Evidence regarding the effect of malnutrition on pregnancy outcomes has been extensively documented(Reference Triunfo and Lanzone1–Reference Abu-Saad and Fraser4), with micronutrient supplement interventions traditionally focused on low- and middle-income countries(Reference Bhutta, Das and Rizvi5). This is in part due to the disproportionate rate of poor perinatal outcomes attributable to malnutrition compared to high-income regions(Reference Beck, Wojdyla and Say6,Reference Kavle and Landry7) . However, suboptimal nutrition also represents a significant public health concern in high-income countries as a result of undernourishment, over-nourishment or micronutrient deficiency(8). Despite this knowledge, women from high-income countries represent an under-researched population(Reference Wolf, Hegaard and Huusom2).
Over recent years, the Cochrane Database of Systematic Reviews has thoroughly examined published research regarding the effect of multiple micronutrient supplements (MuMS)(Reference Keats, Haider and Tam3), folic acid(Reference Lassi, Salam and Haider9), iron(Reference Peña-Rosas, De-Regil and Garcia-Casal10), zinc(Reference Ota, Mori and Middleton11), calcium(Reference Buppasiri, Lumbiganon and Thinkhamrop12), iodine(Reference Harding, Peña-Rosas and Webster13) and vitamin D(Reference De-Regil, Palacios and Lombardo14) on pregnancy outcomes. However, the majority of studies exhibited vast heterogeneity in data, cohorts, context and examined outcomes, with many reporting low or no evidence of significant benefits of supplements in low-risk women and high-income countries. Further, each Cochrane review cautiously highlights the need for further randomised controlled trials in view of perceived pregnancy risks and ethical considerations(Reference Keats, Haider and Tam3,Reference Lassi, Salam and Haider9–Reference Harding, Peña-Rosas and Webster13) . Despite these limitations, systematic reviews remain the foundation of clinical practice recommendations(15–17).
This is potentially problematic in that the effects of supplementation in the absence of identified deficiency, low-risk pregnancies and high-income countries are largely unknown. This review and synthesis of contemporary empirical research aimed to determine how, where and when empirical research was conducted, the cohorts studied, and who the major publishers are in this field. In addition, this review identifies what has been most commonly studied in terms of demographics, outcomes, supplements and their timing over the last decade, and synthesises a consensus of findings relating to the effect of micronutrient supplements on the birth outcomes of low-risk pregnancies in high-income countries. Identified research deficits and implications for policy and practice are subsequently discussed.
Methods
The systematic quantitative literature review
Systematic literature reviews are commonly used in the health sector and are an important component of evidence-based health care, assisting in the development and maintenance of guidelines and policies, institutional and personal practice(Reference Boren and Moxley18). However, while systematic reviews aid in the identification of knowledge gaps, they do not necessarily highlight research deficits(Reference Pickering and Byrne19). The systematic quantitative literature review is emerging in a range of scientific disciplines as an appropriate methodology in heterogeneous research contexts. The quantitative approach facilitates an analysis of varying approaches in discipline, design, context, intent and methodology(Reference Pickering and Byrne19). This review is systematic in that a structured framework has been used to establish project parameters, assess the literature and construct a database of literature for review according to PRISMA guidelines(Reference Moher, Liberati and Tetzlaff20).
The Pickering and Byrne(Reference Pickering and Byrne19) approach utilises a unique fifteen-step process that encourages researchers to develop a categorisation framework based on an iterative and inductive process to develop, implement and present their review – a process consistent with but more detailed than traditional systematic reviews(Reference Wakefield21,Reference Whittemore and Knafl22) . A significant benefit of this quantitative methodology is that the examination technique expands rather than refines the depth of the review(Reference Pickering and Byrne19); research gaps are easily identified under these systematic conditions in addition to research ‘hotspots’. This differs from traditional systematic reviews in which the examination commences with a broad foundation, and content is deductively reduced, limiting the scope of the review to collation of current knowledge and relying on deductive reasoning to identify research deficits(Reference Pickering and Byrne19). As such, this approach is appropriate for areas of research with a diversity of scope(Reference Pickering and Byrne19), a feature demonstrated by the literature regarding supplementation during pregnancy, the range of micronutrients, outcomes, settings and demographic groups studied, and the methodologies employed to do so.
This systematic review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) recommendations(Reference Moher, Liberati and Tetzlaff20) and conforms to the systematic quantitative literature review approach described by Pickering and Byrne(Reference Pickering and Byrne19). The PRISMA flowchart (Fig. 1) details the review process; methods and results are detailed according to PRISMA reporting criteria.
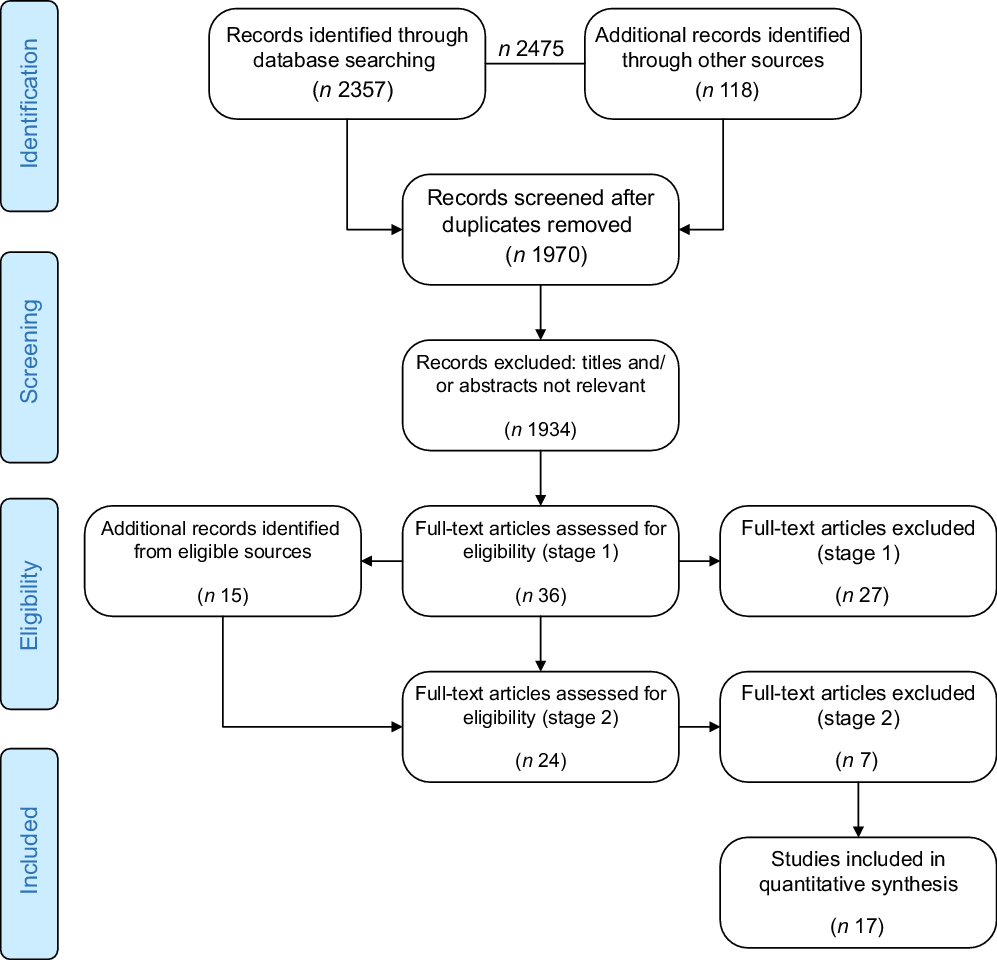
Fig. 1 PRISMA flow diagram(Reference Moher, Liberati and Tetzlaff41)
Search strategy
To identify papers examining the effects of micronutrient supplementation on the birth outcomes of low-risk pregnancies in high-income countries, a systematic search was conducted using the electronic databases CINAHL, EMBASE, MEDLINE, PubMed and Google Scholar from 1 March 2009 to 1 March 2019, a timeframe determined by the recent systematic reviews(Reference Keats, Haider and Tam3,Reference Lassi, Salam and Haider9–Reference Harding, Peña-Rosas and Webster13) . A literature search was conducted using a combination of the following keywords: (supplement* OR vitamin* OR mineral OR micronutrient OR ‘micro nutrient’ OR micro-nutrient) AND (‘high income’ OR developed OR industrial* OR ‘first world’) AND ((birth OR pregnanc*) AND outcomes). Studies were considered eligible if original, peer-reviewed, detailing empirical data pertaining to supplementation in low-risk pregnancies (including the periconception period), examining live birth outcomes in relation to supplementation in either primary or secondary capacity, undertaken on women living in high-income countries according to 2019 indexes(23), and published in English language within the last 10 years.
Selection criteria
All papers were screened by the first author by title and/or abstract for suitability; discussion papers, papers examining supplementation in non-pregnant populations or low- and middle-income regions, or in the presence of known pregnancy risks, or reporting haematological values alone were excluded. Similarly, errant results with no reference to pregnancy outcomes and micronutrient supplementation were excluded. References cited by each study and meeting stage one eligibility were reviewed to identify additional potential studies; systematic reviews were excluded. Full-text versions of all studies passing this initial screening were reviewed in detail. A second screening was employed whereby a study was excluded if it did not meet the established parameters. Included and excluded papers were recorded at each screening stage according to the PRISMA statement. Risk of bias was assessed at a study level; each addressed the potential for bias within the individual research protocol or discussion. Two included papers were known to the authors and included as additional resources. Both papers met the selection criteria and, as such, selection bias was considered minimal. The analysis of reviewed articles is reported in terms of bibliographic details and research design, examined variables and key findings.
Categories
Characteristics regarding the nature and scope of research surrounding the effects of micronutrient supplementation on the outcomes of low-risk pregnancies in high-income countries were measured across a range of emergent categories(Reference Pickering and Byrne19).
The final categories comprised bibliographic details (authors, year, title, journal, genre), settings and methods (country (state, region), study/cohort, year of collection, funding source, number of participants, method (observational/experimental – single/double-blinded randomised controlled trial, single/multisite), descriptive groups (ethnicity, age group, parity, BMI, smoker, education, socioeconomic status), trimester (periconception, trimester 1, 2, 3, 1 & 2, 2 & 3 and 1, 2 & 3), supplementation (MuMS, folic acid, vitamin C/vitamin E, vitamin D, zinc, iron, calcium, DHA, supplement combinations) and birth outcomes (pre-eclampsia, gestational diabetes, induction of labour, birth < 37 weeks, birth > 41 weeks, gestation at birth, low birthweight/small for gestational age, birthweight). Subcategories were created on an emergent basis; all affirmative categories were recorded in a spreadsheet with value 1 and totalled at the end of analysis. The frequency of examined variables was documented along with reference numbers (online supplementary material).
Results
A total of 2475 potentially relevant studies were identified. Of the fifty-one full-text articles meeting stage one and two criteria, seventeen were retained for quantitative analyses in this systematic review(Reference Alwan, Greenwood and Simpson24–Reference Vanderlelie, Scott and Shibl40) (Fig. 1, Table 1). Of the thirty-four papers excluded, sixteen were systematic reviews(Reference Keats, Haider and Tam3,Reference Lassi, Salam and Haider9–Reference Ota, Mori and Middleton11,Reference Harvey, Holroyd and Ntani42–Reference Thorne-Lyman and Fawzi53) , four were systematic reviews with meta-analyses(Reference Wolf, Hegaard and Huusom2,Reference Wen, White and Rybak54–Reference Wagner, McNeil and Johnson56) and two presented meta-analyses alone(Reference Dror and Allen57,Reference Fekete, Berti and Trovato58) . A further six were discussion papers(Reference Buppasiri, Lumbiganon and Thinkhamrop12,Reference Berti, Biesalski and Gärtner59–Reference Curtis, Moon and Harvey63) , four included women with an identified risk(Reference Bánhidy, Dakhlaoui and Dudás64) or diagnosis of pregnancy-related morbidities (pre-eclampsia(Reference Haider, Olofin and Wang65,Reference Haider, Yakoob and Bhutta66) and gestational diabetes(Reference De-Regil, Palacios and Lombardo14)), one study was ceased due to adverse outcomes(Reference Harding, Peña-Rosas and Webster13) and one examined a particular micronutrient administered in a MuMS formulation(Reference Xu, Perez-Cuevas and Xiong67) (online supplementary material).
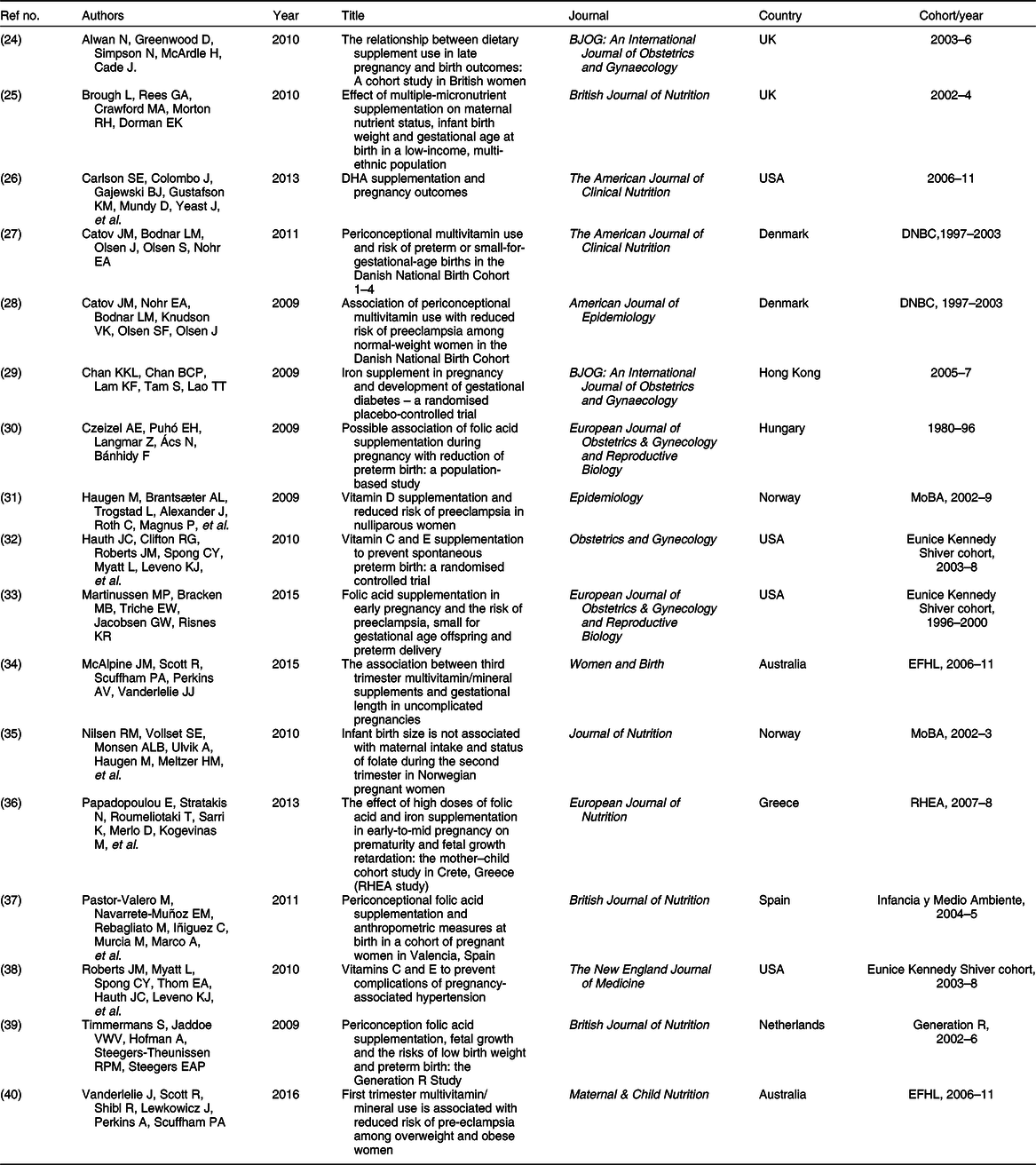
Table 1 List of papers included in this review
Bibliographic details and design
Authors and data pools
A total of 103 authors were credited with authorship across the eligible papers. Sixteen authors were responsible for first authorship of the seventeen; one author was responsible for first authorship of two papers(Reference Catov, Bodnar and Olsen27,Reference Catov, Nohr and Bodnar28) . Large birth cohorts were responsible for generating the majority of data: the Danish National Birth Cohort (DNBC 1997–2003, n 2)(Reference Catov, Bodnar and Olsen27,Reference Catov, Nohr and Bodnar28) , Norwegian Mother and Baby cohort (MoBA 2002–9, n 2)(Reference Haugen, Brantsæter and Trogstad31,Reference Nilsen, Vollset and Monsen35) , Environments for Healthy Living cohort (EFHL 2006–11, n 2)(Reference McAlpine, Scott and Scuffham34,Reference Vanderlelie, Scott and Shibl40) and the New England, USA dataset (collected with support from the Eunice Kennedy Shriver National Institute of Child Health and Human Development’s (NICHD) Maternal-Fetal Medicine Units Network 1996–2008, n 3)(Reference Hauth, Clifton and Roberts32,Reference Martinussen, Bracken and Triche33,Reference Roberts, Myatt and Spong38) , which all accounted for the bulk of eligible papers (Table 1). Additionally, RHEA (2007–8)(Reference Papadopoulou, Stratakis and Roumeliotaki36), Generation R (2002–6)(Reference Timmermans, Jaddoe and Hofman39) and Infancia y Medio Ambiente(Reference Pastor-Valero, Navarrete-Muñoz and Rebagliato37) cohorts (2004–5) contributed to the final data pool (n 147 323 women).
Five countries each contributed one unique cohort and one subsequent publication to the field of research (Table 1); Australia(Reference McAlpine, Scott and Scuffham34,Reference Vanderlelie, Scott and Shibl40) , Denmark(Reference Catov, Bodnar and Olsen27,Reference Catov, Nohr and Bodnar28) and Norway(Reference Haugen, Brantsæter and Trogstad31,Reference Nilsen, Vollset and Monsen35) each contributed one cohort from their respective countries; each of these cohorts contributed data to two unique papers. The United Kingdom added a further two studies on independent cohorts to the research(Reference Alwan, Greenwood and Simpson24,Reference Brough, Rees and Crawford25) . The United States was responsible for three studies originating from one cohort of women(Reference Martinussen, Bracken and Triche33), two of which reported the same intervention on different outcomes(Reference Hauth, Clifton and Roberts32,Reference Roberts, Myatt and Spong38) . The United States contributed an additional unique cohort and intervention to the pool of research(Reference Carlson, Colombo and Gajewski26) (Table 1).
Year of publication and research methodologies
The quantity of empirical studies has declined over the past decade. While 2009 and 2010 both returned five qualifying studies each, the years 2011–16 produced seven empirical studies in total. No research meeting these parameters was found after 2016 (Table 1). The majority were observational studies (n 12); of these, nine were conducted in multicentre collaborations (75·0 %). Multicentre research also accounted for two out of the five experimental studies(Reference Hauth, Clifton and Roberts32,Reference Roberts, Myatt and Spong38) (40 %), with the remainder being conducted with a single-centre approach(Reference Brough, Rees and Crawford25,Reference Carlson, Colombo and Gajewski26,Reference Chan, Chan and Lam29) . All experimental studies were randomised controlled trials; four of these used double-blinded methodologies (Table 1) with the remaining ones being blinded to participants but not researchers(Reference Chan, Chan and Lam29).
Genres and journals
Covering four genres, nutrition(Reference Brough, Rees and Crawford25–Reference Catov, Bodnar and Olsen27,Reference Nilsen, Vollset and Monsen35–Reference Pastor-Valero, Navarrete-Muñoz and Rebagliato37,Reference Timmermans, Jaddoe and Hofman39,Reference Vanderlelie, Scott and Shibl40) (n 8, 47·1 %), medicine(Reference Alwan, Greenwood and Simpson24,Reference Chan, Chan and Lam29,Reference Czeizel, Puhó and Langmar30,Reference Hauth, Clifton and Roberts32,Reference Martinussen, Bracken and Triche33,Reference Roberts, Myatt and Spong38) (n 6, 35·3 %), epidemiology(Reference Catov, Nohr and Bodnar28,Reference Haugen, Brantsæter and Trogstad31) (n 2, 11·8 %) and midwifery(Reference McAlpine, Scott and Scuffham34) (n 1, 5·9 %) journals produced all eligible articles. The British (Reference Brough, Rees and Crawford25,Reference Pastor-Valero, Navarrete-Muñoz and Rebagliato37,Reference Timmermans, Jaddoe and Hofman39) (n 3) and American (Reference Carlson, Colombo and Gajewski26,Reference Catov, Bodnar and Olsen27) Journals of Nutrition (n 2) published eligible research most frequently, along with the European Journal of Obstetrics & Gynecology and Reproductive Biology (Reference Czeizel, Puhó and Langmar30,Reference Martinussen, Bracken and Triche33) (n 2) and the British Journal of Obstetrics and Gynaecology (Reference Alwan, Greenwood and Simpson24,Reference Chan, Chan and Lam29) (n 2). A further eight journals published one study each(Reference Catov, Nohr and Bodnar28,Reference Haugen, Brantsæter and Trogstad31,Reference Hauth, Clifton and Roberts32,Reference McAlpine, Scott and Scuffham34–Reference Papadopoulou, Stratakis and Roumeliotaki36,Reference Roberts, Myatt and Spong38,Reference Vanderlelie, Scott and Shibl40) . These journals were produced by a range of publishers.
Variables examined
Supplements
In total, nine micronutrients – either alone or in combination – were examined with empirical research methodology in the specified timeframe (Table 1). Folic acid was the most frequently examined supplement (n 10). Of these ten studies, three examined the use of folic acid in combination with either a multivitamin (n 2)(Reference Czeizel, Puhó and Langmar30,Reference McAlpine, Scott and Scuffham34) or iron(Reference Papadopoulou, Stratakis and Roumeliotaki36) (n 1). MuMS were examined independently in five studies, iron in two(Reference Chan, Chan and Lam29,Reference McAlpine, Scott and Scuffham34) ; vitamins C and E in combination accounted for two studies(Reference Hauth, Clifton and Roberts32,Reference Roberts, Myatt and Spong38) and one study examined zinc and calcium both independently and in combination with all previously mentioned micronutrients(Reference McAlpine, Scott and Scuffham34). Studies evaluating the roles of DHA(Reference Carlson, Colombo and Gajewski26) (n 1) and vitamin D(Reference Haugen, Brantsæter and Trogstad31) (n 1) in pregnancy outcomes were performed in the absence of other supplements.
Outcomes
Reporting of birth outcomes was limited to gestation at birth (continuous, n 8; categorical, <37 weeks, n 10, >41 weeks, n 1), birthweight (continuous, n 8; low birthweight (<2500 g/small for gestational age <10th centile), n 12), onset of labour (n 2) and the development of hypertensive disorders during pregnancy (including pre-eclampsia, n 6; gestational diabetes, n 2; Table 1).
Timing of supplementation
Supplement use was examined across all three trimesters of pregnancy in six of the studies (Table 1). A further five examined supplement use in the periconception period only, that is, 3 months before conception through until 3 months after conception. Examination exclusively by trimester was performed by the minority (Table 1).
Demographic groups
Maternal age was recorded by all papers and reported as mean and standard deviation of a continuous variable in nine of the seventeen studies (Table 1). Categorisation by maternal age groups was difficult given the lack of consistency in age group categories between the remaining studies. A total of twenty-seven age group categories were reported among the eight studies, citing the division of maternal age into categorical variables for ages <18 to ≥40 years. The age groups ranged from two- to nine-year spans, preventing category reduction through combination and descriptive reporting of age group representation in the cohort as a whole.
Ethnicity was poorly reported in these studies, with seven of the eligible papers not declaring ethnicity (Table 1); a further two recorded the country of birth rather than ethnic background(Reference Alwan, Greenwood and Simpson24,Reference Timmermans, Jaddoe and Hofman39) . For those that recorded ethnic identity, 44 % (n 12 154) of the combined cohort recorded ethnicity as ‘other’. Hispanic was the most frequently recorded ethnic origin (n 4), followed by Caucasian (n 2), African American (n 2) and the generic category ‘Black’ (n 2). Nine individual ethnicities were recorded in these studies (Table 1).
Parity was well reported, with all papers reporting it as a descriptive variable. While 88 % (n 15) of these did not report associations with outcomes (Table 1), two did – one exclusively examined supplementation in nulliparous women(Reference Haugen, Brantsæter and Trogstad31), the other reported differences between women in first and subsequent pregnancies(Reference McAlpine, Scott and Scuffham34). The majority of research was undertaken in nulliparous women (n 78 520, 62 %). Smoking status was reported by 76 % of the papers (n 13); four papers did not provide such details(Reference Brough, Rees and Crawford25,Reference Chan, Chan and Lam29,Reference Czeizel, Puhó and Langmar30,Reference Nilsen, Vollset and Monsen35) . Smokers comprised 15 % (n 18 730) of the total cohort that reported smoking habits (n 122 967).
Reporting of education level and socioeconomic status (SES) was inconsistent. Education level was unrecorded or reported as years completed (±sd) in all but two studies(Reference McAlpine, Scott and Scuffham34,Reference Pastor-Valero, Navarrete-Muñoz and Rebagliato37) . SES was more frequently recorded, with low- (n 4), middle- (n 2) and high-income categories (n 2) examined to some extent (Table 1).
Key findings
In total, fifty-five categorical outcome/supplement combinations were examined across these seventeen publications. Of these, 67·3 % (n 37) found no evidence of increasing or decreasing risk of the selected outcomes due to micronutrient supplementation. A decrease in the risk of suboptimal outcomes was detected in 25·5 % (n 14), while the risk was found to have increased in 7·3 % (n 4) of combinations (Table 2). Two studies reported a reduction in the risk of low birthweight with the use of folic acid in the periconception period(Reference Catov, Bodnar and Olsen27,Reference Timmermans, Jaddoe and Hofman39) and iron supplementation in trimesters 2 and 3(Reference Chan, Chan and Lam29).
Table 2 Number of studies and effects by outcomes
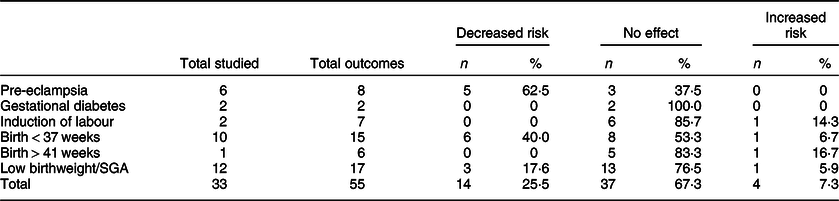
SGA, small for gestational age.
A reduction in risk was found in a majority of studies examining hypertensive disorders of pregnancy and preterm birth. Four of the included studies contributed to this consensus, three of which examined the use of MuMS and/or folic acid(Reference Catov, Nohr and Bodnar28,Reference Martinussen, Bracken and Triche33,Reference Vanderlelie, Scott and Shibl40) . The remaining study reported a reduced risk of pre-eclampsia by vitamin D supplementation in the context of nulliparous pregnancy(Reference Haugen, Brantsæter and Trogstad31). MuMS were found to reduce the risk of preterm birth and pre-eclampsia in the two separate Danish cohort studies; this did not extend to folic acid supplementation(Reference Catov, Bodnar and Olsen27,Reference Catov, Nohr and Bodnar28) .
However, one study examining MuMS across the whole gestation reported an increased risk of preterm birth(Reference Alwan, Greenwood and Simpson24); another reported an increased risk of small-for-gestational-age infants in women using iron supplements in the first and second trimesters(Reference Papadopoulou, Stratakis and Roumeliotaki36). An increased risk for birth beyond 41 completed weeks in women using supplement combinations in the third trimester was reported by one of the two related papers(Reference McAlpine, Scott and Scuffham34); induction of labour was reported to be higher in this study. No evidence of benefit or harm was reported with regard to micronutrient supplementation and the development of gestational diabetes.
Discussion
This review aimed to quantify and characterise recently published empirical studies that examined the effects of micronutrient supplementation on birth outcomes in high-income countries. A systematic quantitative literature review methodology(Reference Pickering and Byrne19) facilitated the identification of a number of challenges faced by research in this field. These include data pool limitations, diversity of methodologies and lack of research in supplement use beyond the first trimester.
Data informing this research originated from about ten countries; however, these ten countries represent only 12 % of the high-income countries based on the World Bank ranking(23). Further, four cohorts provided data for nine of these studies. These cohorts reported data pertaining to a large number of participants, thereby reducing the potential of recruitment bias(Reference Nohr, Frydenberg and Henriksen68–Reference Nohr and Liew71). However, the low number of unique data pools is problematic, in that data typifies its specific population(Reference Mai, Isenburg and Langlois72). This resulted in homogeneity within each data extraction irrespective of the intended investigation and prevented a comparison of the efficacy of interventions between different descriptive groups. Further, heterogeneity was present within the methodology of each study, with a lack of consistency contributing to a lack of cohesion between reported findings.
Another problem faced by researchers in this field is the currency of data available for examination. The most recent empirical data offered by these studies pertained to 2011; some data was collected nearly four decades ago, although getting published in 2009(Reference Czeizel, Puhó and Langmar30). These datasets and their demographic foci are representative of known health risk groups at the time of data collection. However, baseline maternal health and diets have changed substantially in this timeframe(Reference Hanson, Bhutta and Dain73). Physical determinants such as obesity and poor nutrition are driving factors for non-communicable diseases such as essential hypertension, heart disease and diabetes in high-income countries(Reference Firoz, McCaw-Binns and Filippi74). These historically rare concerns represent significant risks during pregnancy and are increasingly affecting birth outcomes(Reference Hanson, Bhutta and Dain73). Determining the effects of dietary supplementation in women who might experience these comorbidities is vital if improvements are to be made in associated perinatal morbidities.
Similarly, cultural factors influencing the nutrition status in high-income countries have changed substantially over time, with increasing immigration and inevitable transference of sociocultural norms across international boundaries(Reference Maskileyson75). This acculturation has resulted in a diversity in baseline health status and food selection practices of pregnant populations in high-income countries(Reference Erten, Van Den Berg and Weissing76–Reference Thomas, Schubert and Whittaker78). Hence, the nutritional needs of pregnant women in contemporary high-income regions differ considerably from those of the cohorts detailed by existing studies. This highlights a knowledge gap regarding the needs and effects of micronutrient supplementation in women from culturally diverse backgrounds. As such, high-quality contemporary data are necessary to ascertain the implications and effects of micronutrient interventions in the context of high-income countries. Randomised controlled trials incorporating baseline nutrition status, biological sampling, supplementation and birth outcomes in specific demographic groups would be a valuable step towards gathering relevant evidence and improving the birth outcomes of culturally diverse women in today’s multicultural societies.
The translation of research into practice in the field of supplementation during pregnancy faces a number of challenges. Global, national and state health organisations rely on data synthesis and systematic reviews to inform clinical recommendations(17). While systematic reviews are a gold standard for evidence-based practice(Reference Boren and Moxley18), they rely on existing data and publications. However, with the exception of periconception folic acid, the level of evidence informing supplementation guidelines is universally poor. This has resulted in guidelines – being founded on consensus-based recommendations – advising the use and timing of specific supplements during pregnancy. This challenges a consistent dissemination of information among and between health professionals and the wider community, resulting in advices influenced by opinions, rhetoric, anecdotes and a widespread belief that supplements are harmless(Reference Marinello, Buckton and Combet79). However, emerging evidence suggests that an inappropriate use of supplements may be detrimental to select birth outcomes(Reference McAlpine, Scott and Scuffham34). Outcomes examined reflect morbidity and mortality associated with pre-eclampsia(Reference Lisonkova, Sabr and Mayer80), preterm labour(Reference Beck, Wojdyla and Say6) and low birthweight(Reference Conde-Agudelo and Díaz-Rossello81) infants. However, gestational length beyond 38 completed weeks has also been found to exhibit a linear increase with poor perinatal outcomes(Reference de Vries, Barratt and McGeechan82). These include an inherent risk of the aging placenta(Reference Smith83) and an increased risk of medical intervention and related sequelae(Reference Walker and Gan84).
Several important supplements were absent from these studies, including those considered beneficial (selenium for the prevention of hypertensive disorders(Reference Xu, Guo and Gu85)) or much recommended (iodine to meet increased need during pregnancy(15)). Both these micronutrients could be toxic if used in excess(Reference Ventura, Melo and Carrilho86), so well-regulated studies would be required if cohort-specific interventions were proposed. However, recommending such supplements in the absence of an identified deficiency would incur an inherent risk. Further, while folic acid supplementation is highly beneficial in the prevention of neural tube defects in the periconception period(Reference Garrett and Bailey87), little evidence exists describing its effect in the subsequent trimesters. Trimester-specific interventions with all supplements were highly variable, causing an inability to determine timely windows of opportunity for specific addition or withdrawal of supplementation to optimise outcomes.
The paucity of research surrounding supplementation during pregnancy will prevent making an accurate estimation of the percentage of women using such supplements. However, the demonstrated knowledge gap along with the reliance of clinicians and consumers on unsubstantiated information would contribute to a high predicted growth of the global pregnancy supplement market, which is estimated to reach a value of $674 million by 2025(88). Since little contemporary empirical research data regarding supplement use during pregnancy is available globally, evidence informing the current policies and recommendations remains questionable. As such, understanding the associations between micronutrient supplements and birth outcomes in high-income countries is a matter of research priority.
Conclusion
Data informing our systematic review pertaining to the effects of micronutrient supplementation on birth outcomes in low-risk women in high-income countries are limited due to a lack of current research, limited data pools, heterogeneity in methodologies and traditional dissemination of research to primary health practitioners. Over two-thirds of reports presented no evidence of benefit or harm to birth outcomes as a result of supplementation in low-risk women in high-income countries. Heterogeneity in methodologies used and lack of specificity regarding demographic grouping confound findings regarding what constitutes appropriate and effective supplementation, challenging the application of such findings to practice. A coordinated, cohesive and uniform empirical approach is required to determine what constitutes appropriate, effective and safe micronutrient supplementation during pregnancy in contemporary cohorts from high-income countries.
Acknowledgements
Acknowledgements: None. Financial support: This research received no specific grant from any funding agency, commercial or not-for-profit sectors. Conflict of interest: None. Authorship: J.M.M. designed the research and the maternal outcomes and nutrition tool, and was responsible for conceptualisation, literature search, systematic analysis and drafting the manuscript. J.J.V., L.V. and A.V.P. contributed to revisions and have read and approved the final manuscript.
Supplementary material
For supplementary materials accompanying this article visit https://doi.org/10.1017/S1368980020000725






