Mexico has one of the highest prevalences of anencephaly in the world, probably due to limited prenatal care and access to elective terminations among others factors, with 5·91 cases per 10 000 live births from 2006 to 2008 according to the International Clearinghouse for Birth Defects Monitoring System( 1 ).
The aetiology of neural tube defects (NTD) is multifactorial, including genetic and environmental factors( Reference Blatter, Van der Star and Roeleveld 2 , Reference Copp and Greene 3 ). Reports have suggested that the risk of NTD is associated with variants in the gene encoding methylenetetrahydrofolate reductase (MTHFR) as well as other genes involved in folate metabolism (e.g. cystathionine-β-synthase or methionine synthase reductase) and nutritional status (e.g. folate and vitamin B levels)( Reference Botto and Yang 4 , Reference Guéant, Guéant-Rodriguez and Anello 5 ). Low levels of folate and vitamin B12, associated with hyperhomocysteinaemia, have been found in mothers of children with NTD. However, the findings of different studies have not been consistent( Reference Gaber, Farag and Soliman 6 – Reference Wild, Schorah and Sheldon 12 ).
MTHFR is an enzyme involved in the metabolism of folic acid via conversion of 5,10-methylenetetrahydrofolate to 5-methyltetrahydrofolate (circulating folate), the methyl donor for methionine synthesis from homocysteine (Hcy). This reaction is catalysed by methionine synthase and uses vitamin B12 as a cofactor. This is important in one-carbon metabolism because methionine is the precursor of S-adenosylmethionine, the methyl group donor in more than 100 reactions( Reference Bagley and Selhub 13 ) necessary for DNA synthesis, cell division and tissue growth( Reference Botto and Yang 4 ). It is also essential for DNA methylation, which plays an important role in gene expression and chromatin structure( Reference Botto and Yang 4 , Reference Boyles, Hammock and Speer 14 ).
The MTHFR gene has two common polymorphisms (677C→T and 1298A→C) that reduce activity of the enzyme. The 677C→T mutation causes an alanine to valine substitution in the predicted catalytic domain of MTHFR, rendering the enzyme thermolabile, with reduced activity under conditions of low folate concentrations( Reference Friso and Choi 15 ). Homozygosity for the 677T allele is associated with an increase in plasma Hcy levels and a decreased methyltetrahydrofolate pool, predominantly in states of folate, cobalamin and riboflavin (vitamin B2) deficiency( 1 – Reference Jakubowski, Boers and Strauss 19 ). The other polymorphism, 1298A→C, which leads to substitution of an adenine by a cytosine, has also been associated with decreased MTHFR enzyme activity, although not as pronounced as that caused by the 677C→T polymorphism( Reference Weisberg, Tran and Christensen 18 , Reference van der Put, Gabreels and Stevens 20 , Reference Ulvik, Ueland and Fredriksen 21 ).
The Mexican population has one of the highest frequencies of MTHFR 677T allele (>50 %) in the world( Reference Blanco-Muñoz, Lacasaña and Cavazos 22 , Reference Mutchinick, Lopez and Luna 23 ). Previous studies by our group( Reference Blanco-Muñoz, Lacasaña and Cavazos 22 ) and investigations in other populations in Mexico( Reference Gonzalez-Herrera, Castillo-Zapata and Garcia-Escalante 24 ) and elsewhere( Reference Kirke, Mills and Molloy 25 – Reference Shaw, Rozen and Finnell 27 ) showed an increased risk of anencephaly and other types of NTD in TT homozygous mothers. This homozygosity, in combination with a diet that is deficient in folate or other B vitamins, could increase the risk in women of reproductive age of adverse reproductive effects, including NTD( Reference Shaw, Rozen and Finnell 27 – Reference Harisha, Devi and Christopher 29 ).
The high prevalence of anencephaly and high frequency of MTHFR 677T allele in the population make Mexico an ideal setting for evaluating the potential effect of gene–nutrient interaction on the risk of this congenital malformation. The objective of the present study was to evaluate the risk of anencephaly associated with the maternal biochemical profile related to the folic acid metabolic pathway and its interaction with MTHFR 677C→T polymorphism, in three states of Mexico with a high prevalence of anencephaly (Mexico State, Puebla and Guerrero).
Materials and methods
Design and study population
The study population and design of the present population-based case–control study (March 2000–February 2001) are described in detail elsewhere( Reference Blanco-Muñoz, Lacasaña and Cavazos 22 , Reference Lacasaña, Vázquez-Grameix and Borja-Aburto 30 ). Briefly, cases and controls were paired (1:1) on maternity clinic, date of birth and state of residence (Mexico State, Puebla or Guerrero). Cases of anencephaly were identified using the Registry of the Mexican Neural Tube Defect Epidemiological Surveillance System (initials in Spanish, SVEDTN). The SVEDTN forms part of the National Epidemiological Surveillance System, which compiles information from all National Health System institutions, e.g. death certificates. In Mexico, there are two types of death certificate: Foetal Death Certificate for babies born dead; and Death Certificate for post-delivery death.
Selection process
All cases of death from anencephaly (WHO International Classification of Diseases, 10th revision, code Q00·0) registered in the local SVEDTN between 1 March 2000 and 28 February 2001 were potentially eligible, including live births and stillbirths, with the exception of cases with <20 weeks’ gestational age, because there is practically no registration of abortions (spontaneous or induced) in Mexico.
For each case, the next child born alive at the same maternity clinic without anencephaly or other congenital malformation was selected as control. Inclusion criteria for cases and controls were residence by the mother in the corresponding state for ≥1 year prior to the birth and the possibility of localizing the mother during the first 3 months postpartum.
During the study period, 189 cases complied with the inclusion criteria, and 157 (83·1 %) of the mothers agreed to participate in the study. Once a case mother signed a consent form, we contacted the mother of the control or, if she refused participation, the mother of the next eligible control. Contact was made with 160 mothers of controls, 151 of them (94·4 %) agreed to participate. For six cases, it was not possible to find a control fulfilling the inclusion criteria. At the end of this process, we obtained complete information for ninety-eight (51·9 %) mothers of cases and ninety-one (56·9 %) mothers of controls. Given the sample's size, there is an 81 % power to detect a significant interaction at 95 % confidence for odds ratios equal to those observed in the present study. The software Power version 43 (National Cancer Institute, Bethesda, MD, USA)( Reference Garcia-Closas and Lubin 31 ) was used for these calculations.
Data collection
The questionnaires were applied by the nursing staff previously trained in each of the participating states. Interviews were conducted in the homes of the cases and the controls. The interviewers helped the mothers to define the periconceptional period of interest which was defined as the period from 3 months prior to the last menstruation to 1 month after.
General questionnaire
A structured questionnaire was administered to mothers of the cases and controls included in the study, containing items on sociodemographic characteristics (age, marital/cohabitant status, maternal education and family income), habits (lifetime and periconceptional use of tobacco and alcohol), presence of chronic or acute disease or fever during periconceptional period, multivitamin supplementation or receipt of medication during periconceptional period, reproductive history (number of pregnancies, history of stillbirths, spontaneous abortions, premature births and malformed children), antenatal care in index pregnancy, family reproductive history, occupational history and domestic exposure to chemical substances during periconceptional period.
Mothers who refused to participate in the study answered a brief questionnaire on their socio-economic characteristics (education, income and occupation) and reproductive history.
FFQ
A standard FFQ was used to assess dietary intake of nutrients during the periconceptional period. It was developed by Willett et al.( Reference Willett 32 ) and adapted to the Mexican population, validated against 24 h recalls in a sample of 134 women from Mexico City( Reference Hernández-Avila, Romieu and Parra 33 ).
Laboratory procedures
Fasting blood samples were collected from the mothers. All blood samples were drawn before 3 months postpartum. Blood was obtained from the antecubital vein by means of the Vacutainer system, using one tube without anticoagulant for measuring serum folate and serum vitamin B12 and an EDTA tube for measuring plasma levels of total Hcy (t-Hcy) and for DNA extraction. Samples were kept refrigerated at 4°C and transported to the National Institute of Perinatology, Mexico DF, where they were centrifuged to separate serum, plasma or buffy coat, which was kept frozen at −70°C until DNA extraction, genotyping and biochemical profile analysis.
Genotyping
DNA was extracted from leucocytes using a Promega™ Wizard® Genomic DNA Extraction Kit (Promega Corporation, Madison WI, USA). MTHFR genotype was analysed by PCR in an Eppendorf™ Mastercycler® Gradient thermal cycler (Eppendorf North America, Hauppauge, NY, USA), using 5 μl of 10x PCR Buffer (Promega), 4 μl of 25 mm-MgCl2, 2·5 μl of dimethyl sulfoxide, 1 μl of 2·5 mm-dNTP mix, 10 pmol of each primer (forward: 5′-GCAGGGAGCTTTGAGGCTGAC-3′, reverse: 5′-AGGACGGTGCGGTGAGAGTG-3′) and 0·5 U of Taq polymerase (Promega) in a total reaction volume of 50 μl. PCR conditions were as follows: denaturation at 92°C for 1 min, alignment at 60°C for 30 s and extension at 72°C for 30 s (35 cycles), followed by a final extension to 72°C (7 min). A 15 μl aliquot of PCR product was incubated at 37°C for 3 h with 1 U of HinfI restriction enzyme (New England Biolabs® Inc., Ipswich MA, USA). Restriction fragments were electrophoresed in a 4 % (w/v) agarose gel stained with ethidium bromide and visualized under UV light in a Fotodyne™ transilluminator (Fotodyne® Inc., Hartland, WI, USA).
Genotyping was performed simultaneously by two technicians and then the results were contrasted.
Determination of red blood cell folate, serum folate, serum vitamin B12 and plasma total homocysteine
Serum folate and red blood cell (RBC) folate were quantified by means of ionic capture using an IMx analyser (Abbott IMx Folate; Abbott Laboratories, Diagnostic Division, Abbott Park, IL, USA). This assay is based on the formation of polyanion–analyte complexes with a negative charge captured through an electrostatic interaction with the matrix, which has a positive electrical charge( 34 ). It is necessary to determine the haematocrit value of each patient and to perform a previous haemolysis to quantify RBC folate.
Vitamin B12 was determined by microparticle enzyme immunoassay (Abbott IMx Vitamin B12; Abbott Laboratories, Diagnostic Division), which uses microparticles coated with an intrinsic factor for vitamin B12 ( 35 ).
Plasma t-Hcy levels were quantified by fluorescence polarization immunoassay technology using the IMx system( 36 ). Laboratory reference values were 175–700 ng/ml for RBC folate, 3–17 ng/ml for serum folate, 200–950 pg/ml for vitamin B12 and 5–16 μmol/l for plasma t-Hcy.
Genotyping and analyses of biochemical profile were performed blind to case and control status.
Ethics approval
The study was approved by the Institutional Review Board of the National Institute of Public Health of Mexico. All participants were given an informed consent letter, which was signed before participation.
Statistical analysis
Differences in sociodemographic, reproductive and lifestyle variables and in folate and vitamin B12 intakes were compared between mothers of cases and controls by using the χ 2 test or, if applicability conditions were not met, by Fisher's exact test.
Concentrations of serum folate, RBC folate, serum vitamin B12 and plasma t-Hcy (medians, means and standard deviations) were compared between mothers of cases and controls as a function of MTHFR 677C→T polymorphism by means of the Kruskal–Wallis non-parametric test, because the distribution of these compounds was skewed to the right and did not fulfil normality criteria (Kolmogorov–Smirnov test). The differences in biochemical profile between case mothers and control mothers were evaluated by means of the Mann–Whitney non-parametric test.
A Spearman correlation analysis was developed between the levels of serum folate, RBC folate, serum vitamin B12 and plasma t-Hcy, because these variables did not have a normal distribution. The Kolmogorov–Smirnov test was applied to assess the normal distribution of the variables.
A multivariate unconditional logistic regression model was constructed to assess the risk of anencephaly associated with maternal concentrations of serum folate, RBC folate, serum vitamin B12 and plasma t-Hcy, including the genotype–nutrient interactions between the maternal biochemical profile (as continuous variables) and MTHFR 677C→T polymorphisms. We constructed both a non-adjusted model and a model adjusted for the following confounding variables: state of residence, age of the mother, social status, reproductive history, exposure to agricultural work, and daily intake during the periconceptional period of folate, vitamin B12 and total energy.
Finally, unconditional logistic regression models were constructed that only included genotype–serum folate interactions. Two distinct approaches were applied: (i) considering serum folate levels as a continuous variable to evaluate the change in risk per unit increase; and (ii) considering the continuous variable in terms of tertiles of serum folate in the controls in order to compare the risk between mothers with high v. low levels of serum folate. In both cases, a non-adjusted model and a model adjusted for confounders were constructed.
We applied the following criteria for inclusion of potential confounding variables in the multivariate model: (i) all the variables associated with anencephaly in the bivariate models with P < 0·20 were selected to evaluate the presence of confounding; (ii) multivariate models were constructed adding the previously selected variables, including in the final model all those variables associated with anencephaly with P < 0·10.
The level of significance was set at 0·05 and 95 % confidence intervals were calculated for odds ratios. SPSS version 14 (SPSS Inc, Chicago, IL, USA) and STATA version 7 (StataCorp LP, College Station, TX, USA) statistical software packages were used for the analyses.
Results
We had complete information on MTHFR 677C→T polymorphisms and serum folate levels for 189 mothers, ninety-eight case mothers and ninety-one control mothers. No significant differences in variables of interest were found between mothers with available data on genotype and biochemical profile and those without these data. Moreover, the distribution of genotypes for MTHFR 677C→T among controls was in Hardy–Weinberg equilibrium (P = 0·83).
Table 1 displays the main characteristics of case and control mothers in relation to the risk of anencephaly, showing significant differences. Case mothers were more often 35 years of age or older, had a lower income, were more often multipara, more often had a history of adverse events, and more frequently reported having worked in agriculture some time in life. The mean gestational age was significantly lower than in controls (7·8 months v. 8·9 months).
Table 1 Sociodemographic, reproductive and lifestyle characteristics of mothers of anencephaly cases and controls, Mexico, Puebla and Guerrero states, Mexico, March 2000–February 2001
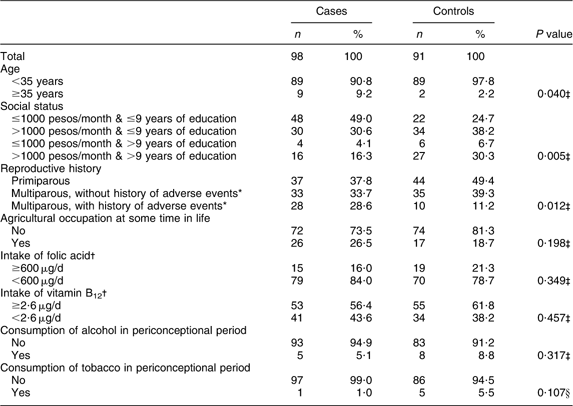
*Includes stilbirths, premature births, congenital malformations and abortions.
†Limits selected in accordance with current daily recommendations for folate and vitamin B12 intake by pregnant women( 37 ).
‡χ 2 test.
§Fisher's exact test.
The median folate intake of case mothers (299·3 μg/d) and control mothers (330 μg/d) was about 50 % of the RDA for pregnant women( 37 ) (600 μg/d).
The Spearman correlation analysis showed a significant positive correlation between serum folate levels, RBC folate and vitamin B12, and a significant negative correlation between maternal concentrations of these nutrients and plasma t-Hcy levels. The highest correlation coefficient was observed between maternal levels of serum folate and RBC folate (r = 0·534). The same pattern was observed in both cases and controls (data not presented).
Median levels of RBC folate, serum folate, serum vitamin B12 and plasma t-Hcy were within the laboratory reference range and did not differ significantly between case and control mothers (P > 0·05). The percentage of cases and controls below the reference range were as follows: serum folate, 2 % of cases and 0 % of controls; RBC folate, 20 % of cases and 22 % of controls; vitamin B12, 11 % of cases and 8 % of controls. Also, 26 % of cases and 18 % of controls had t-Hcy levels above the reference range.
However, when stratified according to MTHFR 677C→T polymorphism, case mothers with 677TT genotype showed significantly lower levels of serum folate and serum vitamin B12 compared with case mothers with 677CC and 677CT genotypes. t-Hcy levels were significantly higher in case mothers with 677TT genotype than in the other case mothers. In contrast, control mothers showed no differences in serum folate, RBC folate, serum vitamin B12 or plasma t-Hcy levels as a function of the MTHFR 677C→T polymorphism (Table 2).
Table 2 Maternal serum and RBC levels of folate, vitamin B12 and t-Hcy as a function of the MTHFR 677C→T polymorphism among mothers of anencephaly cases and controls, Mexico, Puebla and Guerrero states, Mexico, March 2000–February 2001
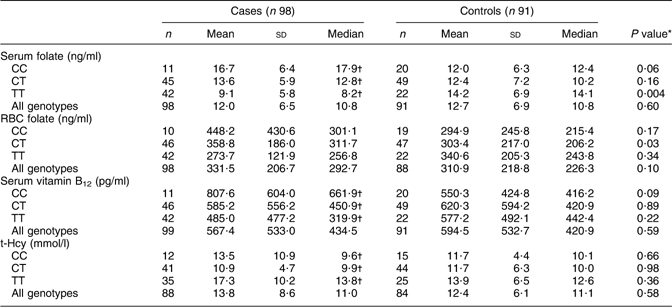
RBC, red blood cell; MTHFR, methylenetetrahydrofolate reductase gene; t-Hcy, total homocysteine.
Laboratory reference range: serum folate, 3–17 ng/ml; RBC folate, 175–700 ng/ml; vitamin B12, 200–950 pg/ml; homocysteine, 5–15 mmol/l.
*Comparison between case and control mothers of the same genotype by Mann–Whitney test.
†Values were significantly different among genotypes (Kruskal–Wallis test): P < 0·05.
Comparison of serum folate levels in case and control mothers according to the MTHFR 677C→T polymorphism showed that only the case mothers with 677TT genotype had significantly lower serum folate levels compared with control mothers (median values: 8·2 v. 14·1, P = 0·004). However, median RBC folate levels were significantly higher in case mothers with 677CT than in the control mothers (medians: 311·7 v. 206·2, P = 0·026), with no differences among remaining genotypes. No significant differences in median serum vitamin B12 and plasma t-Hcy concentrations were found in cases or controls as a function of the MTHFR 677C→T polymorphism (Table 2).
Table 3 shows the odds ratios for anencephaly in the unconditional logistic regression model that included genotype–nutrient interactions between the mother's biochemical profile and the MTHFR 677C→T polymorphism. After adjusting for state of residence, childbirth health-care centre, age, social status, reproductive history, exposure to agricultural work at some time in life and daily dietary intake of folate, vitamin B12 and total energy during the periconceptional period, a significant interaction was found between MTHFR 677C→T polymorphism and serum folate levels (P = 0·02), while the interaction between this polymorphism and RBC folate, vitamin B12 or t-Hcy levels was not significant.
Table 3 Logistic regression models for the risk of anencephaly with interaction between biochemical profile (serum folate, RBC folate, vitamin B12 and t-Hcy levels) and the MTHFR 677C→T polymorphism, Mexico, Puebla and Guerrero states, Mexico, March 2000–February 2001

MTHFR, methylenetetrahydrofolate reductase gene; RBC, red blood cell; t-Hcy, total homocysteine.
Interpretation of the model: the OR for serum folate levels (OR = 1·12) is the probability of having a child with anencephaly for each 1 ng/ml increase in serum folate in women with CC genotype (reference level for the MTHFR 677C→T polymorphism); the same applies to the OR for RBC folate, vitamin B12 and serum t-Hcy.
The risk of having an anencephalic child associated with a 1 ng/ml increase in serum folate in mothers with CT genotype is calculated by multiplying the OR associated with serum folate levels (OR = 1·12) by the OR associated with ‘Serum folate × CT genotype’ (OR = 0·90), OR = 1·12 × 0·90 = 1·01. The same procedure is applicable for ‘Serum folate × TT genotype’, OR = 1·12 × 0·68 = 0·76. The same applies to the OR for the other interaction terms.
*Adjusted by maternal characteristics: state of residence, childbirth health-care centre, age, social status, reproductive history, agricultural work at some time in life, and daily intake of folate, vitamin B12 and total energy during the periconceptional period.
†Genotype CC is the reference level.
Table 4 shows the results of the models of the interaction between serum folate and MTHFR 677C→T polymorphism. The adjusted models show that the risk of anencephaly in mothers with 677TT genotype was reduced by 18 % (OR = 0·82; 95 % CI 0·72, 0·94) for each 1 ng/ml increment in serum folate. However, in mothers with 677CC and 677CT genotypes, an increase in serum folate levels did not significantly reduce this risk. In terms of tertiles, mothers with 677TT genotype with serum folate levels in the upper tertile (>14·1 ng/ml) had a 95 % lower risk (OR = 0·05; 95 % CI 0·01, 0·37) v. those with levels in the lowest tertile (<8·2 ng/ml; P trend = 0·012).
Table 4 Crude and adjusted odds ratios and 95 % confidence intervals for the risk of anencephaly by the interaction between serum folate levels and the MTHFR 677C→T polymorphism, Mexico, Puebla and Guerrero states, Mexico, March 2000–February 2001
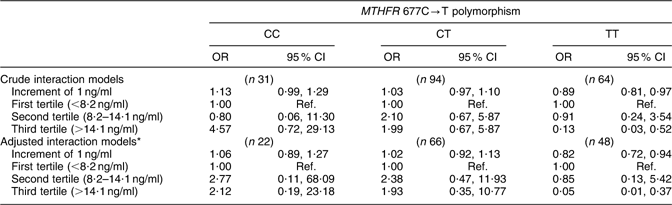
MTHFR, methylenetetrahydrofolate reductase gene; RBC, red blood cell; t-Hcy, total homocysteine; Ref., referent category.
*Adjusted by maternal characteristics: state of residence, childbirth health-care centre, age, social status, reproductive history, agricultural work at some time in life, RBC folate level, serum vitamin B12 level, plasma t-Hcy level, and daily intake of folate, vitamin B12 and total energy during the periconceptional period.
Discussion
Maternal nutritional factors, especially folic acid intake, are known to make a substantive contribution to reduce the probability of occurrence or recurrence of the birth of a child with NTD. However, the aetiology of NTD has not been fully elucidated. In the present study, no significant differences were found between mothers of anencephaly cases and mothers of controls in median postpartum concentrations of serum folate, RBC folate, serum vitamin B12 and plasma homocysteine. However, evaluation of the potential effect of the interaction between biochemical profile and MTHFR 677C→T polymorphism showed a significant protective effect for each 1 ng/ml increment in serum folate levels among mothers with 677TT genotype (adjusted OR = 0·82; 95 % CI 0·72, 0·94).
Inconsistent results have been published on the protective effect of folate. Thus, some studies showed similar serum folate levels between mothers of children with NTD and mothers of controls( Reference Gaber, Farag and Soliman 6 , Reference Suarez, Hendricks and Felkner 8 – Reference Christensen, Arbour and Tran 10 ), whereas others reported lower serum folate levels in case v. control mothers( Reference Kirke, Molloy and Daly 11 , Reference Cech and Burau 38 ). There has even been a recent report from Brazil of significantly lower serum folate levels in control mothers and their children than in mothers of NTD patients and the NTD patients themselves, which the authors attributed to a greater dietary intake of folate by the case group( Reference Félix, Leistner and Giugliani 7 ).
Median RBC folate levels were similar between case and control mothers in the present study, as also observed in other Mexican populations( Reference Suarez, Hendricks and Felkner 8 , Reference Martínez de Villarreal, Delgado-Enciso and Valdéz-Leal 9 ), although Martinez-Villarreal et al.( Reference Martínez de Villarreal, Delgado-Enciso and Valdéz-Leal 9 ) observed a higher percentage of case than control mothers with RBC folate levels <160 ng/ml. However, studies in other countries reported significantly lower RBC folate concentrations in case mothers than in control mothers( Reference Christensen, Arbour and Tran 10 – Reference Wild, Schorah and Sheldon 12 , Reference Cech and Burau 38 – Reference Ren, Zhang and Hao 40 ).
Evidence has emerged over the past few decades of an independent role for vitamin B12 in NTD risk, with numerous studies reporting significantly lower vitamin B12 levels in mothers of NTD cases compared with control mothers( Reference Gaber, Farag and Soliman 6 – Reference Suarez, Hendricks and Felkner 8 , Reference Christensen, Arbour and Tran 10 , Reference Kirke, Molloy and Daly 11 , 36 ). However, vitamin B12 levels were similar between case and control mothers in the present study, although the percentage of mothers with vitamin B12 deficiency (<200 pg/ml) was slightly higher in cases than in controls (57·9 % v. 42·1 %).
A limitation of many of the above studies was that they did not jointly consider the effect of the genotype and biochemical profile of the mothers. In our study, stratification by MTHFR 677C→T polymorphism showed that case mothers with 677TT genotype had significantly lower levels of serum folate and serum vitamin B12 and significantly higher levels of t-Hcy in comparison with case mothers with 677CC and 677CT genotypes, with a lower intake of folate and vitamin B12 by case mothers with 677TT genotype compared with the other case mothers. However, the control mothers showed no significant difference in biochemical profile as a function of the MTHFR 677C→T polymorphism, probably because there were no differences in folate or vitamin B12 intake as a function of the genotype. This finding supports the hypothesis that serum levels of folate and vitamin B12 can be attributed to both genetic and nutritional factors( Reference Christensen, Arbour and Tran 10 , Reference Mitchell, Duffy and Duffy 41 ).
However, case and control mothers with 677TT genotype showed no significant differences in median RBC folate concentrations compared with mothers with 677CT and CC genotypes (P = 0·103 and P = 0·354 for cases and controls, respectively), as also reported by other authors( Reference Christensen, Arbour and Tran 10 , Reference van der Put, Gabreels and Stevens 20 , Reference Molloy, Mills and Kirke 42 , Reference Narayanan, McConnell and Little 43 ).
Finally, in the multivariate analysis a significant effect was found for the interaction between serum folate levels and MTHFR 677C→T polymorphism, but not for the interaction between RBC folate, serum vitamin B12 and plasma t-Hcy levels. The case mothers with 677TT genotype and serum folate levels >14·1 ng/ml (third tertile) showed a 95 % reduction in the risk of having a child with anencephaly v. those with levels <8·2 ng/ml (first tertile), and a significant dose–response relationship was observed (OR = 0·05; 95 % CI 0·01, 0·37; P trend = 0·012). Although numerous studies have suggested this effect of the interaction between this MTHFR polymorphism and folate status on the risk of NTD, the small sample size of most of them prevented a statistical analysis of this interaction. Only Christensen et al.( Reference Christensen, Arbour and Tran 10 ) analysed this interaction in a study in Montreal of children with NTD and their mothers in comparison with a control group. They reported a higher risk of NTD for children with 677TT genotype and low RBC folate levels (OR = 13·43; 95 % CI 2·49, 72·83), although the difference in maternal levels did not reach significance (OR = 3·28; 95 % CI 0·84, 12·85). Nevertheless, some human and animal studies did not find any effect of this interaction( Reference Félix, Leistner and Giugliani 7 , Reference Perez, D'Almeida and Vergani 44 , Reference Li, Pickell and Liu 45 ).
The mechanism by which the 677TT genotype and serum folate levels increase the risk of anencephaly has yet to be elucidated. In the present study, the case mothers with 677TT genotype and serum folate levels <10·4 ng/ml (median value in controls) had higher t-Hcy levels than the other case mothers (P < 0·07). However, in the multivariate model no interaction effect between the 677TT genotype and plasma homocysteine levels was observed. So, although a teratogenic effect has been attributed to Hcy( Reference Bennett, Vanwaes and Moser 46 , Reference Padmanabhan, Shafiullah and Benedict 47 ), this hypothetical mechanism does not seem to be reflected in the results of our study. Heidenreich et al.( Reference Heidenreich, Reedy and Brauer 48 ) demonstrated that the increase in intracellular Ca2+ signalling could be responsible for the above-mentioned teratogenic effect.
Human and animal studies have shown that the cause of NTD is multifactorial and that folate metabolism, although important, is not the only factor( Reference Blom 49 ). Although polymorphism in other genes involved in folate metabolism (e.g. methionine synthase reductase (MTRR) 66A→G) appeared to be a potential risk factor for NTD( Reference Wilson, Platt and Wu 50 ) and mutated homozygotes for 66A→G have hyperhomocysteinaemia( Reference Lucock, Daskalakis and Hinkins 51 , Reference Relton, Wilding and Pearce 52 ), no association with NTD was found in the study of three different polymorphisms in MTRR performed by O'Leary et al.( Reference O'Leary, Mills and Pangilinan 53 ).
Until now, more than 200 genes have been found to be involved in the development of NTD in the mouse, some of them being energy metabolism gene (UCP2), vitamin B metabolism (Transcoalbumin), over-expression of sonic hedgehog signalling and loss of function of non-canonical Wnt gene( Reference Copp and Greene 3 , Reference Boyles, Hammock and Speer 14 ).
In our study population, serum folate, RBC folate, vitamin B12 and t-Hcy levels were measured before 3 months postpartum; therefore they may not reflect levels during the periconceptional period, specifically up to day 25 of gestation when the neural tube closes at its rostral pole( Reference Blom, Shaw and den Heijer 54 ). Moreover, the mean gestational age was significantly lower in cases (7·8 months) than in controls (8·9 months), and therefore serum folate, RBC folate and vitamin B12 were likely higher in cases than in controls at the end of the pregnancy and thus in the postpartum period. Nevertheless, vitamin B12, RBC folate and Hcy concentrations return to periconceptional levels at 6 weeks postpartum( Reference Blom, Shaw and den Heijer 54 ). On the other hand, although serum folate levels also show a slight decrease during pregnancy, they do not reach periconceptional levels until 6 months postpartum( Reference Bruinse and van den Berg 55 ). This can be explained by the haemodilution process produced during pregnancy, by lactation, by hormonal influences or by oral contraception methods( Reference Cikot, Steegers-Theunissen and Thomas 56 – Reference O'Rourke, Redlinger and Waller 58 ). Therefore, a potential bias due to gestational age and lactation (only controls are breast-fed) may lead to an underestimation of the effect of the associations observed. In fact, the effect of folic acid deficiency on anencephaly risk and the effect of interaction with the TT genotype may be even greater than reported herein.
One of the limitations of our study is that specific information regarding the ethnicity of the women was not available, thus we cannot rule out the presence of ethnic stratification, nor control for this factor in the analysis; this could have led to a certain degree of residual confounding. However, given that each control was selected from the same state and in the same childbirth health-care centre where the case was identified, we think that both come from the same population and that, if stratification exists, the distribution of the strata would be similar between the case and the control mothers and the value of association would not be biased.
Another potential limitation of the study could be if the biochemical profile established for the mothers was influenced by a differential multivitamin supplementation at postpartum between cases and controls and did not reflect nutritional status during organogenesis of the neural tube. However, this is unlikely because folic acid supplementation was very infrequent in these women, and only three mothers received it during the period of neural tube organogenesis. Therefore it is unlikely that mothers of the cases were receiving multivitamin supplementation to avoid a recurrence of NTD or other adverse reproductive effects.
A further possible study limitation may be that we analysed a subpopulation of cases and controls, i.e. those for whom complete information was available on the biochemical profile and MTHFR 677C→T genotype. However, no differences were found between this population and the original study population in maternal characteristics associated with anencephaly that might act as confounders or covariables (age, social status, reproductive history, agricultural work, daily intake of folate and vitamin B12, use of alcohol and tobacco during the periconceptional period).
Among the main strengths of our study is that the outcome studied is one type of congenital malformation while most previous studies have analysed NTD as if they were a homogeneous group, reducing the power to find associations between the genetic marker and the disease( Reference Relton, Wilding and Jonas 59 ), since different NTD may have distinct aetiologies( Reference Khoury, Erickson and James 60 , Reference Detrait, George and Etchevers 61 ). Thus, the main mechanism for closure of the neural tube is neurulation at the anterior pole and canalization at the posterior pole( Reference Elwood, Little and Elwood 62 ). Hence, risk factors may act in different ways at distinct levels and it is more useful to evaluate the combined effect of polymorphism and folate status on a specific NTD( Reference Padmanabhan, Shafiullah and Benedict 47 ).
These results are of considerable public health interest, especially in Mexico, where there is a high risk of anencephaly, a high prevalence of 677T allele and a low level of preventive interventions (e.g. periconceptional supplementation and fortification with folic acid). The percentage of NTD preventable by folic acid fortification of wheat and maize flour in 2008 was only 25 %( Reference Bell and Oakley 63 ). Our study revealed that the dietary intake of folate was below the RDA for pregnant women (600 μg/day)( 37 ) in 83·3 % of our cases and 77·2 % of controls, and the median folate intake of cases (299·3 μg/d) and controls (330 μg/d) was about 50 % of the RDA. These findings emphasize the need for folic acid supplementation and fortification programmes in the Mexican population to modify the genetic risk of having a child with anencephaly.
Acknowledgements
This project was supported by the National Council of Science and Technology of Mexico (grant number 28203-M). The authors declare that there are no conflicts of interest. Each author made a significant contribution to the paper and meets the criteria for authorship. M.L. assumes the responsibility for correspondence. The authors thank the mothers of the cases and controls, without whom the study would have been impossible. They are also grateful to the Health Services of Mexico State, Puebla and Guerrero for logistical support.






