Fluoride (F) is present in small amounts in the soil, water, plants and animals; therefore, it is naturally present as a trace element in the diet(Reference Smolin and Grosvenor1). An extensive body of evidence has proven a clear reduction in dental caries prevalence in communities exposed to F, and it has been added to the diet via water or salt for human consumption as part of community fluoridation programmes to prevent dental caries(Reference Sampaio, Levy and Buzalaf2). Like other nutrients and trace elements in the diet, F has both beneficial and detrimental effects: with low exposures, it prevents and controls dental caries, while higher exposures can lead to hard-tissue changes such as dental fluorosis(Reference DenBesten, Li and Buzalaf3). Given that the risk for developing dental fluorosis is present only during critical periods of tooth development, dietary F intake has been extensively and traditionally monitored in children(Reference Buzalaf, Levy and Buzalaf4), but rarely in other age groups. There is, however, emerging evidence on potential adverse effects of prenatal F exposure(Reference Grandjean5). Associations between F concentration in urine during pregnancy and poor neurodevelopmental outcomes in the offspring have been reported, not only in populations with endemic fluorosis and exposed to high levels of F(Reference Jiménez, Guzmán and Flores6) but also in populations exposed to low levels, such as the ones considered optimal for community water and salt fluoridation programmes(Reference Bashash, Thomas and Hu7–Reference Green, Lanphear and Hornung9). This newly emerging evidence on the potential side effects of prenatal F exposure suggests monitoring of F intake in other susceptible groups, such as pregnant women, may be warranted.
In 1997 and based on data collected in children and non-pregnant adults, the USA’s Institute of Medicine recommended an adequate intake (AI) for F of 3 mg/d (0·05 mg/kg per d). Using a pre-pregnancy body weight for women >19 years of ∼61 kg as a reference, the recommendation for both pregnant and non-pregnant women was also set as 3 mg F/d(10). Given the existing knowledge gap on F intake in the context of the dietary and physiological changes of pregnancy, there is a need for observational studies of F intake in populations of pregnant women. For instance, pregnant women are encouraged to increase the dietary intake of foods and supplements containing nutrients that are beneficial for maternal and fetal health, such as Ca, Fe and folate(10). In contrast, the dietary intake of F is not particularly encouraged or discouraged during pregnancy and has no reported benefits for fetal health. In a previous investigation on the concentration of F in foods and beverages available in Mexico City, we found small amounts of F in Mexican dietary staples (cereals, legumes and animal products)(Reference Cantoral, Luna-Villa and Mantilla-Rodriguez11). These foods are rich sources of folate, Ca, Fe and protein(Reference Roselló-Soberón and Casanueva12) and their frequent consumption may increase the total daily dietary intake of F. We hypothesised that Mexican pregnant women, attempting to meet dietary recommendations, increase their dietary intake of F. Understanding how F intake changes over the course of pregnancy and, whether meeting the requirements of key beneficial nutrients also increases the dietary intake of F, can serve the purpose of informing future dietary recommendations for pregnancy.
The objectives of the current study were to estimate dietary F intake over the course of pregnancy and the overall adjusted difference of dietary F intake by pregnancy stage and levels of compliance with Mexican dietary recommendations.
Methods
Study sample and setting
The study population was pregnant women participating in the Early Life Exposures in Mexico to ENvironmental Toxicants (ELEMENT) project, which has been described previously(Reference Perng, Tamayo-Ortiz and Tang13). ELEMENT comprises three mother–child pregnancy and birth cohorts, initiated in the 1990s to study early life exposures and health outcomes in Mexico City. In the current analysis, we included pregnant women from Cohort 3, who were recruited between 2001 and 2003 (n 670). Pregnant women attending three clinics of the Mexican Institute of Social Security (IMSS) were invited to participate, but only those with gestational age <14 weeks, a healthy singleton pregnancy, no history of systemic diseases (hypertension, diabetes) and intention to stay in Mexico City who agreed to participate through informed consent were included and followed thereafter. Socio-demographic questionnaires included several questions on variables such as age, educational attainment, parity, marital status and self-reported weight before pregnancy. Details and demographics of the entire project can be found in the ELEMENT project’s profile publication(Reference Perng, Tamayo-Ortiz and Tang13). The project has a dedicated research facility next to Mexico City’s ABC Medical Center and participating women were invited to attend the facility at the early, middle and late stages of their pregnancy(Reference Perng, Tamayo-Ortiz and Tang13). Cohort 3 was originally designed as a double-blind randomised clinical trial (RCT) to examine the effects of Ca supplementation on blood lead levels during pregnancy and up to 1 year postpartum(Reference Ettinger, Lamadrid-Figueroa and Mercado-García14). Women were randomised to receive either the Ca supplement (1200 mg Ca/d) or placebo. During each pregnancy visit, each woman was interviewed by a social worker, who performed anthropometry (weight and height) using calibrated instruments, applied a general demographic questionnaire and a FFQ. Only women from Cohort 3 with FFQ and all variables of interest available in the database were included for the analyses (n 568). All participants who were included had data available for at least two pregnancy stages, and 511 had data for all three.
Measurement of flouride in foods, water and other beverages
Mexico City has naturally occurring water fluoride levels <0·7 ppm(Reference Martínez-Mier, Soto-Rojas and Buckley15) and does not have community water fluoridation as used in other countries. Instead, since 1981 a salt fluoridation programme was implemented as a method to deliver F for caries prevention (250 ppm of F/kg) in regions with water fluoride levels <0·7 ppm(16,17) . Therefore, the main sources of dietary F intake in Mexico City are foods with intrinsic F content and those containing fluoridated salt added either during the cooking process and/or at the table before consumption. A database was developed specifically for the ELEMENT project by analysing the F content of typical foods and beverages in the Mexican diet(Reference Cantoral, Luna-Villa and Mantilla-Rodriguez11). Details on the methodology for the collection of food and beverages and the analysis of F can be found in the publication by Cantoral et al.(Reference Cantoral, Luna-Villa and Mantilla-Rodriguez11). Briefly, fruits and vegetables were bought from three different major markets in Mexico City. Meat products, processed foods, juices, beverages and industrialised foods were bought from four large supermarket chains. Natural juices were purchased from street vendors, flavoured waters (a traditional Mexican beverage prepared with water, fruit and sugar) were bought from ice cream parlors and dairy was purchased in creameries. To measure the concentration of F in water drank by participating women, a trained research assistant visited the household of study participants of the ELEMENT project (n 552) and collected water samples (∼5 ml). To standardise the concentration of F in the water and salt in foods that are consumed cooked (meats, rice, pasta, legumes), these were boiled using water containing negligible amounts of F (<0·01 mg/l) and fluoridated salt (250 ppm of F/kg), following standardised recipes from the National Health and Nutrition Survey. Traditional foods (e.g., tamales and maize-based foods) were purchased cooked from street vendors. Fluoride analyses were conducted at the Fluoride Research Laboratory at the Oral Health Research Institute, Indiana University School of Dentistry using a modification of the hexamethyldisiloxane method(Reference Taves18) as modified by Martinez-Mier et al (Reference Martínez-Mier, Cury and Heilman19). Participants reported the intake of mainly bottled and tap water, which had mean F levels ±sd of 0·16 ± 0·13 ppm and 0·14 ± 0·09 ppm, respectively. These two average values were the ones included in the database for FFQ-derived estimations of F intake from water.
Estimation of dietary intake of fluoride, macro- and micronutrients
Dietary F intake (mg F/d) was assessed through a semiquantitative questionnaire consisting of 104 items, adapted from the Willett semi-quantitative FFQ(Reference Willett, Sampson and Stampfer20) to include foods and beverages commonly consumed in the 1983 Dietary Survey of the Mexican National Institute of Nutrition(Reference Hernández, Aguirre and Serrano21) and validated to estimate dietary intake over the previous month in Mexican women of child-bearing age (15–44 years)(Reference Hernández-Avila, Romieu and Parra22). The FFQ was applied by a trained social worker at each study visit once per trimester, using visual and measuring aids (spoons, cups) for the identification of foods and portion sizes. Estimates of dietary intake of macro- and micronutrients (in µg or mg/d) were calculated through software developed at the National Institute of Public Health (INSP) using methods for the analysis of dietary data from the Mexican National Health and Nutrition Survey(Reference Rodríguez-Ramírez, Mundo-Rosas and Jiménez-Aguilar23). To generate estimates of daily dietary intake, the software utilises the following data: (1) the average content of nutrients and trace elements (including F) of each food/beverage item reported the INSP-compiled nutrient composition database(24) and the ELEMENT F database, and (2) the reported frequencies and portion sizes of foods and beverages from the FFQ. Further details on the development, validation and calculations derived from the FFQ are available in the publication by Hernandez-Avila et al.(Reference Hernández-Avila, Romieu and Parra22). Although the FFQ did not include quantitative estimations of table salt intake, it did include a dichotomous question on whether table salt was added to foods right before eating them (yes/no), which we used for this secondary data analysis. Given that this cohort was originally designed as a Ca supplementation RCT and women may had chosen to ingest other dietary supplements as well, a variable with group assignment to the RCT (placebo/supplemented) and another one specifying intake of other dietary supplements (yes/no) was available to control for potential confounding.
Assessment of compliance with dietary recommendations
Dietary F- intake in Mexican women is neither contraindicated nor encouraged in Mexican pregnancy dietary recommendations. The Mexican Official Norms regulating health services for pregnant women, which were current for the cohort, highly encouraged an increase in the dietary intake of key nutrients(Reference Roselló-Soberón and Casanueva12) in order to meet the AI or estimated average requirement (EAR) as recommended by the dietary reference intakes by the USA Institute of Medicine(10). Recommendations on dietary intake during pregnancy were made according to pre-pregnancy BMI and pregnancy stage(25). The four key nutrients encouraged in the dietary recommendations were Ca (AI: 1000 mg/d), Fe (EAR: 22 mg/d), folate (EAR: 520 µg/d) and protein (EAR: 0·88 g/kg per d). Intake was recommended from various dietary sources for all nutrients(26); in the case of Ca, folate and Fe, the recommendation included intake from both diet and supplements. The dietary intake of each nutrient was estimated only from the intake reported in the FFQ. Since one of the objectives of the current study was focused on the relationship between compliance with nutrients from dietary sources and dietary F intake, supplement sources were excluded from the calculation.
Women were classified as compliant with an individual nutrient if their estimated daily dietary intake was equal to or above the AI or EAR for that particular nutrient during pregnancy. Overall compliance at each stage of pregnancy was categorised according to individual-nutrient compliance, as follows: non-compliance if non-compliant with all the nutrients (0/4); moderate compliance if compliant with one or two nutrients (1/4 or 2/4) and high compliance if compliant with three or all the four nutrients (3/4 or 4/4).
Covariates
The selection of covariates was based on our knowledge of factors that may influence dietary F intake and bivariate analyses. These included pregnancy stage, compliance with dietary recommendations, group allocation in the Ca supplementation RCT, intake of other supplements, addition of salt at the table, total daily energy intake, pre-pregnancy BMI and educational attainment.
Data analyses
Study participants were stratified by pregnancy stage. Differences in key dietary variables across pregnancy stages were assessed with Friedman test (for continuous, non-normally distributed repeated measures variables) or χ 2 tests (for categorical variables). Differences in dietary F intake between the RCT allocation groups and according to table salt use were tested with the non-parametric Mann−Whitney test. To estimate the overall adjusted difference in dietary F intake by pregnancy stage (early, middle, late) and levels of compliance with Mexican dietary recommendations (none, moderate, high), we fitted multivariate regression models for panel data using the xtreg command in STATA. Panel data models were chosen because they examine both individual- and time-specific effects to deal with unobserved heterogeneity or individual effects. Regression diagnostics revealed significant differences in the individual- and time-specific variance components (panel effect; Breusch–Pagan LM test P < 0·05) and no correlation between individual effects and the regressors (Hausman test, P > 0·05); therefore, one-way random effects GLS regressions provided the best fit. After the model’s estimates were obtained, adjusted predictions for dietary F intake were calculated for each pregnancy stage using the margins command. The association between dietary F intake and individual intake of nutrients (Ca, Fe, folate, protein) was assessed following the same approach. All analyses were conducted with STATA v16.0 (StataCorp.).
Results
Characteristics of the sample and dietary flouride intake by pregnancy stage
Table 1 summarises and compares the characteristics of women included in the analytical sample (n 568) and those who were excluded (n 102). Overall, the median age of the study participants at the time of recruitment was 26·4 years and the median gestational age for women who completed FFQ at the early, middle or late stages of pregnancy was 13·6, 25·4 and 34·3 weeks, respectively. Median weight ranged between 60·5 and 69·4 kg during the three pregnancy stages and most women (57·7 %) had a pre-pregnancy BMI categorised as normal according to the guidelines that were current at the time of data collection(Reference Ettinger, Lamadrid-Figueroa and Mercado-García14). Most women (64·3 %) had been pregnant more than once; and their highest educational level was secondary school (67·8 %). Finally, in the analytical sample, 289 women were allocated to the treatment group of the Ca supplementation RCT, whereas 279 were allocated to the placebo group. The characteristics of women who were excluded were not significantly different from those who were included.
Table 1 Characteristics of women with complete data for all variables of interest that were included in the analytical sample (included) and women who were excluded because they had incomplete data‡

* Total number of women with data available for each variable.
† Number of women assigned to the Ca supplementation group in the randomised clinical trial.
‡ Comparisons between included and excluded women were performed with Mann-Whitney tests for continuous variables and χ 2 tests for categorical variables.
Detailed descriptive statistics of dietary F intake by pregnancy stages and for all available observations are provided in Table 2. This sample of pregnant women living in Mexico City had a median dietary F intake of 0·69 mg/d, ranging between a minimum of 0·11 and a maximum of 3·73 mg/d (for a total of 1649 observations at all pregnancy stages).
Table 2 Dietary fluoride intake (mg/d) by pregnancy stages. The ‘all stages’ category represents data for all observations available during pregnancy

Bivariate statistics for key dietary variables by pregnancy stages
Table 3 summarises bivariate statistics of key dietary variables by pregnancy stages. We found variability in both dietary F intake and total energy intake over the course of pregnancy. Women had a median dietary F intake that ranged from 0·64 (IQR 0·38) in the early stage to 0·72 (0·44) mg F/d in the late stage, and the tendency to increase was statistically significant. Considering that the median weight across pregnancy was 65 kg, this range of median F intake throughout pregnancy corresponds to 0·01 mg/kg per d. Estimates of dietary F intake between those who reported adding salt at the table to their meals and those who did not were not significantly different (0·67 mg/d interquartile range 0·47 v. 0·70 mg/d, interquartile range 0·40, respectively) (data not shown in Table 3). In contrast to dietary F, median total energy intake significantly decreased during the middle stage of pregnancy. There were also variations in overall compliance and individual-nutrient compliance across pregnancy stages. Compliance with both Ca and Fe was associated with pregnancy stage. For Ca, compliance rose from 51·2 % in the early, 58·6 % in the middle and 62·0 % in the late stages of pregnancy (P < 0·001), whereas for Fe it went from 6·0 % in the early, 7·1 % in the middle and, 5·5 % in the late stage − although for the latter, no statistically significant association was found. In contrast, compliance with protein was associated (P < 0·001) with pregnancy stage (68·7, 62·6 and 56·8 % for the early, middle and late stages of pregnancy, respectively). Compliance with folate intake from dietary sources experienced a slight increase towards the middle stage, followed by a decrease towards the end of gestation (5·8, 6·3 and 3·8 % for the early, middle and late stages of pregnancy, respectively), although this change was not associated with pregnancy stage (P = 0·151). Over the course of pregnancy, women were mostly moderately compliant with dietary recommendations (P = 0·412). Only a slight increase in the proportion of women who were non-compliant towards the end of pregnancy was observed (not statistically significant). Towards the end of pregnancy, the proportion of women reporting adding table salt to meals was lower (30·3 % in early v. 22·6 % in late-pregnancy; P = 0·012), whereas the proportion of women reporting the use of dietary supplements was higher (23·4 % in early v. 37·9 % in late pregnancy; P < 0·001) (Table 3). Median F intake from dietary sources for women allocated to the Ca supplementation group in the RCT was significantly higher than that of women allocated to the placebo group (overall median 0·71 v. 0·67, P = 0·03; data not shown in Table 3); therefore, we also included RCT allocation group as a covariate in the models.
Table 3 Bivariate statistics for key dietary variables by pregnancy stages
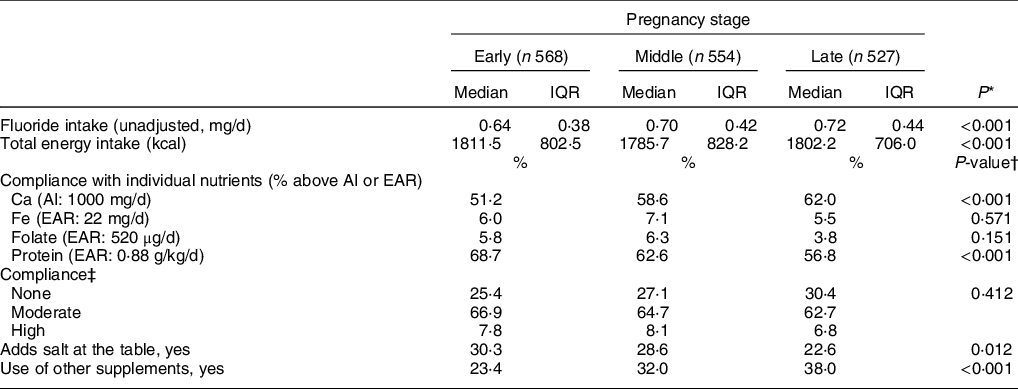
IQR, interquartile range.
* Friedman test (n 511).
† χ 2 test.
‡ None: non-compliant with all of the nutrients (0/4); moderate: compliant with one or two nutrients (1/4 or 2/4); and high: compliant with three or all the four nutrients (3/4 or 4/4).
Adjusted dietary flouride intake by pregnancy stages and compliance with dietary recommendations
After adjustment for covariates, the association between dietary F intake and pregnancy stages was significant (P < 0·001, Table 4). Compared with the early stage, women in the middle and late stages of their pregnancy ingested on average, 0·04 and 0·08 more mg F/d, respectively. The adjusted predictions of dietary F intake from foods and beverages during pregnancy therefore increase from 0·72 (95 % CI 0·70, 0·74) in the early stage, 0·76 (95 % CI 0·74, 0·77) in the middle stage, to 0·80 (95 % CI 0·78, 0·82) in the late stage (Fig. 1). Women who reported intake of dietary supplements other than the Ca supplement provided for the RCT supplementation group ingested, on average, 0·03 less mg F/d, compared with women who did not take other supplements (P = 0·027). Furthermore, compared with non-compliant women, those who were moderately and highly compliant with dietary recommendations ingested on average, 0·04 and 0·14 more mg F/d, respectively (Table 4). The covariates included in the model were total energy intake, allocation group in the Ca supplementation RCT, intake of other supplements and pre-pregnancy BMI.
Table 4 Adjusted associations between daily fluoride (mg/d), pregnancy stage and compliance with dietary recommendations*
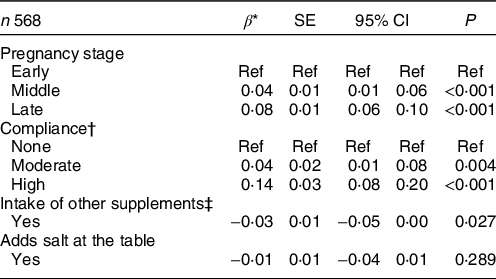
* Estimates from a one-way random effects GLS regression model adjusted for total energy intake, allocation group in the Ca supplementation RCT, intake of other supplements and pre-pregnancy BMI.
† None: non-compliant with all of the nutrients (0/4); moderate: compliant with one or two nutrients (1/4 or 2/4) and high: compliant with three or all the four nutrients (3/4 or 4/4).
‡ Supplements other than the one provided for the Ca supplementation RCT.
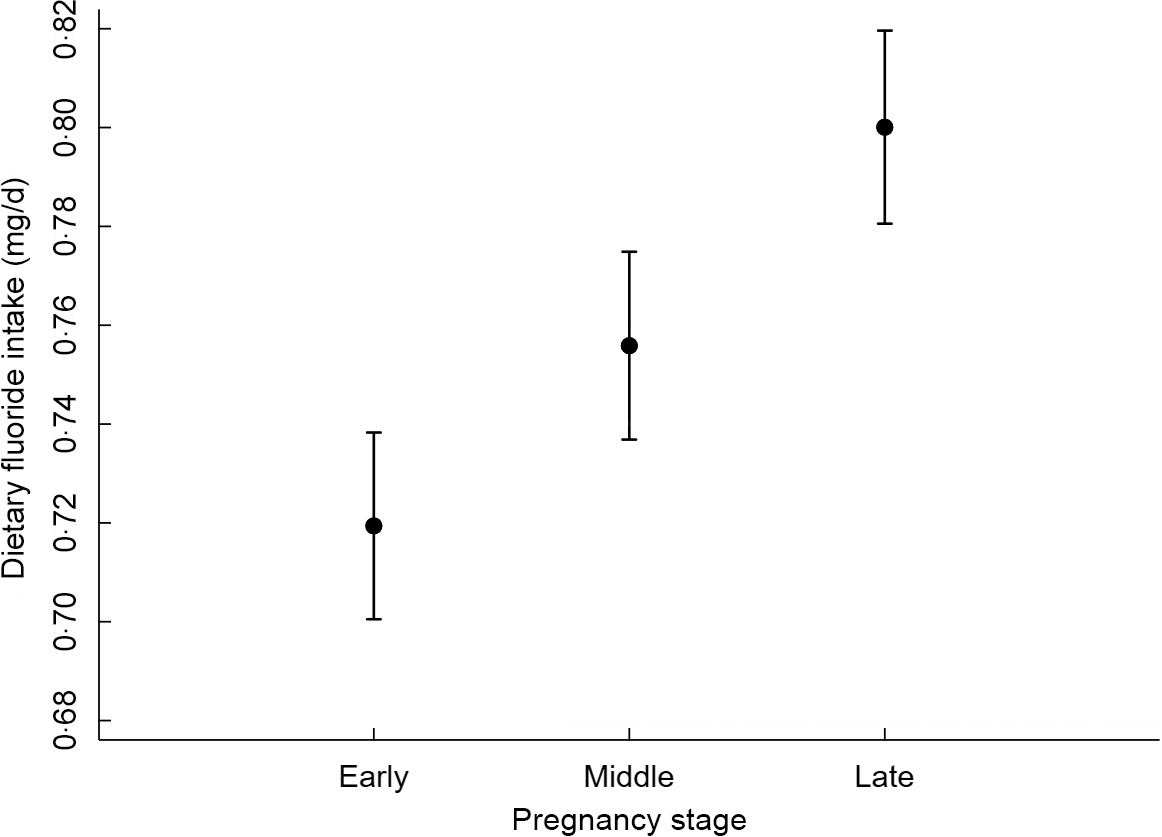
Fig. 1 Estimated fluoride intake (95 % CI) by pregnancy stage*. *Estimates from a one-way random effects GLS regression model adjusted for total energy intake, allocation group in the Ca supplementation RCT, intake of other supplements and pre-pregnancy BMI
In order to understand which nutrients were associated with changes in dietary F intake, we also assessed the association between compliance with individual nutrients and dietary F intake. Compared with women who did not meet Ca and Fe recommendations, those who were compliant with Ca and Fe recommendations ingested on average, 0·12 and 0·18 more mg F/d, respectively (P < 0·001). In contrast, women who were compliant with folate recommendations ingested on average, 0·12 less mg F/d, compared with those who did not meet recommendations (P = 0·009) (Table 5). Compliance with protein intake recommendations was not associated with dietary F intake.
Table 5 Adjusted associations between daily fluoride intake and compliance with individual key nutrients*
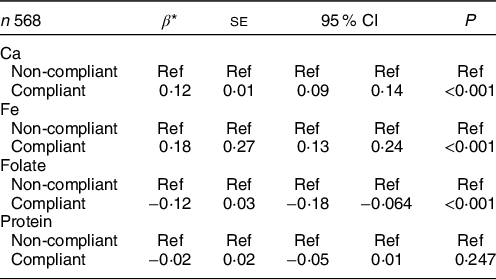
* Estimates from a one-way random effects GLS regression model adjusted for pregnancy stage, allocation group in the Ca supplementation RCT, intake of other supplements, total energy intake, addition of salt at the table (yes/no), pre-pregnancy BMI and educational attainment.
Discussion
Median dietary F intake in this sample of pregnant women living in Mexico City was 0·69 (min − max: 0·11–3·73) mg/d or 0·01 mg/kg per d. To date, only two other studies have reported dietary F intake during pregnancy in large samples of pregnant women. In Canada, F intake from beverages was assessed in 162 pregnant women living in communities with access to fluoridated water, with a reported intake of (mean ± sd) 0·93 ± 0·43 mg/d(Reference Green, Lanphear and Hornung9). Another study, conducted in Spain in 575 pregnant women also living in a community with access to fluoridated water, reported a median F intake from beverages of 0·02 mg/kg per d (min − max: 0·005–0·043)(Reference Jiménez-Zabala, Santa-Marina and Otazua27). While the median dietary F intake reported in our study is lower than in the other study populations, there are differences that limit the ability to make direct comparisons between these three studies. First, the study samples were drawn from populations exposed to different vehicles for community fluoridation. In Mexico City, community-wide fluoridation of salt is used, while in the Canadian and Spanish studies, women had access to fluoridated drinking water. Second, the studies from Canada and Spain only report F intake from beverages, while we report F intake from both foods and beverages, including bottled and tap water. Nonetheless, all three study populations found dietary F intakes in pregnancy below the USA-Institute of Medicine’s AI recommendation from all sources.
Our report has several strengths in the area of F exposure assessment, including repeated measures reported over the course of pregnancy, use of a validated instrument of dietary assessment and a F database specific to the population under study. Limitations of the current study include those inherent to FFQ-derived estimates and secondary data analyses. First, low F water (<0·01 mg/l) and fluoridated salt with standard F levels (250 ppm/kg)(16) were used to prepare foods that are consumed cooked. There may be variation in the natural levels of fluoride in water and even among and within brands of salt(Reference Martínez-Mier, Soto-Rojas and Buckley15), with the potential to add uncertainty to the estimates. And second, we lack quantitative estimates of salt added after cooking. We believe, however, that as fluoride levels in Mexico City are low (<0·7 ppm)(Reference Martínez-Mier, Soto-Rojas and Buckley15) and levels of F in salt are regulated by the government(16,17) , the lack of a quantitative estimate of F in salt after cooking is the main source of uncertainty in the present study, which most likely underestimates the true dietary F intake. To control for potential differences that could be explained by the habit of adding salt at the table, we used a dichotomous question on the practice of adding table salt to meals after the cooking process (yes/no) that was available in the FFQ. This variable allowed us to make a rough calculation of what total F intake would look like in women who add salt to their meals. Using a quantitative report of added table salt by Mexican non-pregnant women aged 23–50 years in the State of Mexico (5·4 g of salt/d)(Reference Martinez-Salgado, Tovar-Zamora and Chavez-Villasana28) and assuming all salt gets ingested at a concentration of 250 ppm F, women in our study who reported adding table salt to their meals (median dietary F intake of 0·67 mg/d) would be ingesting about 1·31 mg F/d (0·02 mg F/kg per d) – approximately double the amount of those who reported not adding extra salt. This rough calculation, however, should be interpreted with caution, as quantitative estimations of salt intake after cooking were not made in the sample under study. We recommend for future studies in populations of pregnant women exposed to fluoridated salt, the inclusion of quantitative measures of table salt intake. Furthermore, the median dietary intake found for this population (0·69 mg/d) constitutes the contribution of only intrinsic F in foods and F from salt added to foods during the cooking process. To provide a broader perspective of fluoride exposure, future research should also consider the contribution of other sources of F, such as occupational exposures and the unintentional intake of fluoridated oral hygiene products.
Dietary F intake in this sample of pregnant women increased with the progression of pregnancy, suggesting that dietary F intake does change during gestation − as would be expected given increased consumption by pregnant women to meet the nutrient demands of the growing fetus. We were interested in testing whether the increase observed in F intake throughout pregnancy remained after controlling for covariates that explain dietary F intake such as total energy intake, the practice of addition of salt at the table and compliance with dietary recommendations. We chose these variables because most nutrients, including F, have a positive linear relationship with total energy intake(Reference Willet, Hofman, Marmot and Samet29); pregnant women increase their total energy intake towards the end of gestation responding to an increased RMR(Reference Most, Dervis and Haman30), and there is tendency to change dietary behaviours with the progression of pregnancy(Reference Saunders, Rehbinder and Carlsen31). In fact, controlling for total energy intake, women ingested more F in the middle and late stage of pregnancy compared with the early stage, plus 0·14 mg F/d (∼0·2 mg/d total) if they were compliant with pregnancy dietary recommendations. The biological significance of an increase of ∼0·2 mg/d of F intake during the third trimester of pregnancy (˜7 % of the current recommendation) is, however, still unknown and should be considered for future research. Studies on the association between maternal dietary F intake and health outcomes in the offspring are needed to investigate the biological significance of the current levels of intake. Only one study has reported that a 1-mg increase in maternal F intake from beverages was associated with a 3·7 decrease in intelligence scores among boys and girls(Reference Green, Lanphear and Hornung9), which would be of public health significance for about 10 % of the women in the present study (90th percentile, Table 1). Further research on the effect of lower prenatal F exposure levels on neurodevelopment − such as the median dietary intake found in the current study (0·69 mg/d), is needed to inform future dietary F intake recommendations for pregnant women.
We were interested in understanding whether Mexican women, attempting to meet dietary recommendations, increase their dietary intake of F. We found an association between women who were compliant with recommendations for both Ca and Fe and increased F intake levels (Table 5). This observation could be explained by the frequent consumption of Ca- and Fe-rich foods with low-to-moderate amounts of fluoride, which can lead to an overall increase in F intake. In Mexico, foods rich in both Ca and Fe with a moderate content of F include milk, maize-based products and legumes(Reference Cantoral, Luna-Villa and Mantilla-Rodriguez11). We also found that women who reported to consume dietary supplements had lower dietary F intakes. A plausible explanation for this negative association is that individuals who proactively take supplements tend to eat lower amounts of nutrient-dense foods(Reference Anders and Schroeter32). Therefore, it is possible that pregnant women in this sample who reported supplement intake relied on supplementation to meet their dietary goals instead of choosing more nutrient-dense foods. Socio-demographic factors have also been reported to influence dietary F intake(Reference Franco, Martignon and Saldarriaga33); however, although we found no association, we cannot entirely tease out their effect on dietary intakes of the current study’s population given that it included a relatively homogeneous group of women attending the clinics of the Social Security System in Mexico (IMSS) that serves a low-to-middle income population.
Within the limitations of the current study, we conclude that the levels of dietary F intake were below the current AI, were greater towards the end of gestation and in women who were moderately and highly compliant with Mexican dietary recommendations. Given the mounting evidence of potential adverse effects(Reference Bashash, Thomas and Hu7–Reference Green, Lanphear and Hornung9) and multiple sources of exposure to F, additional assessment and monitoring of dietary intakes and exposures from other community sources, especially in vulnerable populations such as pregnant women and children, should be considered in future dietary recommendations for F intake.
Acknowledgements
Acknowledgements: The authors acknowledge the American British Cowdray (ABC) Hospital in Mexico City, Mexico, for providing research facilities for the ELEMENT project. We also acknowledge the active participation of women from the ELEMENT cohort 3 and the members of the research team involved in the ELEMENT project. Financial support: Indiana University’s President’s International Research Award (PIRA 23-140-39). NIH RO1ES021446 and NIEHS/EPA P01ES022844 RD8354360. PhD in Dental Sciences Program, Indiana University School of Dentistry. Instituto Nacional de Salud Pública de México (INSP). Conflict of interest: There are no conflicts of interest. Authorship: G.A.C. participated in the conception and design of the study, analysis of data, statistical modeling, interpretation of results and writing of the manuscript. T.V.M.-R. participated in the management of the ELEMENT database, analysis of data, statistical modeling interpretation of data and, writing of the manuscript. A.C., A.M. and M.M. T.-R. participated in the conception and design of the study, interpretation of results and writing of the manuscript. H. H. was a co-founder of the ELEMENT cohort and PI of R01ES021446, and Karen Peterson is PI of NIEHS/EPA P01ES022844/RD8354360; both provided oversight of original dietary data collection and participated in interpretation of data and preparation of the manuscript. M.M.T.-R and A.M.-G. supervised and coordinated field data collection. A.M.-G. and A.E. participated in the design and implementation of the original cohort study and participated in interpretation of data and preparation of the manuscript. Ethics of human subject participation: The current study was conducted according to the guidelines laid down in the Declaration of Helsinki and the ethics committee of the INSP, Indiana University, Harvard University and the University of Michigan approved all procedures involving research study participants. Written informed consent was obtained from all study participants.









