significantly associated with GDM in the normal-weight group, but not in the underweight or obese group( Reference Cho, Hur and Lee 15 ). Another study showed that GWG is a significant risk factor for GDM in overweight or obese women but not in women who were underweight or had a normal pre-pregnancy BMI( Reference Gibson, Waters and Catalano 16 ). At present, it remains unclear whether trimester-specific GWG prior to glucose screening is associated with an elevated risk of GDM in women with different pre-pregnancy BMI.
GWG guidelines were published by the US Institute of Medicine (IOM) in 1990( 17 ) and revised in 2009( 9 ). However, they may be of limited use in Asian populations because they are largely based upon studies of Caucasian women. Furthermore, the WHO international BMI cut-off points defining pre-pregnancy BMI categories in the IOM guidelines are not directly applicable to Asians, especially the Chinese. Most importantly, the IOM does not take the pregnancy outcome of GDM into consideration. A study to define optimal GWG ranges for the avoidance of GDM was needed for Chinese women.
Methods
Study sample and design
Participants for the present analysis were derived from a population-based prospective study conducted in Sichuan Provincial Hospital for Women and Children, Southwest China, which was designed to investigate the effects of GWG and serum lipids on GDM. All participants provided written informed consent when recruited to the cohort.
Women who had their first prenatal visit during the first trimester were invited to join this cohort in 2017. Initially, 2211 pregnant women with weight measurement at 12 ± 2 weeks of gestation were recruited for the study. Of all these participants, 178 were excluded for induced labour (n 143), multiple births (n 32) and diabetes diagnosed before pregnancy (n 3). Participants who had missing data on maternal height or pre-pregnancy weight (n 2) or who did not have information about the result of the oral glucose tolerance test (OGTT; n 121) were also excluded. Therefore, 1910 pregnant women (86·4 % of the eligible sample) were eventually obtained for the statistical analyses.
Data collection and anthropometric measurements
All participants completed a questionnaire about maternal demographics, weeks of gestation, parity, family history of diabetes, pre-pregnancy drinking and smoking habits, physical activity, psychological state, sleep quality during early pregnancy and dietary intake in the last 3 d. The questionnaire was given by trained staff in face-to-face interviews when participants enrolled. Physical activity, sleep quality, dietary intake and weeks of gestation in the second trimester were also recorded when participants had their prenatal care visit at 24–28 weeks of gestation. The 75-g OGTT was conducted at 24–28 weeks of gestation. The OGTT result was extracted from the electronic medical record system maintained by the hospital.
Maternal pre-pregnancy weight was based on self-reporting. High correlations were found between self-reported and measured pre-pregnancy weight( Reference Headen, Cohen and Mujahid 18 ). Height in metres was measured on standardized height-measuring stations at the first prenatal visit. Weight in kilograms was measured on standardized digital weighing scales by trained staff at the first prenatal visit and at 24–28 weeks of gestation.
Gestational weight gain
GWG-F was calculated by subtracting pre-pregnancy weight from maternal weight at 12 ± 2 weeks of gestation. The rate of gestational weight gain in the second trimester (RGWG-S) was calculated as follows: RGWG-S = (measured weight at 24–28 weeks − measured weight at 12 ± 2 weeks)/weeks between the two measurements. GWG-F and RGWG-S were classified by the cut-offs of the IOM recommendations( 9 ) into three groups: below, within and above the IOM recommendations. In recent years, most hospitals have used the BMI cut-off points in China when applying IOM GWG recommendations to Chinese women. Therefore, the BMI cut-off points in China were adopted to determine pre-pregnancy weight status when applying IOM GWG recommendations to classify participants into the three groups of below, within and above the IOM recommendations. For instance, in terms of GWG-F, the IOM recommends the same range regardless of pre-pregnancy BMI. Therefore, our study classified pregnant women as below when GWG-F was less than 0·5 kg, within when GWG-F was between 0·5 and 2 kg, and above when GWG-F was more than 2 kg.
Diagnostic criteria of gestational diabetes mellitus
At 24–28 weeks of gestation, the 75-g OGTT was conducted. GDM was diagnosed according to the standards issued by the Ministry of Health of China in 2011, which were based on the International Association of Diabetic Pregnancy Study Group guidelines( Reference Yang, Sun and Cu 19 ). Briefly, GDM was defined as having one or more abnormal values from a 75-g, 2-h OGTT between 24 and 28 weeks of gestation, with cut-off points of 5·1 mmol/l (92 mg/dl) for fasting, 10·0 mmol/l (180 mg/dl) for 1-h post-load and 8·5 mmol/l (153 mg/dl) for 2-h post-load glucose. In China, women diagnosed with GDM receive both diet and physical activity guidelines at the hospital, which was the basis of selecting women for the groups with GWG in the first and second trimesters prior to the diagnosis of GDM.
Additional information
On the basis of self-reported pre-pregnancy weight and height measured at the first prenatal visit, pre-pregnancy BMI was calculated as self-reported pre-pregnancy weight in kilograms divided by squared height in metres and was categorized as underweight (BMI < 18·5 kg/m2), normal-weight (18·5 ≤ BMI < 24·0 kg/m2), overweight (24·0 ≤ BMI < 28·0 kg/m2) or obese (BMI ≥ 28·0 kg/m2) according to the Chinese criteria( Reference Zhou 20 ). As a result of the descriptive similarities and small numbers, overweight and obese women were combined in the analysis.
Gestational age was calculated based on the earliest ultrasonogram obtained. Educational level was classified as ≤12, 13–15 and ≥16 years according to the number of completed schooling years. Drinking before 6 months of pregnancy was considered positive when the woman had drunk alcohol before 6 months of pregnancy. Exposure to a second-hand smoking environment frequently before 6 months of pregnancy was defined as staying in a second-hand smoking environment more than three times per week before 6 months of pregnancy. Regular exercise before pregnancy referred to exercise more than three times per week before pregnancy.
Statistical analysis
Continuous variables are presented as means and standard deviations. Categorical variables are expressed as frequencies and percentages. The χ 2 test was performed to analyse the relationship between variables. Participants were categorized into three groups (below, within and above the IOM recommendations) on account of the GWG-F and RGWG-S status. Multivariable logistic regression was used to assess the effects of GWG-F and RGWG-S on GDM stratified by pre-pregnancy BMI, adjusting for the following confounding variables: maternal age (<25, 25–29 and 30–34 years, ≥35 years as reference), educational level (≤12 and 13–15 years, ≥16 years as reference), parity (primiparous or multiparous), employment (yes or no), family history of diabetes (yes or no), family history of hypertension (yes or no), family history of thyroid diseases (yes or no), average personal income per month (<3000, 3000–4999 and 5000–9999 Yuan, ≥10 000 Yuan as reference), regular exercise before pregnancy (yes or no), frequent exposure to a second-hand smoking environment (yes or no) and drinking before 6 months of pregnancy (yes or no). Studies have found that these variables, including regular exercise before pregnancy, family history of thyroid diseases, average personal income per month, frequent exposure to a second-hand smoking environment and drinking before pregnancy, were related to GDM( Reference Ouyang, Shen and Jiang 21 – Reference Zhao, Che and Jia 25 ), so our study adjusted for them in the final models. Women whose GWG-F and RGWG-S were within the IOM recommendations were used as the reference group. All confidence intervals were estimated at the 95 % level and P < 0·05 was considered statistically significant.
To explore the optimal GWG ranges for the avoidance of GDM for Chinese women, the prevalence of GDM was calculated when GWG increased by a certain unit. The present study defined the optimal GWG ranges stratified by China-specific BMI categories( Reference Zhou 20 ) as the corresponding GWG ranges when the prevalence of GDM was lowest. As a result of the descriptive similarities and small numbers, overweight and obese women were also combined in this analysis. All data were analysed using the statistical software package IBM SPSS Statistics version 21.0.
Results
Among the 1910 participants in the study, 620 (32·5 %) were diagnosed with GDM. The mean maternal age was 28·7 (sd 4·0) years and most of the women were primiparous (75·9 %). Compared with women in the non-GDM group, women with GDM were more likely to be older, to have a higher pre-pregnancy BMI and to have a family history of diabetes or hypertension (P < 0·05). A higher proportion of unemployment was observed among women with GDM (P < 0·05). The mean GWG-F and RGWG-S were 0·7 (sd 2·5) kg and 0·5 (sd 0·2) kg/week, respectively. GWG-F was significantly higher in the GDM group, but RGWG-S was lower than the non-GDM group (Table 1).
Table 1 Characteristics of the study sample of pregnant women (n 1910) from Southwest China, 2017

GDM, gestational diabetes mellitus; GWG-F, gestational weight gain in the first trimester; RGWG-S, rate of gestational weight gain in the second trimester.
* The data are presented as mean and sd for continuous variables or as n and % for categorical variables.
† Tests for differences between GDM and non-GDM were performed using the Wilcoxon two-sample test for non-normally distributed continuous variables or the χ 2 test for categorical variables; P < 0·05 indicates significance.
Table 2 shows the results of a multivariable logistic regression analysis for the effects of GWG-F and RGWG-S on GDM stratified by pre-pregnancy BMI. After adjusting for maternal age, educational level, parity, employment, family history of diabetes, family history of hypertension, family history of thyroid diseases, average personal income per month, regular exercise before pregnancy, frequent exposure to a second-hand smoking environment and drinking before 6 months of pregnancy, our study found that GWG-F above the IOM recommendations increased the risk of GDM among underweight (OR = 2·500; 95 % CI 1·106, 5·655), normal-weight (OR = 1·396; 95 % CI 1·023, 1·906) and overweight/obese women (OR = 3·017; 95 % CI 1·118, 8·138) compared with women within IOM recommendations. No significant difference was observed between RGWG-S and GDM (P > 0·05) in different pre-pregnancy BMI groups after adjusting for GWG-F based on the previous model.
Table 2 The effects of gestational weight gain in the first trimester (GWG-F) and rate of gestational weight gain in the second trimester (RGWG-S) on gestational diabetes mellitus (GDM), stratified by pre-pregnancy BMI, in the study sample of pregnant women (n 1910) from Southwest China, 2017
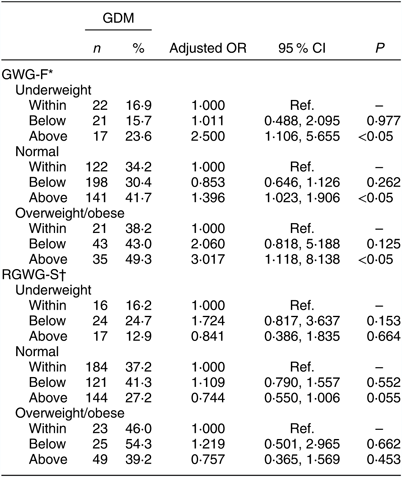
Ref., reference category.
* Adjusted for maternal age, educational level, parity, employment, family history of diabetes, family history of hypertension, family history of thyroid diseases, average personal income per month, regular exercise before pregnancy, frequent exposure to a second-hand smoking environment before 6 months of pregnancy, and drinking before 6 months of pregnancy.
† Adjusted for GWG-F based on the previous model.
The prevalence of GDM in different pre-pregnancy BMI groups when GWG-F increased by a certain level was calculated. To ensure a quantitative balance between groups, the interval unit for underweight and normal-weight groups was determined to be 0·4 kg. The interval unit for the overweight/obese group was 0·35 kg because the total GWG-F was less than in the other two groups. The optimal GWG-F ranges stratified by China-specific BMI categories were set as the corresponding ranges when the prevalence of GDM was lowest. The lowest prevalence of GDM occurred in women who gained 0·8–1·2, 0·8–1·2 and 0·35–0·7 kg in the first trimester among underweight, normal-weight and overweight/obese women, respectively (Table 3). The optimal GWG-F for the avoidance of GDM was 0·8–1·2 kg in both underweight and normal-weight women, while overweight/obese women had the optimal GWG-F range of 0·35–0·7 kg. The optimal ranges along with corresponding IOM recommendations for different pre-pregnancy BMI groups are given in Table 4. The optimal GWG-F ranges in our study were within IOM recommendations, while the upper limit for each pre-pregnancy BMI group was lower than that in the IOM recommendations.
Table 3 The prevalence of certain levels of gestational weight gain in the first trimester (GWG-F) according to pre-pregnancy BMI among the study sample of pregnant women (n 1910) from Southwest China, 2017
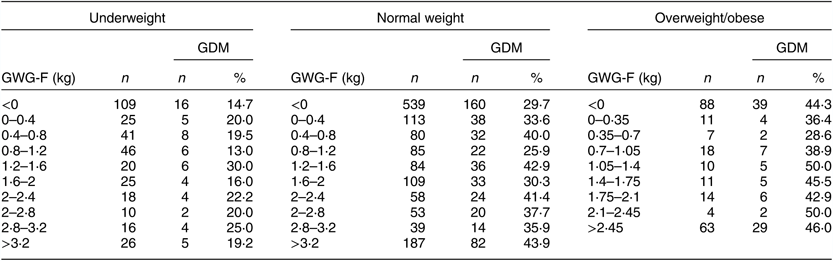
GDM, gestational diabetes mellitus.
Table 4 Optimal ranges of gestational weight gain in the first trimester (GWG-F) for the avoidance of gestational diabetes mellitus in the study sample of pregnant women (n 1910) from Southwest China, 2017
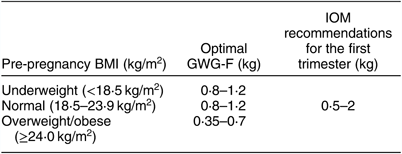
IOM, Institute of Medicine.
Discussion
The present study is the first to investigate the optimal GWG ranges for the avoidance of GDM stratified by China-specific BMI categories. Our study found that excessive GWG-F in women with different pre-pregnancy BMI, rather than RGWG-S, was associated with an increased risk of GDM. The optimal GWG-F ranges for the avoidance of GDM were 0·8–1·2 kg in both underweight and normal-weight women, while overweight/obese women had the optimal GWG-F range of 0·35–0·7 kg. The IOM recommends the same range of weight gain in the first trimester regardless of pre-pregnancy BMI. This is the greatest difference between our study and the IOM recommendations. The optimal GWG-F ranges in our study were within IOM recommendations, while the upper limit for each pre-pregnancy BMI group was lower than that in the IOM recommendations.
Our finding that excessive GWG-F increased the risk of GDM in women with different pre-pregnancy BMI is consistent with several studies( Reference Hedderson, Gunderson and Ferrara 12 , Reference Dong, Yu and Wei 26 – Reference Zhong, Li and Chen 28 ). In a prospective cohort study, Dong et al. found that excessive weight gain in the first trimester was significantly related to a higher risk of developing GDM, with an OR of 2·655 (95 % CI 1·265, 5·571)( Reference Dong, Yu and Wei 26 ). Hedderson et al. found that greater rate of GWG, especially in the first trimester, increased the risk of GDM after adjusting for potential confounders( Reference Hedderson, Gunderson and Ferrara 12 ). Zhong et al. demonstrated that greater early pregnancy weight gain was associated with increased risk of GDM( Reference Zhong, Li and Chen 28 ). Carreno et al. reported that only normal-weight women with excessive early GWG had a higher increase in the risk of developing GDM. Similar results were not found in underweight and overweight or obese women( Reference Carreno, Clifton and Hauth 29 ). However, Saldana et al. have suggested that obese women may already be at a high insulin resistance level or have β cells closer to a point of exhaustion at the start of their pregnancy, thus limiting the potential effect on glucose intolerance of additional weight gain during pregnancy( Reference Saldana, Siega-Riz and Adair 30 ). Gibson et al. found that GWG before GDM diagnosis was a significant risk factor for GDM in overweight or obese women but not in women who were underweight or had a normal BMI before pregnancy( Reference Gibson, Waters and Catalano 16 ). Excessive GWG-F may result in an increase in maternal fat mass, which decreases insulin sensitivity and increases insulin resistance, leading to the development of GDM. However, Herring et al. found that higher weight gain prior to GDM diagnosis conferred an increased risk of impaired glucose tolerance but not GDM when they examined the association between GWG before GDM diagnosis and maternal hyperglycaemia( Reference Herring, Oken and Rifas-Shiman 31 ).
Because the weight gain composition during gestation (fat and fetal mass proportions) and the maternal metabolism changes vary by trimester( Reference Hedderson, Gunderson and Ferrara 12 , Reference Van Raaij, Peek and Vermaat-Miedema 32 ), it seems that the timing of excessive weight gain during the different trimesters of pregnancy has different effects on GDM. In the current study, we found that RGWG-S was not associated with GDM in any pre-pregnancy BMI group, similar to a recent study. Those authors examined the relationship between weight gain trajectories in the first and second trimesters of pregnancy and GDM among 806 women with GDM and 4819 randomly sampled women without GDM in a case–cohort study, concluding that the gain trajectory of the second trimester was not associated with GDM (OR = 1·1; 95 % CI 0·9, 1·3)( Reference Macdonald, Bodnar and Himes 33 ). Morisset et al. found that the rate of GWG until GDM diagnosis was a risk factor for GDM. However, only first-trimester weight gain was an independent predictor of GDM. In fact, second-trimester weight gain was not associated with GDM risk( Reference Morisset, Tchernof and Dube 11 ). Liu et al. demonstrated that elevated weight gain in mid-pregnancy increased the risk of GDM only among pregnant women with greater weight gain in the first trimester( Reference Liu, Ao and Yang 14 ). However, Hantoushzadeh et al. explored the effects of changes in indicators of adiposity and found that gains in gestational weight increased the risk of GDM( Reference Hantoushzadeh, Sheikh and Bosaghzadeh 27 ), although they did not consider the effect of the interaction of weight gain between both periods and risk of GDM. As weight gain in the first trimester is disproportionately composed of adipose tissue compared with that in later pregnancy( Reference Van Raaij, Peek and Vermaat-Miedema 32 ), it can be hypothesized that the increasing fat mass in the first trimester may play a relatively important role in glucose intolerance.
The present study has also analysed RGWG-F and GWG-S, producing the same results as GWG-F and RGWG-S in relation to the risk of GDM. To be consistent with the IOM recommendations and the pattern of weight gain during pregnancy, GWG-F and RGWG-S were used as eventual indicators. Moreover, they would be more properly applied in clinical guidance in terms of practicability and availability.
In addition, our study explored the optimal GWG-F ranges stratified by China-specific BMI categories for preventing the development of GDM. Currently, the quartile method, receiver-operating characteristic curves or predicted risk is used when establishing the optimal GWG ranges. Since the quartile method depends on the average level in the population and GWG has increased in recent years in China( Reference Zhang, Dong and Zhang 7 , Reference Zhu, Fan and Yang 8 ), higher ranges would have been obtained if this method had been used. Receiver-operating characteristic curves are more suitable to explore upper or lower limits. Therefore, the prevalence of GDM was calculated to explore optimal GWG-F ranges( Reference Bracero and Byrne 34 , Reference Juan, Qi and Min 35 ). The optimal GWG ranges were defined as the corresponding GWG ranges when the prevalence of GDM was lowest after GWG had increased by a certain amount.
The optimal GWG-F ranges were 0·8–1·2 kg in both underweight and normal-weight women, while overweight/obese women showed an optimal GWG-F range of 0·35–0·7 kg. Compared with that for underweight and normal-weight women, the optimal GWG-F range for overweight/obese women was lower. This result is consistent with previous findings( Reference Zhou, Li and Ye 36 , Reference Wong, Tang and Lau 37 ). Zhou et al., based on the Asian BMI classification, found that the mean GWG among Chinese women decreased as pre-pregnancy BMI increased. Zhang et al. observed that GWG-F was 0·92 kg among normal-weight women in China without any pregnancy complications( Reference Zhang, Huang and Ming 38 ), which is similar to our findings. The IOM recommends the same range of weight gain in the first trimester regardless of pre-pregnancy BMI. This is the aspect of the IOM guidelines that is most different from our findings. Abrams et al. suggested that both the rate and the shape of the weight gain curve for relatively heavier women might differ from that of women with lower pre-pregnancy BMI( Reference Abrams, Carmichael and Selvin 39 ). Therefore, it was necessary to provide optimal GWG-F ranges according to the pre-pregnancy BMI. The optimal GWG-F ranges in our study were within IOM recommendations, while the upper limit for each pre-pregnancy BMI group was lower than that in the IOM recommendations.
Strengths and limitations should be considered when interpreting our findings. In terms of strengths, the present study shows the biological plausibility of a strong association between GWG-F and risk of GDM. Our study also adjusted for the effect of GWG-F on GDM when analysing the effect of RGWG-S on the risk of GDM. Further, in our investigation of the effects of GWG-F and RGWG-S on the risk of GDM and our exploration of the optimal GWG ranges for the avoidance of GDM, the pre-pregnancy BMI cut-off points were based on the China-specific BMI categories, which are more suitable for application in Chinese women. Another strength of the present study is that it established the first optimal GWG-F ranges stratified by China-specific BMI categories, which can be used for the avoidance of GDM and provide certain evidence for the establishment of GWG standards for Chinese women. Nevertheless, the present study also has some limitations. First, it used self-reported pre-pregnancy weight. Although high correlations have been found between self-reported and measured pre-pregnancy weight( Reference Hedderson, Gunderson and Ferrara 12 ), there may be potential misclassifications of pre-pregnancy BMI. There were also an insufficient number of women with high BMI to allow analyses of overweight and obese women separately, due to the demographics of overweight/obese women in Southwest China. In addition, our data are not from a nationally representative sample; the study used only a prospective cohort study in Chengdu, which is in the Southwest region of China, to eliminate the potential for selection bias. Due to the considerably high prevalence of GDM, the generalizability of our findings requires further investigation and more studies conducted in different areas or combined with another population are needed to verify the optimal ranges. Moreover, given the small sample sizes in the underweight and overweight/obese groups, the applicability of optimal GWG-F ranges for underweight and overweight/obese women is still limited. Further large studies are needed for verification as well.
Conclusions
Our study suggests that excessive GWG in the first trimester, rather than the second trimester, is associated with an increased risk of GDM regardless of pre-pregnancy BMI. The optimal GWG ranges in the first trimester for the avoidance of GDM are 0·8–1·2, 0·8–1·2 and 0·35–0·7 kg for underweight, normal-weight and overweight/obese women, respectively. Identifying potential strategies for prevention of excessive GWG-F may have far-reaching benefits. Obstetricians should provide more pre-emptive nutrition guidance and lifestyle interventions in achieving adequate GWG-F. Further studies are needed to verify the generalizability of the ranges as well.
Acknowledgements
Acknowledgements: All participants are gratefully acknowledged. Financial support: All phases of this study were supported by a research grant from the Danone Nutrition Center Dietary Nutrition Research and Education Fund (grant number DIC2016-06). The funders had no additional role in the design, analysis or writing of this article. Conflict of interest: The authors reported no potential conflict of interest. Authorship: G.Z. designed the research; X.L. performed initial data analyses and wrote the manuscript; Y.Z., H.D. and J.Z. edited the manuscript; F.Z., Y.B. and C.C. performed data entry; R.Z., D.B. and X.P. provided critical input on the data analyses; G.Z. supervised the study. All authors critically reviewed and approved the final manuscript. Ethics of human subject participation: This study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects were approved by the Ethics Committee of Sichuan University. Written informed consent was obtained from all the participants before they took part in the study.






