Periodontal disease is defined as an inflammatory condition of the periodontal tissues. Periodontal pocketing can occur with inflammation and swelling of the gingiva and/or destruction of the connective tissue and bone that support the teeth (Fig. 1). Advanced disease with deep periodontal pockets affects 10 to 15 % of the world's population(Reference Petersen and Ogawa1) and is a major cause of tooth loss in adults(Reference Locker, Ford and Leake2). The main aetiological agent in the causation of periodontal disease is dental plaque. According to previous reports, there are several risk factors for periodontal disease: hypoalbuminaemia(Reference Iwasaki, Yoshihara and Hirotomi3), hyperglycaemia (diabetes)(Reference Taylor4) and smoking(Reference Axelsson, Paulander and Lindhe5). Periodontal disease is characterized by hyper-inflammation, involving excess release of reactive oxygen species (ROS) by host neutrophils(Reference Chapple and Matthews6). Increased ROS production causes oxidative stress(Reference Sies7) and contributes to tissue destruction by damaging DNA and proteins, causing lipid peroxidation and oxidation of other enzymes (e.g. antiproteases), and stimulating pro-inflammatory mediators through activation of the transcription factors NF-κB and activator protein 1(Reference Chapple and Matthews6, Reference Chapple8, Reference Chapple9). Studies have demonstrated the in vitro ability of ROS to degrade extracellular matrix components of the periodontal connective tissues (e.g. proteoglycans)(Reference Waddington, Moseley and Embery10). Moreover previous study results indicate that ROS act as an intracellular signal mediator for osteoclast activation and differentiation(Reference Lee, Choi and Baik11). These findings suggest that oxidative stress plays a significant role in the pathogenesis of periodontal disease. Oxidative stress is also associated with a higher risk of several chronic inflammatory diseases which relate to periodontal disease, such as diabetes(Reference Maritim, Sanders and Watkins12), rheumatoid arthritis(Reference Ozkan, Yardym-Akaydyn and Sepici13) and CHD(Reference Wolfram, Oguogho and Palumbo14).
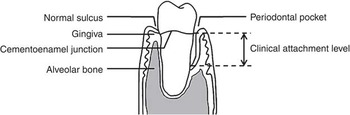
Fig. 1 Periodontal tissues
Dietary antioxidants, such as vitamin C, vitamin E and carotenoids, have been shown to be protective in a number of diseases involving chronic inflammation(Reference Hertog, Feskens and Hollman15–Reference Thurnham19). The mechanisms via which antioxidants protect biological systems from oxidative damage include the direct scavenging of ROS and the sequestration of free catalytic metal ions, which promote ROS formation(Reference Waddington, Moseley and Embery10). There is some evidence of a negative association between antioxidant status and periodontal disease. For example, the association between low vitamin C intake and periodontal disease among smokers has been demonstrated in a cross-sectional study(Reference Nishida, Grossi and Dunford20). However, there are few data on the relationship between other antioxidant nutrients (e.g. vitamin E and carotenoids) and periodontal disease. Studies have demonstrated that both local and systemic antioxidant capacities were compromised in patients with periodontal disease(Reference Chapple, Milward and Dietrich21–Reference Sculley and Langley-Evans23). It is unclear from these cross-sectional analyses whether this reflects a response to the neutrophil hyper-reactivity or is the result of reduced intake of dietary antioxidants, malabsorption or metabolic compromise because of polymorphisms in key redox-regulating enzymes(Reference Chapple and Matthews6, Reference Chapple, Brock and Milward24, Reference Kim, Park and Chung25). To date, there are no data on the relationship of dietary antioxidants to changes in the periodontal condition over time.
Investigating the relationship between dietary antioxidants and the development of periodontal disease in a longitudinal study will help further elucidate the potential role of dietary modification in the prevention and treatment of periodontal disease and the ultimate prevention of tooth loss. The hypothesis of the present study was that a high intake of dietary antioxidant vitamins is associated with a lower risk of periodontal disease progression. The study aimed to determine if there was a longitudinal relationship between intake of dietary antioxidants and periodontal disease in community-dwelling older Japanese. A further aim was to determine if the intakes of vegetables and fruits, primary food sources of dietary antioxidants, were associated with periodontal disease progression in this group of older people.
Materials and methods
Study design and selection of study participants
The current investigation was a subset study of the Niigata Study over the period 2003 to 2005. Briefly, the Niigata Study is a prospective community-based study initiated in 1998 to evaluate relationships between systemic health status and history of dental diseases. In April 1998, all 4542 Niigata citizens aged 70 years (2099 men and 2443 women) were sent a written request to participate in the survey. The invitation was mailed again to non-respondents 3 weeks later and, consequently, 81·4 % (n 3695) responded positively to participate in the survey. Considering the availability of resources, examination appointments could be arranged for 600 individuals. The final study sample was randomly recruited from several areas of Niigata in order to have an approximately equal number of men (n 306) and women (n 294)(Reference Iwasaki, Yoshihara and Hirotomi3).
During 1998–2003, 230 of the 600 original participants were not available for the current study due to the following reasons: (i) 149 refused, (ii) forty-five were hospitalized, (iii) twenty-seven had died and (iv) nine had moved out of Niigata City; therefore, 370 individuals underwent dietary intake assessments, dental and medical examinations, interview and anthropometric evaluation in 2003 as part of the baseline assessment for the current study. At baseline, people who were already edentulous (i.e. having no teeth; n 32) or who did not submit complete data (n 4) were excluded, leaving 334 eligible individuals to enter the study. Two years later, participants underwent follow-up examinations including dental assessment at which sixty-nine were not available to participate (sixty-four refused, four had died and one had moved out of Niigata City) and one became edentulous. Data were therefore analysed for the 264 dentate participants (141 men, 123 women) completing the follow-up examination in 2005. Figure 2 presents the flow diagram of study participation. The study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects were approved by the Ethics Committee of the Faculty of Dentistry, Niigata University. Written informed consent was obtained from all study participants.
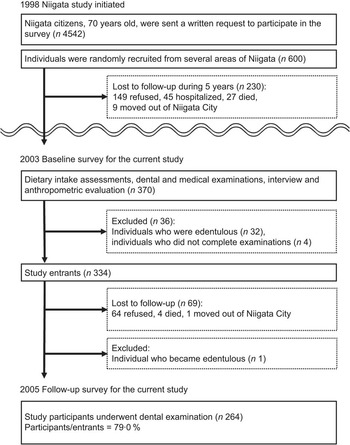
Fig. 2 Flow diagram of participation in the study
Dietary intake assessment
Dietary habits during the preceding month were assessed with a brief-type self-administered diet history questionnaire (BDHQ)(Reference Sasaki26). Responses to the BDHQ were checked for completeness and, where necessary, clarified by direct questioning of the participant.
The BDHQ is a validated questionnaire that enquires about the frequency of consumption of a total of fifty-six food and beverage items, with specified serving sizes described in terms of the natural portion or the standard weight and volume measurement of servings commonly consumed in general Japanese populations(Reference Murakami, Mizoue and Sasaki27). The BDHQ was developed based on a comprehensive version of a self-administered diet history questionnaire(Reference Sasaki, Ushio and Amano28–Reference Sasaki, Yanagibori and Amano30). Estimates of mean daily intakes of energy, vitamin C, vitamin E, α-carotene and β-carotene were calculated using an ad hoc computer algorithm for the BDHQ, which was based on the Standard Tables of Food Composition in Japan (31). Information on dietary supplement use was not available to the investigators and therefore antioxidant nutrient intake from dietary supplements was not incorporated into the analysis. Mean daily intakes of vegetables and fruits were also evaluated from the information reported in the BDHQ. Dietary antioxidant, vegetable and fruit intakes were energy-adjusted (i.e. amount per 4184 kJ/1000 kcal for each variable).
Dental examination
Dental examinations were carried out at baseline (2003) and after 2 years of follow-up (2005). The same methods as in the baseline survey were used in the follow-up dental examination. The number of teeth present and periodontal condition were assessed. Periodontal condition was specifically assessed as measured clinical attachment level (CAL). CAL was recorded by calibrated examiners as previously reported(Reference Iwasaki, Yoshihara and Hirotomi3). Teeth were examined at six sites per tooth for all teeth present, and measurements were recorded to the nearest millimetre.
CAL refers to the amount of space between the attached periodontal tissue and the cementoenamel junction. As periodontal disease progresses, loss of periodontal attachment can occur through the inflammatory destruction of the periodontal ligament and its adjacent alveolar bone, subsequently leading to pathological periodontal pockets (Fig. 1). Therefore, CAL reflects the severity of periodontal disease and longitudinal increases in CAL values can be used as an indicator to estimate the progression of periodontal disease over time. In the present study, loss of attachment of 3 mm or greater over the 2-year study period (e.g. CAL 1 mm at baseline + 3 mm increase = 4 mm at follow-up) at an interproximal site for each tooth was considered periodontal disease progression(Reference Brown, Beck and Rozier32, Reference Tonetti and Claffey33). Then the number of teeth with periodontal disease progression (i.e. demonstrating a longitudinal loss of proximal attachment of ≥3 mm) was summed up for each participant.
Biochemical examination of blood
Biochemical values, including serum levels of albumin and glycated Hb (HbA1c), were evaluated. Hypoalbuminaemia was defined as albumin ≤4·0 g/dl and hyperglycaemia was defined as HbA1c ≥6·5 %.
Interview and anthropometric evaluation
An interview was conducted on all participants including establishment of smoking status, with those reporting any smoking history classified as smokers. Information about socio-economic status (household income and years of school attendance) and oral health-related behaviours (brushing frequency (<2/d or ≥2/d), use of devices for inter-dental cleaning (yes or no) and the pattern of visits to a dentist) was also gathered. Lower income was defined as annual household income <2 000 000 Japanese Yen ($US 16 667; $US 1 = 120 Japanese Yen; hourly minimum wage in Niigata = 641 Japanese Yen in 2003) and lower education was defined as school attendance ≤10 years. Regular dental visits were defined as having regular periodontal examination and professional teeth cleaning at least once annually(Reference Nordstrom, Bergman and Borg34).
Anthropometric evaluation included measurements of weight and height to calculate BMI (weight (kg)/[height (m)]2).
Statistical analyses
Differences in selected characteristics between study participants and withdrawals were compared with the use of independent t tests and χ 2 tests.
The primary outcome for the analyses was the number of teeth with periodontal disease progression, modelled as count data. Univariable and multivariable Poisson regression analyses were performed to evaluate associations between intake of dietary antioxidants and the number of teeth with periodontal disease progression. As the main exposure in the analyses, dietary antioxidant nutrient, vegetable and fruit intakes were modelled as tertiles. Potential confounders controlled for included the number of teeth present at baseline, mean CAL at baseline, gender, lower income, lower education, BMI, hypoalbuminaemia, hyperglycaemia, smoking status, brushing frequency, use of devices for inter-dental cleaning and the pattern of visits to a dentist. Effect modification by gender and smoking was evaluated using interaction terms.
The level of significance was set at α = 0·05. All calculations and statistical analyses were performed using the STATA™ software package version 10 (Stata Corp., College Station, TX, USA).
Results
Selected characteristics of study participants and withdrawals are listed in Table 1. No significant differences were found between the two groups. Median (25th, 75th percentile) CAL of the average study participant was 3·1 (2·5, 3·7) mm at baseline and 3·1 (2·6, 3·9) mm at follow-up, respectively. Table 2 presents participants’ dietary antioxidant, vegetable and fruit intakes.
Table 1 Selected characteristics of study participants and withdrawals; individuals aged 75 years in 2003, Niigata City, Japan
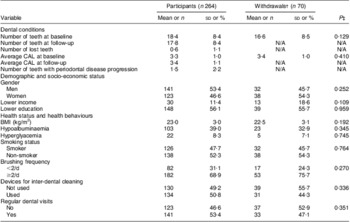
CAL, clinical attachment level; N/A, not available.
Lower income = annual household income <2 000 000 Japanese Yen; lower education = school attendance ≤10 years; hypoalbuminaemia = albumin ≤4·0 g/dl; hyperglycaemia = HbA1c ≥6·5 %; regular dental visits = having regular periodontal examination and professional teeth cleaning at least once annually.
†Withdrawal = person who participated in baseline examination but did not return for follow-up (n 69) and person who became edentulous during the study (n 1).
‡P value for the comparison of selected characteristics between participants and withdrawals.
Table 2 Intakes of dietary antioxidants, vegetables and fruits at baseline; dentate individuals (n 264) aged 75 years in 2003, Niigata City, Japan
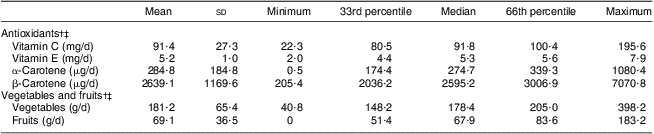
†Energy-adjusted (i.e. amount per 4184 kJ/1000 kcal for each variable).
‡Participants’ mean energy intake = 9314 (sd 2657) kJ/2226·1 (sd 635·0) kcal.
The frequencies of participants by the number of teeth with periodontal disease progression are presented in Fig. 3.
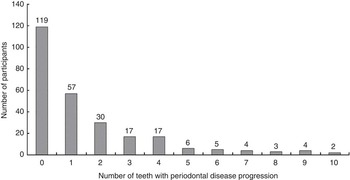
Fig. 3 Frequency of participants by the number of teeth with periodontal disease progression; dentate individuals (n 264) aged 75 years in 2003, Niigata City, Japan
Associations between the number of teeth with periodontal disease progression and dietary antioxidant intakes
Table 3 shows crude and adjusted associations between the number of teeth with periodontal disease progression and dietary antioxidant intakes. There were no interactions of dietary antioxidants with gender and smoking status. In univariable models, significant inverse relationships of the number of teeth with periodontal disease progression with daily intakes of vitamin C, vitamin E, α-carotene and β-carotene were found (Table 3). A high dietary intake of antioxidants was significantly associated with a smaller number of teeth with periodontal disease progression. The crude incidence rate ratios (IRR) for the mean number of teeth with periodontal disease progression in the first, second and third tertiles were, respectively: 1·00, 0·73 (95 % CI 0·58, 0·92) and 0·61 (95 % CI 0·48, 0·77) for vitamin C; 1·00, 0·71 (95 % CI 0·57, 0·89) and 0·47 (95 % CI 0·36, 0·61) for vitamin E; 1·00, 0·87 (95 % CI 0·70, 1·10) and 0·72 (95 % CI 0·56, 0·92) for α-carotene; and 1·00, 0·92 (95 % CI 0·74, 1·15), and 0·64 (95 % CI 0·50, 0·82) for β-carotene (Table 3). Vitamin C, E and β-carotene remained significant after simultaneously adjusting for the number of teeth present at baseline, mean CAL at baseline, gender, lower income, lower education, BMI, hypoalbuminaemia, hyperglycaemia, smoking status, brushing frequency, use of devices for inter-dental cleaning and the pattern of visits to a dentist. The multivariate-adjusted IRR in the first, second and third tertiles were, respectively: 1·00, 0·76 (95 % CI 0·60, 0·97) and 0·72 (95 % CI 0·56, 0·93) for vitamin C; 1·00, 0·79 (95 % CI 0·62, 0·99) and 0·55 (95 % CI 0·42, 0·72) for vitamin E; and 1·00, 1·02 (95 % CI 0·81, 1·29) and 0·73 (95 % CI 0·56, 0·95) for β-carotene (Table 3). There was no statistical association between the number of teeth with periodontal disease progression and dietary α-carotene in the multivariable model (Table 3).
Table 3 Associations of intakes of dietary antioxidants, vegetables and fruits with the number of teeth with periodontal disease progression; dentate individuals (n 264) aged 75 years in 2003, Niigata City, Japan
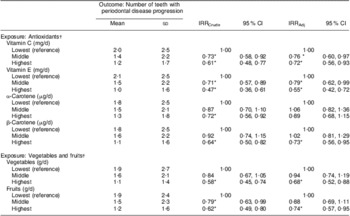
IRRCrude, unadjusted incidence rate ratio for the associations of antioxidant, vegetable and fruit intakes with the number of teeth with periodontal disease progression; IRRAdj, incidence rate ratio for the associations of antioxidant, vegetable and fruit intakes with the number of teeth with periodontal disease progression, simultaneously taking into account the number of teeth present at baseline, mean clinical attachment level at baseline, gender, lower income, lower education, BMI, hypoalbuminaemia, hyperglycaemia, smoking status, brushing frequency, use of devices for inter-dental cleaning and the pattern of visits to a dentist as covariates.
*P < 0·05.
†Energy-adjusted (i.e. amount per 4184 kJ/1000 kcal for each variable).
Associations between the number of teeth with periodontal disease progression and vegetable and fruit intakes
Table 3 shows crude and adjusted associations between the number of teeth with periodontal disease progression and vegetable and fruit intakes. In multivariable modelling, significant inverse associations were found between intakes of vegetables and fruits and the number of teeth with periodontal disease progression. The multivariate adjusted IRR in the first, second and third tertiles were, respectively: 1·00, 0·94 (95 % CI 0·74, 1·19) and 0·68 (95 % CI 0·52, 0·88) for vegetables; and 1·00, 0·88 (95 % CI 0·69, 1·11) and 0·74 (95 % CI 0·57, 0·95) for fruits (Table 3).
Discussion
The present study is the first longitudinal one to show a relationship between dietary antioxidants and periodontal disease. The findings show that higher intakes of the antioxidants vitamin C, vitamin E and β-carotene were associated with lower number of teeth with periodontal disease progression in older Japanese people. These effects were independent of several potential confounders. Although not all antioxidant intake tertile categories had statistically significantly different individual-level periodontal disease progression compared with the lowest tertile reference group, there was a tendency for a smaller number of teeth with periodontal disease progression with higher antioxidant intake. For vitamins C and E there were also significant decreased risks in the middle compared with the lowest tertile. However, differences between middle and lowest tertiles did not reach significance for β-carotene. There are several explanations. First, sample size limitations may lead to the lack of statistical significance of the three-category specification of β-carotene intake. Second, there may be a threshold for the amount of β-carotene intake that contributes to protection against periodontal disease.
A number of studies have demonstrated that oxidative stress can be mitigated by antioxidants, including vitamin C, vitamin E and carotenoids(Reference Burton and Ingold35–Reference Weber, Bendich and Schalch40), which could help explain the observed association between antioxidants and periodontal disease. Dietary antioxidants might play a beneficial role by reducing the levels of oxidative stress in periodontal tissues. It has been demonstrated that dietary intakes of vitamin C (mean 54·2 (sd 35·4) mg/d), vitamin E (mean 5·8 (sd 2·0) mg/d) and β-carotene (mean 1·7 (sd 1·4) mg/d) were negatively associated with oxidative stress biomarkers among 704 participants aged 70 years in a 7-year Swedish cohort study(Reference Helmersson, Arnlov and Larsson41). The potential relationship between dietary antioxidants and periodontal disease is also supported by a randomized trial that examined the efficacy of an antioxidant supplement on periodontal disease among smokers. Grossi and colleagues demonstrated significant improvements in CAL among those taking single-dose vitamin C/vitamin E/grape seed extract (1100 mg vitamin C, 135 mg vitamin E, 42 mg grape seed) and double-dose supplements compared with the placebo group(Reference Grossi, Nowadly and Takemura42). However, these are pharmaceutical doses that cannot be achieved through consumption of a normal diet. The present study results suggest that antioxidant nutrient levels which can be obtained through diet have a positive impact on the periodontal tissues.
The mean levels of intake of antioxidants (not including supplements) in the present study were 202·4 (sd 79·9) mg/d for vitamin C, 11·5 (sd 4·2) mg/d for vitamin E, 625·4 (sd 416·9) μg/d for α-carotene and 5841·7 (sd 2951·1) μg/d for β-carotene. These levels are higher than those reported in other countries. In the British National Diet and Nutrition Survey (NDNS) of people aged 65 years or over (n 1054)(Reference Bates, Hamer and Mishra43), the mean dietary intakes were 71·1 (sd 70·5) mg/d among men and 65·4 (sd 57·1) mg/d among women for vitamin C, and 9·5 (sd 8·2) mg/d among men and 10·7 (sd 39·4) mg/d among women for vitamin E. In the National Health and Nutrition Examination Survey (NHANES) 1999–2000, for the US population aged 60 years and over (n 1537)(Reference Ervin, Wright and Wang44), the mean dietary intakes were 104 (sd 4·6) mg/d for vitamin C and 8·3 (sd 0·3) mg/d for vitamin E. These differences may in part be due to between-survey differences in dietary methodology as FFQ have a tendency to overestimate absolute levels of intake. This is supported by the observation that the levels of antioxidant intakes among participants in the present study are also higher than those reported for Japanese people aged 75 years and over in the National Nutrition Survey in Japan (J-NNS)(Reference Katanoda and Matsumura45). In the J-NNS of people aged 75 years or over (n 1168), the mean dietary intakes were 138 (sd 127) mg/d for vitamin C and 9·9 (sd 28·9) mg/d for vitamin E. Unfortunately, neither the UK NDNS nor the US NHANES survey reports intake of vitamins adjusted for energy intake, which makes comparison with the present data difficult. However, the data from the J-NNS suggest that Japanese older people consume a higher level of nutrients than Western older populations.
In the present study, a high intake of fruits and vegetables was protective against periodontal disease. This concurs with previous data from Japan (n 261, 70 years) which showed the intake of vegetables was negatively correlated with periodontal disease(Reference Yoshihara, Watanabe and Hanada46). Moreover, a significant inverse dose–response relationship between intake of vegetables other than yellow and green vegetables (such as cabbages, radishes and onions) and the prevalence of tooth loss was demonstrated among 1002 Japanese women(Reference Tanaka, Miyake and Sasaki47). From the these findings, it may therefore be concluded that dietary modification to increase the consumption of fruits and vegetables and other foods containing vitamin C, vitamin E and carotenoids may be beneficial to oral health and have a therapeutic and preventive role in periodontal disease.
In this cohort, a homogeneous group restricted to the age of 75 years at baseline was selected to exclude the influence of race and age variation in the results. Among 334 study entrants (173 men and 161 women) seventy did not complete the study because they were unable to participate in the follow-up survey or became edentulous (Fig. 2). Individuals who withdrew were excluded from the following statistical analyses. There was no significant difference in dental conditions, demographic, socio-economic and health status and health behaviours between withdrawals and study participants; however, there is the potential risk that our final sample analysed (n 264) may not be representative of the originally sampled cohort (n 600). At baseline, four of 370 individuals did not submit complete data. We could not access the information on oral and systemic health and nutrient intakes of these individuals, and therefore we could not assess whether there were differences in these characteristics between the 334 study entrants and the four individuals who did not submit complete data. Additionally, the edentulous group (n 32) who were excluded from further analysis had a higher prevalence of hyperglycaemia and poorer oral health behaviour (i.e. did not visit a dentist regularly; data not shown). During 1998–2003, 230 of the 600 original participants were withdrawn from the study. Compared with the 370 individuals who undertook baseline assessments for the current study, the 230 withdrawals had poorer oral health behaviour in 1998 (data not shown). In this context, the current sample may be healthier than the general population; however, the healthy cohort effect would probably bias the results toward the null hypothesis and not lead to overestimation of the observed longitudinal associations.
There are several other limitations to the present study. First, our study period was only 2 years. In the present study, it is not possible to assess the effects of dietary antioxidants on periodontal conditions that could occur further out than 2 years. Over- or underestimation of the observed association between dietary antioxidants and periodontal disease caused by this short study period remains a risk. Second, the participants were restricted to the age of 75 years. At this age, they might have already changed their usual dietary intake due to tooth loss or use of dentures(Reference Krall, Hayes and Garcia48). The mean number of teeth in the study participants was 18·4 (sd 8·4) at baseline and 17·8 (sd 8·4) at follow-up. Reverse causality (i.e. dentition status affects nutrient intake) may weaken any true association as the limited number of teeth among the study participants could lead to a reduced consumption of vegetables and fruits(Reference Yoshihara, Watanabe and Nishimuta49) and therefore low intake of antioxidant vitamins. Future work with larger, more diversified, full dentate samples and over longer periods of time would be necessary to substantiate our findings. Third, information on nutritional supplement use was not available; therefore, it was not possible to assess with complete accuracy the amount of dietary antioxidants, leading to potential misclassification. In the National Health and Nutrition Survey Japan 2003 of people aged 70 years or over (n 1800)(50), the mean intake of vitamins from supplemental sources was 21 (sd 215) mg/d for vitamin C and 4·6 (sd 37·6) mg/d for vitamin E. Finally, because other information regarding participants’ oral health status (e.g. dental plaque scores, subgingival biofilm, history of periodontal treatment), medication use or physician's diagnosis of related diseases (e.g. diabetes(Reference Taylor4)) was not collected, a number of other potentially important risk factors could not be assessed in the analyses. Although the most important confounders were assessed, overestimation of the observed association between antioxidant intake and periodontal disease caused by residual confounding remains a risk.
Conclusions
Within the limitations of the present study, the reported findings suggest an independent relationship of intake of dietary antioxidants, and intake of fruits and vegetables, with periodontal disease progression among older Japanese. Further studies are necessary to substantiate these findings and evaluate the effectiveness of dietary modification to increase antioxidant intake in controlling periodontal disease.
Acknowledgements
This work was supported by grants-in-aid from the Ministry of Health and Welfare of Japan (H10-Iryo-001, H13-Iryo-001 and H16-Iryo-020) and a grant for promotion of Niigata University Research Projects (23C068). The authors have no conflict of interests to report. The authors’ contributions were as follows: M.I., corresponding author; P.M., conception and design of study, interpretation of data; M.C.M., conception and design of study, analysis and interpretation of data; G.W.T., conception and design of study; A.Y., conception and design of study, collection of data; K.M., collection of data; R.W., collection of data; H.M., conception and design of study, collection of data ethics.








