The rapid increase in the prevalence of overweight and obesity in many parts of the world is now characterized as a global pandemic and so has become one of the more important contemporary public health issues( Reference Kelly, Yang and Chen 1 , Reference Finucane, Stevens and Cowan 2 ). Recent evidence suggests that the status of micronutrients such as vitamin A( Reference Jeyakumar, Vajreswari and Giridharan 3 ), vitamin D( Reference Foss 4 ), vitamin B( Reference Gunanti, Marks and Al-Mamun 5 ) and Zn( Reference Marreiro, Fisberg and Cozzolino 6 ) may affect energy balance and so play a role in obesity. Obese individuals have lower blood micronutrient concentrations while micronutrient deficiencies are associated with increased fat deposition in both animal models and epidemiological studies( Reference Garcia, Long and Rosado 7 ). Micronutrient supplementation of obese adults can lead to reductions in body weight, BMI and abdominal obesity, and improve serum lipid profiles, possibly through increased energy expenditure and fat oxidation( Reference Li, Wang and Zhu 8 ).
Obese adults have been found to have significantly lower serum Zn concentrations while Zn deficiency has been found to be a risk factor for central adiposity among Indian men( Reference Ghayour-Mobarhan, Taylor and New 9 , Reference Singh, Beegom and Rastogi 10 ). Similarly, children with low hair Zn concentrations in Guatemala, New Zealand, Malawi and Ghana have been found to have higher weight-for-age Z-scores, higher BMI and lower mid upper-arm muscle area (MAMA) compared with children with greater hair Zn concentrations( Reference Cavan, Gibson and Grazioso 11 – Reference Ferguson, Gibson and Opare-Obisaw 13 ). Other studies, in contrast, have found no differences in serum Zn concentrations between obese and normal-weight individuals( Reference Galan, Viteri and Bertrais 14 , Reference Weisstaub, Hertrampf and Lopez de Romana 15 ). However, Zn supplementation has been found to be associated with greater weight gain and increased lean tissue mass simultaneously with increased linear growth among malnourished and stunted children( Reference Ninh, Thissen and Collette 16 – Reference Golden and Golden 18 ).
Zn may determine body adiposity through its role in energy metabolism, appetite control and adipokine regulation. Marginal Zn deficiency solely or in conjunction with a low-protein diet is associated with reduced lean body mass, decreased appetite and excess adiposity, each of which can be altered or restored by Zn supplementation( Reference McClain, Stuart and Kasarskis 19 , Reference Golden and Golden 20 ). Zn is an important component of enzymes involved in energy metabolism and Zn metalloenzymes essential for nucleic acid and protein synthesis and new tissue synthesis( 21 ). It also regulates the hormones leptin, ghrelin, insulin and adiponectin, which, in turn, regulate adiposity and fat mass( Reference Marreiro, Geloneze and Tambascia 22 – Reference Hashemipour, Kelishadi and Shapouri 24 ). Any changes in tissue-specific adipokine concentrations associated with Zn status may subsequently determine changes in adipose tissue mass and in turn modify the risk of obesity.
Clinical and epidemiological evidence suggests that Zn status may be contributing to the increased burden of obesity reported in countries passing through the epidemiological and nutrition transitions due to its effect on body composition( Reference Singh, Beegom and Rastogi 10 , Reference Golden and Golden 18 ). Meta-analyses have provided strong evidence for the growth-limiting effect of Zn deficiency( Reference Brown, Peerson and Allen 25 ). There is increasing evidence that Zn status may play a role in determining both growth patterns and body adiposity( Reference Golden and Golden 18 , Reference Park, Grandjean and Antonson 26 ). Malnutrition in early childhood is associated with a greater risk of obesity and numerous chronic diseases later in life, possibly due to metabolic ‘programming’ and other physiological adaptations in response to nutrition constraints( Reference Gluckman and Hanson 27 , Reference Barker 28 ). Such metabolic adaptations accompanying childhood stunting affect body fatness, growth rate, energy balance and fat oxidation, which can lead to obesity in the context of later availability of abundant energy (food)( Reference Hoffman, Sawaya and Verreschi 29 , Reference Sawaya, Grillo and Verreschi 30 ). Pre-existing Zn deficiency may be contributing to the development of obesity via its relationship with fat deposition associated with childhood stunting and subsequent short stature.
There has been no systematic review to date of the effects of Zn supplementation on body adiposity and composition. Therefore, we carried out a review of the literature to clarify what is understood about the effects of Zn supplementation on children’s body composition and address what factors may modify these associations. We also reviewed studies which reported the effect of Zn supplementation on adipokines and other hormones involved in adipogenesis that may be the mechanisms underlying associations between Zn status, body composition and risk of obesity.
Methods and procedures
Search strategy
A five-stage comprehensive search of the literature was conducted in the databases Cochrane Library, PubMed, Ovid Medline, CINAHL and EMBASE, for studies published before 28 February 2015. The search strategy for conducting the systematic review used the PICOS (Population, Intervention, Comparison, Outcome and Setting) method, in which search terms are separately developed for each component( Reference Schardt, Adams and Owens 31 ) to identify studies which assessed children as the study population (P) and supplementation with Zn alone or in combination with other micronutrients as the intervention/comparison (I/C). Zn could have been given with other micronutrients and the comparison group received the micronutrients (so that Zn was the only difference between the groups). Body composition, adiposity, leptin, ghrelin, insulin, adiponectin and adipokines are the outcomes (O) and studies conducted either in a community- or hospital-based setting from developing and developed countries (S). During the first stage the above databases were searched using the following keywords: ‘Zinc’ or ‘Zn’ or ‘Zinc supplementation’ or ‘Zn supplementation’ or ‘Zinc therapy’, AND ‘child’ or ‘children’ AND ‘body composition’ or (‘adiposity’, ‘fat’, ‘fat free mass’, ‘weight’, ‘BMI’, ‘fat mass’) or (‘adipocytokine’, ‘adipokine’, ‘insulin’, ‘adiponectin’, ‘ghrelin’, ‘leptin’).
In the second stage the total hits from the databases were pooled and duplicates were removed. This was followed by screening of the retrieved articles by reading the article ‘title’ in the third stage and the article ‘abstract’ in the fourth stage. In the fifth stage individual manuscripts were screened and those not satisfying inclusion criteria were excluded. To obtain additional data, a manual search of the reference lists of articles selected in the fifth stage was performed. This search process was conducted independently by two reviewers (I.R.G. and K.Z.L.) and the final group of articles to be included in the review was determined after an iterative consensus process. The present review was prepared in accordance with the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines( Reference Liberati, Altman and Tetzlaff 32 ).
The assessment of risk of bias within each study and the quality appraisal of each trial were evaluated using the quality checklists protocol of the CONSORT (Consolidated Standards of Reporting Trials) Statement 2001 for randomized controlled trials( Reference Altman, Schulz and Moher 33 ). We appraised the methods section of the articles as the key quality parameters considered in the review (data not shown). However, since no quality evaluation of the studies was made, we did not exclude any studies due to concern about their quality.
We used the following inclusion and exclusion criteria: studies were included if they were controlled trials in human subjects, randomized trials of the effect of Zn supplementation efficacy (either as a single micronutrient or in combination with other micronutrients) on body composition and adiposity-related hormone levels among children aged from infant to adolescent, from both developing and developed countries, in peer-reviewed, English-language journals. Papers were excluded if they were single clinical case studies or clinical discussion papers, or not primary sources. Book chapters and review papers were not considered primary sources and were excluded. The reference lists and citations of articles identified from the electronic search were assessed to ensure the inclusion of additional relevant studies. No communications with individual researchers were made.
The outcomes of interest for the present review were the different measures of body composition defined as the characteristic size and distribution of the component parts of total body weight( Reference Hills, Lyell and Byrne 34 ). Body composition in studies included in the review was assessed using two methods: ‘direct’ and ‘indirect’ methods. Studies employing direct methods used such methods as dual-energy X-ray absorptiometry (DXA) with the use of a densitometer and total body water (TBW) by bio-impedance analysis (BIA) and 2H dilution. These direct methods provide greater accuracy and rapid, non-invasive estimates of fat-free mass (FFM), fat mass (FM) and percentage body fat (%BF)( Reference Gibson 35 ). The indirect method of assessing body composition in the reviewed studies was anthropometric measurements( Reference Gibson 35 ). The anthropometric methods used in the reviewed studies included BMI determined by the relationship of body weight to body height, and skinfold thickness. Skinfold measurements can provide estimates of the size of the subcutaneous fat depot and body composition after applying equations to these measurements( Reference Wells and Fewtrell 36 ). We included studies which reported on FFM outcomes using anthropometric measures of mid upper-arm circumference (MUAC), mid upper-arm fat area (MAFA) and MAMA, which are derived from MUAC and triceps skinfold (TSF)( Reference Gibson 35 ).
Zn plays a role in the regulation of adipokines and inflammatory responses, a mechanism which may underlie the effect of Zn supplementation on adiposity. Accordingly, studies that reviewed the effects of Zn on adipokines and other hormones involved in adipogenesis (leptin, ghrelin, adiponectin and insulin) were also screened as secondary outcomes for the present review.
Evaluation of studies
Information on study outcomes, body composition measures, and the location, study design, sample size and characteristics was extracted from each included study. The reviewed studies were heterogeneous in design and outcomes; therefore statistical pooling was not possible. Instead, findings are presented in narrative form in which bivariate and/or multivariate associations between Zn supplementation and adiposity and adiposity-related hormones are summarized in tables and text to aid data presentation where appropriate. As such, an effect of Zn supplementation was defined as a significant difference in body composition between Zn and control groups for at least one parameter of body composition or adiposity-related hormone.
Results
Search results
The initial search identified 116 articles of which forty-two were identified as potentially eligible for inclusion after the screening of titles and abstracts. These articles were retrieved and reviewed in full, leading to the selection of sixteen articles reporting the effect of Zn supplementation on children’s body composition in randomized controlled trials and five studies reporting Zn supplementation on adiposity-related hormones. Two of the studies on Zn supplementation on body composition( Reference Arsenault, Lopez de Romana and Penny 37 , Reference Diaz-Gomez, Domenech and Barroso 38 ) were considered as randomized controlled trials although the children were randomized into different treatment arms/multiple treatments instead of a placebo group. Two of the studies of Zn supplementation on body composition( Reference Castillo-Duran, Garcia and Venegas 17 , Reference Walravens, Hambidge and Koepfer 39 ) and one study of Zn supplementation on adiposity-related hormones( Reference Ninh, Thissen and Collette 16 ) were excluded due to their focus on childhood growth, morbidity and motor development, with no findings reported on the outcomes of interest. One study( Reference Hashemipour, Kelishadi and Shapouri 24 ) was also excluded due to duplicate publication. This left fourteen studies on body composition and three studies( Reference Arsenault, Havel and Lopez de Romana 40 – Reference Kelishadi, Hashemipour and Adeli 42 ) on adiposity-related hormone outcomes meeting the inclusion criteria and so were included in the present review (Fig. 1).
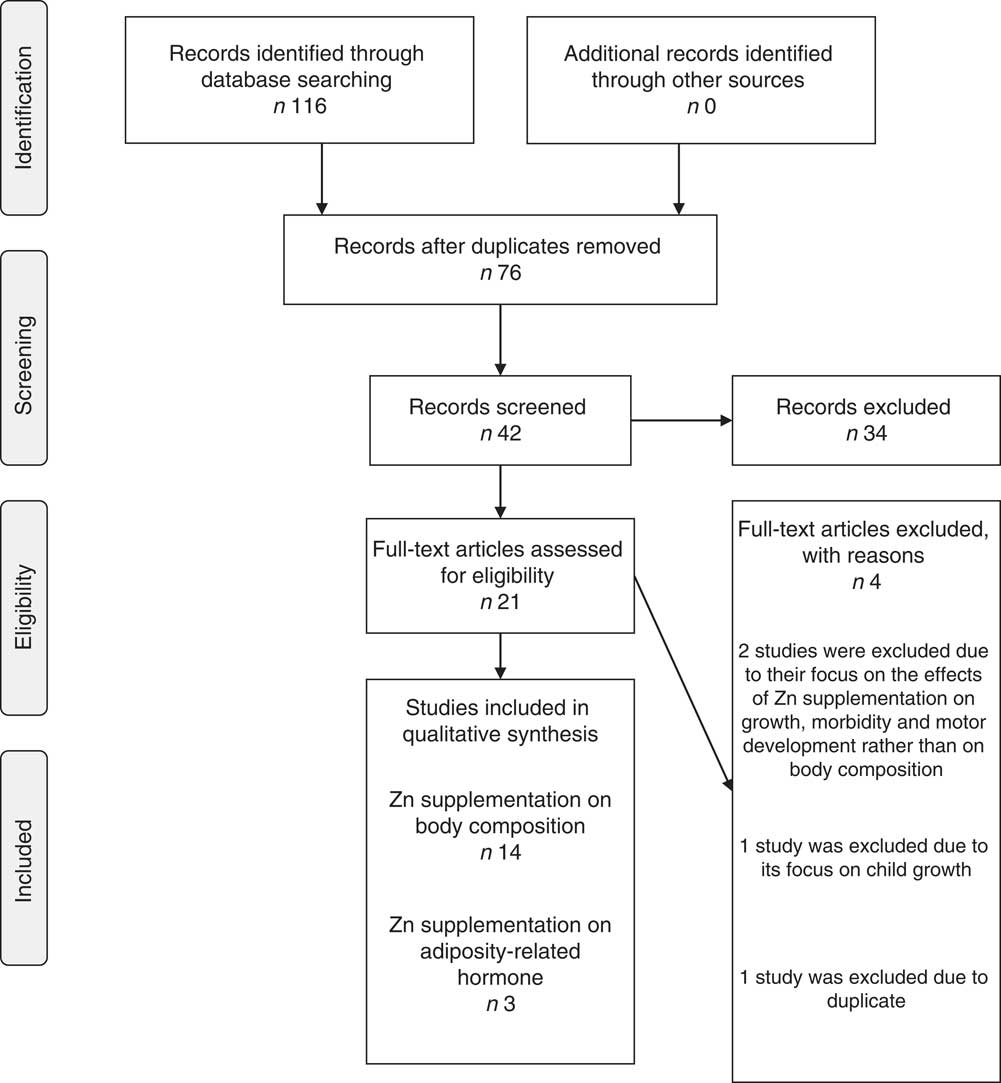
Fig. 1 Systematic review flowchart (adapted from Moher D, Liberati A, Tetzlaff J, Altman DG; The PRISMA Group (2009) Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med 6, e1000097. doi:10.1371/journal.pmed1000097)
Overview of findings
Table 1 presents the summary characteristics of reviewed studies and Table 2 presents the method used for assessing body composition and main findings. Out of the fourteen studies of Zn supplementation on body composition, ten studies( Reference Arsenault, Lopez de Romana and Penny 37 , Reference Diaz-Gomez, Domenech and Barroso 38 , Reference Kelishadi, Hashemipour and Adeli 42 – Reference Radhakrishna, Hemalatha and Geddam 49 ) reported an overall or subgroup effect on at least one parameter of body composition, while the remaining four found no effects( Reference Rosado, Lopez and Munoz 50 – Reference Ruz, Castillo-Duran and Lara 53 ).
Table 1 Zinc supplementation studies on body composition

HAZ, height-for-age Z-score; LAZ, length-for-age Z-score; SES, socio-economic status; CDC, US Centers for Disease Control and Prevention; MUAC, mid upper-arm circumference; TSF, triceps skinfold; MAMA, mid upper-arm muscle area; TWB, total body water; CRP, C-reactive protein.
Table 2 Zinc supplementation studies and body composition outcome

DXA, dual-energy X-ray absorptiometry; FFM, fat-free mass; FM, fat mass; %BF, percentage body fat; MUAC, mid upper-arm circumference; TSF, triceps skinfold; MAFA, mid upper-arm fat area; MAMA, mid upper-arm muscle area; BIA, bio-impedance analysis; TBW, total body water; %FM, percentage fat mass; LAZ, length-for-age Z-score; WAZ, weight-for-age Z-score; HAZ, height-for-age Z-score; SES, socio-economic status; SSF, subscapular skinfold; SE, standard estimate; LSM, least-square mean.
Among the ten studies( Reference Arsenault, Lopez de Romana and Penny 37 , Reference Diaz-Gomez, Domenech and Barroso 38 , Reference Kelishadi, Hashemipour and Adeli 42 – Reference Radhakrishna, Hemalatha and Geddam 49 ) which reported an effect of Zn on body composition, three studies used direct measurements of body composition as well as the indirect measures( Reference Arsenault, Lopez de Romana and Penny 37 , Reference Diaz-Gomez, Domenech and Barroso 38 , Reference Zemel, Kawchak and Fung 46 ). Diaz-Gomez et al.( Reference Diaz-Gomez, Domenech and Barroso 38 ) reported higher mean values of TBW estimated by BIA among Zn-supplemented preterm infants in 6 months. No significant differences in subcutaneous fat accretion were found between children in the Zn and placebo groups, suggesting a positive effect of supplementation on FFM. Similarly, hospitalized children aged 4–10 years with sickle cell anaemia who were supplemented with Zn over 12 months were found to have greater MUAC and MAMA Z-scores and improved rates of linear growth compared with children receiving the placebo, while no significant differences were found for body composition (whole-body FFM, FM and %BF) when using DXA( Reference Zemel, Kawchak and Fung 46 ). However, girls who received the Zn supplement had a significant 0·87 kg increase in FFM. In the study by Arsenault et al.( Reference Arsenault, Lopez de Romana and Penny 37 ), no effect of Zn supplementation was found on overall body composition among Peruvian children aged 6–8 months in 6 months. However, children with mild-to-moderate stunting (length-for-age Z-score <−1·1) who received a Zn supplement with Fe-fortified porridge had a greater increase in FFM and increase in mean TBW compared with children in the two other groups (Fe-fortified porridge plus Zn and control groups). These findings suggested that Zn supplementation may have a beneficial effect on FFM, especially among children with pre-existing growth failure.
Seven out of the ten studies( Reference Arsenault, Lopez de Romana and Penny 37 , Reference Diaz-Gomez, Domenech and Barroso 38 , Reference Kelishadi, Hashemipour and Adeli 42 – Reference Radhakrishna, Hemalatha and Geddam 49 ) which reported an effect of Zn on body composition used only indirect measures of body composition. Friis et al.( Reference Friis, Ndhlovu and Mduluza 44 ) reported that Zn-supplemented children (6–17 years old) in 12 months had significantly greater increases in their MAMA-for-age Z-score (an indication of increased FFM) compared with children receiving the placebo. Their study also reported significant increases in weight gain while no effect was found on FM and linear growth. Rivera et al.( Reference Rivera, Ruel and Santizo 47 ) in their study among rural Guatemalan infants (6–9 months) found that Zn supplementation for 7 months was associated with an increase of 0·61 cm2 in MAMA while at the same time it increased linear growth of children who were stunted at baseline. A 15-month Zn supplementation trial among Gambian children aged 6 months to 2 years reported a linear increase in body weight and a very small (2 %) but significant increase in MUAC or MAMA among Zn-supplemented children( Reference Bates, Evans and Dardenne 48 ). Kikafunda et al.( Reference Kikafunda, Walker and Allan 45 ) found that Zn supplementation among Ugandan children (aged 33–89 months) in 6 months significantly increased MUAC but had no effect on height, weight and height-for-age Z-score. A study carried among obese Iranian children (aged 6–10 years) in 8 weeks reported a significant decrease in mean weight and mean BMI and BMI Z-score, respectively, after Zn supplementation( Reference Kelishadi, Hashemipour and Adeli 42 ). In a study carried out in peri-urban communities of Guatemala, Cavan et al.( Reference Cavan, Gibson and Grazioso 43 ) reported that primary-school children aged 6–7 years supplemented with Zn for 6 months had significantly increased median triceps skinfold Z-score (a measure of fat status) but a small decrease in median MUAC Z-score. Radhakrishna et al.( Reference Radhakrishna, Hemalatha and Geddam 49 ) reported that Zn supplementation of full-term normal infants (<37 weeks) for a mean period of 190 d had significant effect on skinfold thicknesses, but not on linear growth.
The four studies that found no significant effect of supplementation on measures of body composition were carried out in very different settings; all of them used indirect measures of body composition (Table 3). Rosado et al.( Reference Rosado, Lopez and Munoz 50 ) reported the lack of effect of a 12-month Zn supplementation among Mexican children (18–36 months) on anthropometry measurements. A study in Ethiopia carried out by Umeta et al.( Reference Umeta, West and Haidar 51 ) found that Zn supplementation among children aged 6–12 months for 6 months did not have any measurable effects on body composition although it increased the length of stunted infants. Heinig et al.( Reference Heinig, Brown and Lonnerdal 52 ) found no effect of Zn on anthropometric indices among breast-fed infants (4–10 months) followed for 10 months. Similarly, Ruz et al.( Reference Ruz, Castillo-Duran and Lara 53 ) reported no effect of Zn supplementations on body composition among pre-school children (aged 27–50 months) followed for 14 months, although there was a significant trend for increased MAMA among supplemented boys.
Table 3 Studies that did not find any effect of zinc supplementation on body composition

MUAC, mid upper-arm circumference; TSF, triceps skinfold; MAFA, mid upper-arm fat area; MAMA, mid upper-arm muscle area; SE, standard estimate.
Three studies concerned with the effect of Zn supplementation on adiposity-related hormone levels were included in the present review (Table 4). Kelishadi et al.( Reference Kelishadi, Hashemipour and Adeli 42 ) found that supplementing children (aged 6–10 years) with 20 mg Zn/d in 8 weeks was associated with significantly reduced serum leptin and insulin. In contrast, Arsenault et al.( Reference Arsenault, Havel and Lopez de Romana 40 ) found no effect on plasma leptin, ghrelin and insulin concentrations among 6-month-old children supplemented with 3 mg ZnSO4/d in 7 months. The third study by Bueno et al.( Reference Bueno, Bueno and Moreno 41 ) found no effect of Zn supplementation for 6 months on serum leptin concentrations among newborns with intra-uterine growth retardation and asymmetric growth retardation. Interestingly, serum leptin concentrations correlated significantly with changes in skinfold measurements and weight-for-age Z-score among children in the placebo group. There was no clear association between Zn formulation and its dosage on hormonal levels from those three studies.
Table 4 Zinc supplementation studies on adiposity-related hormonal levels

LAZ, length-for-age Z-score; CDC, US Centers for Disease Control and Prevention; TBW, total body water; IGF-I, insulin-like growth factor-I, IGFBP-3, insulin-like growth factor binding protein-3; WC, waist circumference; hs-CRP, high-sensitivity C-reactive protein; IR, insulin resistance.
Discussion
The present systematic review of the effects of Zn supplementation on childhood body composition can only be inconclusive at this point due to the small number of trials and their diverse study designs. There is evidence that Zn supplementation overall has an effect on body composition when determined by anthropometric measurements, i.e. increased MUAC( Reference Cavan, Gibson and Grazioso 43 , Reference Kikafunda, Walker and Allan 45 , Reference Zemel, Kawchak and Fung 46 , Reference Bates, Evans and Dardenne 48 ), TSF( Reference Cavan, Gibson and Grazioso 43 , Reference Radhakrishna, Hemalatha and Geddam 49 ), subscapular skinfold( Reference Radhakrishna, Hemalatha and Geddam 49 ) and MAMA( Reference Friis, Ndhlovu and Mduluza 44 , Reference Zemel, Kawchak and Fung 46 , Reference Rivera, Ruel and Santizo 47 ), and decreased BMI( Reference Kelishadi, Hashemipour and Adeli 42 ). Zn supplementation also showed an effect on body composition when measured by a direct method: i.e. increased mean values of TBW estimated by BIA( Reference Arsenault, Lopez de Romana and Penny 37 , Reference Diaz-Gomez, Domenech and Barroso 38 ), especially among children with mild-to-moderate stunting( Reference Arsenault, Lopez de Romana and Penny 37 ); increased FFM estimated by TBW( Reference Arsenault, Lopez de Romana and Penny 37 ); and increase in FFM assessed by DXA in girls who received the Zn supplement( Reference Zemel, Kawchak and Fung 46 ). Only one study( Reference Kelishadi, Hashemipour and Adeli 42 ) found that Zn supplementation was associated with reduced serum leptin and insulin. Thus, Zn supplementation may have a beneficial effect on body composition, especially on FFM among children with pre-existing growth failure.
These findings suggest that the effect of Zn supplementation on body composition may be less consistent than its effect on growth. Three previously published meta-analyses of Zn supplementation trials on growth have reported strong evidence of the growth-limiting effect of Zn deficiency( Reference Brown, Peerson and Allen 25 , Reference Brown, Peerson and Rivera 54 , Reference Stammers, Lowe and Medina 55 ). Zn directly influences the growth hormone and insulin-like growth factor-I systems( Reference Dorup and Clausen 56 ), affects bone metabolism( Reference Nishi 57 ) and is involved in DNA synthesis, all of which may affect linear growth( Reference Bunce 58 ). However, the effect of Zn supplementation on body composition may depend on the pre-existing nutritional status of children. The study by Arsenault et al.( Reference Arsenault, Lopez de Romana and Penny 37 ) suggests that pre-existing Zn deficiency may be contributing to the development of obesity via its relationship with fat deposition associated with childhood stunting.
It is not clear whether the observed changes of body composition resulted from changes in FM or FFM and whether stunted children are at greater risk of obesity as a result of such changes, since only a few studies reported the findings for FFM and FM( Reference Arsenault, Lopez de Romana and Penny 37 , Reference Zemel, Kawchak and Fung 46 ) from direct measures and most of the studies used the anthropometric method in determining body composition. However, Zn is essential for lean body mass synthesis and its deficiency was reported to increase the energy cost of tissue deposition( Reference Golden and Golden 18 ). Zn deficiency may also cause altered fatty acid metabolism leading to an increase in FM( Reference Golden and Golden 18 , Reference Park, Grandjean and Antonson 26 ). It is important, therefore, to concurrently study how the effect of Zn supplementation on body fat or FFM relates to its effect on linear growth.
Reduced Zn absorption and bioavailability, the combination of Zn with other micronutrients and the form by which Zn is delivered may be determining the efficacy of Zn on body composition( Reference Lonnerdal 59 ). Zn and Fe, for example, compete for mucosal binding sites as well as in the absorption process( Reference Sandstrom, Davidsson and Cederblad 60 ). Dietary Fe:Zn greater than 2:1 will inhibit Zn absorption, as the Fe carrier, transferrin, which also carries Zn, becomes saturated( Reference Fleet 61 ). Zn is absorbed most efficiently from aqueous solutions when Zn is in solution form, but not when it is part of a complex meal( Reference Brown, Rivera and Bhutta 62 ). Zn-fortified foods generally produce a small reduction in fractional absorption, although a positive impact on net absorption( Reference Lopez de Romana, Salazar and Hambidge 63 ). The study by Arsenault et al.( Reference Arsenault, Lopez de Romana and Penny 37 ) included Zn in a liquid multivitamin supplement and in a wheat-based, Fe-fortified porridge, both of which could reduce Zn absorption.
Zn status can have an effect on adipokines and other hormones involved in adipogenesis which can determine body composition and risk of obesity. The adipokine leptin that is produced primarily in adipose tissue regulates food intake and energy expenditure( Reference Rohner-Jeanrenaud and Jeanrenaud 64 ) and is positively associated with body weight( Reference Klok, Jakobsdottir and Drent 65 ), BMI, %BF and FM among children( Reference Ellis and Nicolson 66 , Reference Dencker, Thorsson and Karlsson 67 ). Zn deficiency leads to reduced serum leptin concentration in rats( Reference Baltaci, Mogulkoc and Halifeoglu 68 ) and man( Reference Mantzoros, Prasad and Beck 69 ), and reduced leptin secretion by rat adipocytes while repletion reversed this effect( Reference Chen, Song and Lin 70 ). However, two of the three studies that examined the effect of Zn on adipokines and other hormones involved in adipogenesis found no clear effect.
A child’s overall adiposity may contribute to this lack of an effect of Zn on leptin and subsequently body composition, since leptin levels reflect the amount of energy stored in somatic adipose tissue in man and other mammals( Reference Mantzoros 71 , Reference Friedman and Halaas 72 ) and are highly correlated with body fat in both adults and children( Reference Moore, Falorni and Bini 73 , Reference Considine, Sinha and Heiman 74 ). Sex may also modify the effect of Zn on leptin since studies have reported that women have markedly higher leptin concentrations than men for any given degree of FM( Reference Jequier 75 ) and this may be present at birth( Reference Jaquet, Leger and Levy-Marchal 76 ). In addition, infection and/or inflammation may alter the effect of Zn on leptin since the induction of leptin is part of the acute-phase response to inflammatory stimuli such as lipopolysaccharide and pro-inflammatory cytokines( Reference Sarraf, Frederich and Turner 77 ).
Sources of study outcome heterogeneity
There were important differences between studies in factors that may have modified associations between Zn supplementation and body composition and so contributed to the inconsistent findings. Most importantly, methods used in measuring body composition varied between the different studies. All of the Zn supplementation studies included in the present review used anthropometry as indirect measures to determine body composition while only three studies used more direct methods such as DXA, BIA and 2H dilution in assessing body composition( Reference Arsenault, Lopez de Romana and Penny 37 , Reference Diaz-Gomez, Domenech and Barroso 38 , Reference Zemel, Kawchak and Fung 46 ). Anthropometric methods have poor accuracy and precision when used alone and so have reduced utility for predicting body adiposity outcomes compared with direct methods( Reference Wells and Fewtrell 36 ). The direct method of body composition assessment is more ideal where the aim is to quantify FM or FFM with greater accuracy( Reference Wells and Fewtrell 36 ).
The initial nutritional status of children varied between the different studies and so may have modified the effects of Zn supplementation. An effect on body composition was found in three( Reference Kikafunda, Walker and Allan 45 – Reference Rivera, Ruel and Santizo 47 ) of the six studies where study subjects were growth stunted( Reference Arsenault, Lopez de Romana and Penny 37 , Reference Kikafunda, Walker and Allan 45 , Reference Rivera, Ruel and Santizo 47 , Reference Rosado, Lopez and Munoz 50 , Reference Umeta, West and Haidar 51 ) and in children with sickle cell disease whose stature was below 2 sd ( Reference Zemel, Kawchak and Fung 46 ). The study by Arsenault et al.( Reference Arsenault, Lopez de Romana and Penny 37 ) did not find an overall effect of Zn supplementation on FFM but did find an increase in FFM among mild-to-moderately stunted children (length-for-age Z-score <−1·1). Interestingly, this finding suggests that a positive response to Zn supplementation of body composition is more likely to be apparent among children with pre-existing growth failure. Diaz-Gomez et al.( Reference Diaz-Gomez, Domenech and Barroso 38 ) found an improvement in TBW among premature infants in the Zn group (birth weight between 1000 and 2500 g). Two of four studies conducted among generally healthy normal children also found a positive effect of Zn supplementation on lean body mass( Reference Friis, Ndhlovu and Mduluza 44 ) and a very small but significant increase in MUAC or MAMA( Reference Bates, Evans and Dardenne 48 ).
It is not clear whether children’s initial Zn status and serum adipokine concentrations may also have modified the effect of Zn supplementation on body composition. One( Reference Cavan, Gibson and Grazioso 43 ) out of two studies( Reference Cavan, Gibson and Grazioso 43 , Reference Rosado, Lopez and Munoz 50 ) where children had a high initial Zn status (>13·5 µmol/l) reported an effect of Zn supplementation on body composition; while an effect was also found in three studies among children with low initial Zn status( Reference Kelishadi, Hashemipour and Adeli 42 , Reference Friis, Ndhlovu and Mduluza 44 , Reference Zemel, Kawchak and Fung 46 ). The studies concerned with the effect of Zn on adiposity-related hormones did report the initial Zn status of children but did not consistently report on the initial hormone levels.
The inclusion of other micronutrients in the control group may also contribute to the inconsistent findings. Five studies compared the effect of Zn supplementation on body composition with the effects produced with multi-micronutrients( Reference Arsenault, Lopez de Romana and Penny 37 , Reference Diaz-Gomez, Domenech and Barroso 38 , Reference Cavan, Gibson and Grazioso 43 , Reference Radhakrishna, Hemalatha and Geddam 49 , Reference Rosado, Lopez and Munoz 50 ) while nine studies compared Zn with a placebo group. The supplementation regimen for the treatment groups in two studies( Reference Arsenault, Lopez de Romana and Penny 37 , Reference Arsenault, Havel and Lopez de Romana 40 ) was a wheat-based, Fe-fortified porridge and a liquid multivitamin. Zn supplementation was found to have an effect on body composition (increase in FFM) among the subset of children with mild-to-moderate stunting. In the study by Cavan et al.( Reference Cavan, Gibson and Grazioso 43 ), significantly higher median Z-scores for MUAC and TSF were found in the Zn-supplemented group when children in both the Zn and placebo groups were provided with multi-micronutrient supplements. In contrast, Zn and Zn plus Fe supplements had no effect on growth and/or body composition in the study by Rosado et al.( Reference Rosado, Lopez and Munoz 50 ). Diaz-Gomez et al.( Reference Diaz-Gomez, Domenech and Barroso 38 ) reported an improvement in TBW among children in the Zn group fed with a standard term formula supplemented with Zn (final content: 10 mg/l) and a small quantity of Cu (final content: 0·6 mg/l); the placebo group received the same formula without supplementation (final content of Zn: 5 mg/l; final content of Cu: 0·4 mg/l), and an Fe supplement was given to all children once daily during the study period. These findings suggest that combining micronutrients may dilute the effects of Zn( Reference Lonnerdal 59 ). A trial that supplemented Chinese obese women with Zn combined with other micronutrients did report reductions in body weight, BMI and abdominal obesity and improvement in serum lipid profiles( Reference Li, Wang and Zhu 8 ).
Children’s age and sex may be a source of heterogeneity in findings. For example, two studies( Reference Cavan, Gibson and Grazioso 43 , Reference Friis, Ndhlovu and Mduluza 44 ) conducted among school-aged children found an effect of Zn supplementation while one study( Reference Kikafunda, Walker and Allan 45 ) reported an effect among pre-school children. Studies that included infants( Reference Diaz-Gomez, Domenech and Barroso 38 ), 5–7-month-old children( Reference Arsenault, Lopez de Romana and Penny 37 ) and 4–10-year-old children( Reference Zemel, Kawchak and Fung 46 ) found no effect. The modification of Zn supplementation effect on hormone levels by age is difficult to determine since there were only limited studies of Zn supplementation on adiposity-related hormone levels( Reference Arsenault, Havel and Lopez de Romana 40 – Reference Kelishadi, Hashemipour and Adeli 42 ). There are significant sex differences in body composition before the onset of puberty. Prepubertal girls generally have higher total body fat and %BF but lower FFM( Reference Mast, Kortzinger and Konig 78 ) than do boys matched for age, weight and height. Fat distribution also differs between sexes, with prepubertal girls generally having greater trunk fat than do boys( Reference Arfai, Pitukcheewanont and Goran 79 ). Thus, the pattern of the effect of Zn supplementation on body composition might not be consistent if studies have children of different age groups. In certain age ranges children might have different impacts by Zn supplementation on body composition.
The formulation, dosage and duration of Zn supplementation may also have contributed to the inconsistencies in the effect of Zn, since these varied considerably between the different studies. An effect of Zn on body composition was found in seven studies when ZnSO4 was used as a supplement. Studies that used Zn as an amino acid chelate( Reference Cavan, Gibson and Grazioso 43 ), elemental Zn( Reference Kelishadi, Hashemipour and Adeli 42 ), and Zn acetate and Zn gluconate( Reference Bates, Evans and Dardenne 48 ) also showed an effect. The dosages of Zn supplementation varied considerably (from 3 to 70 mg/d) between the different studies. Dosages of 10 mg/d given to school-aged children( Reference Cavan, Gibson and Grazioso 43 ), 10 mg/d given to pre-school children( Reference Kikafunda, Walker and Allan 45 ) and 30 and 50 mg/d given to schoolchildren whose weight was below 29·5 kg and ≥29·5 kg, respectively( Reference Friis, Ndhlovu and Mduluza 44 ), all had effects on body composition. Studies that gave 20 mg Zn/d to pre-school children, 10 mg Zn/d to school-aged children( Reference Zemel, Kawchak and Fung 46 ) and 10 mg Zn/d to infants( Reference Diaz-Gomez, Domenech and Barroso 38 ) found no effect on body composition. Another study by Arsenault et al.( Reference Arsenault, Lopez de Romana and Penny 37 ), which supplemented children with 3 mg Zn/d (either in liquid supplement or fortified porridge), showed no effect. Four studies( Reference Diaz-Gomez, Domenech and Barroso 38 , Reference Cavan, Gibson and Grazioso 43 , Reference Kikafunda, Walker and Allan 45 , Reference Rivera, Ruel and Santizo 47 ) which supplemented children with Zn for 6–7 months and three studies( Reference Friis, Ndhlovu and Mduluza 44 , Reference Zemel, Kawchak and Fung 46 , Reference Bates, Evans and Dardenne 48 ) which supplemented children with Zn for 10–15 months reported an effect on body composition. The findings suggest that at least 6 months of Zn supplementation can have an effect on body composition but differing dosages and the formulation of Zn have no clear association with body composition.
Additional sources of heterogeneity that may modify study outcomes are dietary Zn intake, dietary factors influencing Zn absorption and morbidity/illness. Although the influence of diet and morbidity were not adequately tested in the reviewed analyses, ten studies( Reference Arsenault, Lopez de Romana and Penny 37 , Reference Diaz-Gomez, Domenech and Barroso 38 , Reference Friis, Ndhlovu and Mduluza 44 – Reference Bates, Evans and Dardenne 48 , Reference Umeta, West and Haidar 51 – Reference Ruz, Castillo-Duran and Lara 53 ) carried out dietary data collection to determine mean daily intakes of energy, protein, Zn( Reference Friis, Ndhlovu and Mduluza 44 – Reference Zemel, Kawchak and Fung 46 ), phytic acid( Reference Kikafunda, Walker and Allan 45 ) and Cu( Reference Diaz-Gomez, Domenech and Barroso 38 ). In addition, the children were generally selected from low socio-economic areas( Reference Friis, Ndhlovu and Mduluza 44 , Reference Kikafunda, Walker and Allan 45 , Reference Rivera, Ruel and Santizo 47 – Reference Radhakrishna, Hemalatha and Geddam 49 ) in which Zn deficiency prevalence is usually high due to poor dietary intake. Only one study( Reference Arsenault, Havel and Lopez de Romana 40 ) concerned with the effect of Zn supplementation on adipokines measured the dietary intake of Zn and the relative content of phytic acid to Zn in ingested food, although it was reported in another paper( Reference Arsenault, Lopez de Romana and Penny 37 ) that reported the body composition results.
Another source of outcome heterogeneity was compliance. For example, the study by Cavan et al.( Reference Cavan, Gibson and Grazioso 43 ) had a low average number of treatment days because of children’s frequent absences from school. Zn supplementation was administered only on school days in the studies by Friis et al.( Reference Friis, Ndhlovu and Mduluza 44 ) and Kikafunda et al.( Reference Kikafunda, Walker and Allan 45 ), resulting in sporadic intake. Similarly, the mild effect of Zn supplementation on MUAC reported by Kikafunda et al.( Reference Kikafunda, Walker and Allan 45 ) may partly result from high study attrition and low compliance.
There are limitations of the current review, which include publication bias from selective inclusion of studies published in English and the lack of statistical pooling of the studies.
Conclusions
Overall, the present review has found that the effect of Zn supplementation on body composition may not be consistent. The review has suggested that Zn supplementation may have a beneficial effect on body composition, especially on FFM among children with pre-existing growth failure. However, variable findings resulting from technical difficulties in measuring body composition in community settings still need to be addressed. A majority of the studies could not accurately address whether alterations in the FM and/or FFM components of the body were responsible for the observed weight or body composition changes due to the use of anthropometry when determining body composition.
Further well-designed studies which measure body composition directly and address the limitations from previous studies are required. The determination of how adiposity-related hormone concentrations relate to body composition among supplemented children that differ in nutritional status may further clarify these relationships. Confirmation that Zn has an effect on body composition would allow the development of new public health interventions that may contribute to efforts to reduce the long-term risk of stunting, obesity and related diseases in the context of the global obesity pandemic.
Acknowledgements
Acknowledgements: The authors acknowledge the assistance of Assistant Professor Geoff C. Marks for his input on the research method. Financial support: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors. Conflict of interest: None. Authorship: I.R.G. conceived the study and developed the overall research plan under supervision of K.Z.L. and A.A.-M. at all stages of its implementation. I.R.G. and K.Z.L. wrote the manuscript and have primary responsibility for final content. L.S. and A.A.-M. contributed in the final content. All authors read and approved the final manuscript. Ethics of human subject participation: Not applicable.








