According to a global estimate based on data from 187 countries, the prevalence of anaemia has decreased from 40·2 % in 1990 to 32·9 % in 2010( Reference Kassebaum, Jasrasaria and Naghavi 1 ). The decline in prevalence was in both sexes and for all severities of anaemia. However, measurements of total years of life lived with disability (YLD), reflecting population size and disability weights, showed that anaemia was responsible for 68·3 million YLD in 2010. This was greater than 65·5 million YLD in 1990( Reference Kassebaum, Jasrasaria and Naghavi 1 ). The anaemia YLD in 2010 accounted for 8·8 % of the total for all conditions, and was more than for major depression (8·2 %), chronic respiratory diseases (6·3 %) and injuries (6·3 %)( Reference Kassebaum, Jasrasaria and Naghavi 1 ). The children under 5 years were the age group that showed the highest prevalence of anaemia; this age group was the only age group with increased anaemia prevalence from 1990 to 2010( Reference Kassebaum, Jasrasaria and Naghavi 1 ).
Children of pre-school age are the most vulnerable to the detrimental long-term effects of anaemia( Reference Kassebaum, Jasrasaria and Naghavi 1 ). Worldwide, it is estimated that 293·1 million (47·4 %) pre-school children are anaemic( 2 ). In developing countries 30–80 % of pre-school children are anaemic at 1 year of age( 3 ). In the Eastern Mediterranean Region, 46·7 % (0·8 million) of pre-school children are anaemic, which is higher than the prevalence of 21·7 % reported from Europe and 23·1 % reported from the Western Pacific Region( 2 ). The WHO classifies the severity of the public health problem of anaemia in a population into severe (≥40 % prevalence), moderate (20–39·9 % prevalence) or mild (5–19·9 % prevalence) based on the population anaemia prevalence, with a normal prevalence being defined as a prevalence of ≤4·9 %( 4 ). Out of 192 countries surveyed, the public health problem of anaemia in children of pre-school age was identified as severe, moderate and mild in sixty-nine, eighty-one and forty countries, respectively( 2 ).
Anaemia in children has significant public health consequences. It impairs the cognitive and physical development of the children and increases the mortality and morbidity rates in this population( 5 , Reference Kotecha 6 ). Breast-feeding and feeding practices, presence of infectious diseases( Reference Juyal, Osmamy and Black 7 , Reference Siegel, Stoltzfus and Khatry 8 ), presence of inherited conditions such as thalassaemia( 9 ), presence of environmental pollutants such as Pb( Reference Choi and Kim 10 ) and tobacco smoke( Reference Hong, Betancourt and Ruiz-Beltran 11 ), nutritional status, mother’s age at childbirth, maternal Hb and level of education( Reference Faber, Swanevelder and Benade 12 , Reference Moench-Pfanner, de Pee and Bloem 13 ), economic status, sanitation, and also the geographic region of residence can contribute directly or indirectly to lowering of the Hb concentration among children( Reference Osório 14 ).
From 1990 to 2010, Fe deficiency was the top-ranking cause of anaemia in children of both sexes( Reference Kassebaum, Jasrasaria and Naghavi 1 , Reference Demaeyer and Adiels-Tegman 15 ). Other causes of anaemia in children and in all time periods in the 187 countries were hookworm, sickle cell disorders, thalassaemias, schistosomiasis and malaria. The lowest burden of anaemia from Fe deficiency was in North America (2·9 %) while its highest burden was in Central Asia (64·7 %), South Asia (54·8 %) and Andean Latin America (62·3 %)( Reference Kassebaum, Jasrasaria and Naghavi 1 ). Fe-deficiency anaemia can usually be prevented at a low cost and preventive programmes for this type of anaemia are recognized as among the most beneficial of public health interventions( Reference Kotecha 6 ). According to the WHO 2013 guidelines, targeted daily Fe supplementation should be considered a greater priority in high-risk populations such as children of pre-school age, women and high-prevalence populations( 16 ). Protein deficiency reduces the Hb concentration by 20 %( Reference Warrier, Dole and Warrier 17 ) and vitamin A deficiency and Fe-deficiency anaemia are known to usually coexist( Reference Lynch 18 , Reference Bloem 19 ). Food fortification is one of the well-recognized strategies for daily supplementation with multiple micronutrients including Fe to combat micronutrient deficiencies. It is described as one of the most cost-effective and sustainable strategies available to improve the micronutrient status at the population level( Reference Horton, Alderman and Rivera 20 ). Systematic reviews of data from randomized controlled clinical trials and quasi-experimental studies have shown a positive impact of the consumption of foods fortified with Fe, folic acid and multiple micronutrients on the Fe status and Hb concentration( Reference Jai, Rehana and Salam 21 – Reference Gera, Sachdev and Boy 23 ).
In Jordan, deficiencies of vitamin A, Fe, Zn and iodine have been identified as public health problems. To address these nutritional problems, the government has implemented different nationwide food fortification programmes, including fortification of salt and wheat flour( 24 ). Evidence is needed from developing countries on the impact of wheat flour fortification with multiple micronutrients on the health of children. The range of Hb values for non-anaemic persons overlap with values for persons with Fe deficiency, leading to misclassification of Hb concentration status. Therefore, although it is a widely used test, measuring Hb concentration should not be used as a sole indicator for Fe-deficiency anaemia( Reference Gibson 25 ). Regardless of Fe concentration, there are different factors playing a significant role in lowering or increasing the concentration of Hb. These factors include biological variation, age and sex, pregnancy, higher altitudes, parasitic infections such as Plasmodium falciparum, chronic diseases such as HIV/AIDS, micronutrient deficiencies and smoking( Reference Gibson 25 ). Smoking and higher altitudes are associated with a higher concentration of Hb( Reference Gibson 25 ). In the present paper we describe the prevalence of anaemia and its correlates among pre-school children, assessed 16–20 months and 34–36 months after the initiation of wheat flour fortification with multiple micronutrients in Jordan, using two nationally representative samples collected in 2007 and 2009.
Methods
Wheat flour fortification in Jordan
The first nationwide fortification programme implemented in Jordan was the nationwide salt iodization programme launched in 1995. In 2002, the Jordanian Government mandated that all Mowahad wheat flour be fortified with only Fe and folic acid. In March 2006, the Mowahad wheat flour fortification programme was expanded to include multiple micronutrients and vitamins in addition to Fe and folic acid. In 2010, vitamin D was also added to the micronutrient premix formulation added to the wheat flour( 24 ). Mowahad wheat flour is the most widely consumed wheat flour in Jordan, accounting for more than 90 % of all the wheat flour consumed in this country, and it is the only wheat flour subsidized by the government. In 2010, the total annual cost to the government for procurement of the premix to be distributed to millers for fortifying wheat flour was approximately 1·2 million Jordanian Dinar (JD), or approximately 0·19 JD (~$US 0·27) per capita( 24 ). Nutritional strengthening of wheat flour by the addition of more micronutrients in addition to Fe and folic acid in Jordan represents one of the most nutritionally comprehensive food fortification programmes in the world. The changes in the micronutrients added to Mowahad wheat flour from 2002 to 2011 are presented in Table 1 ( 24 ).
Table 1 Fortification of Mowahad wheat flour in 2002, 2006 and 2011 in Jordan
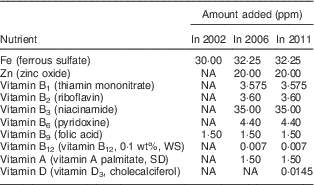
NA, not applicable (micronutrient was not added); WS, water-soluble; SD, spray-dried.
Data sources
We used data from two consecutive Jordan Population and Family Health Surveys carried out in 2007 (JPFHS-07)( 26 ) and 2009 (JPFHS-09)( 27 ) in Jordan. The Hb testing in JPFHS-07 was executed from June to November 2007( 26 ) during the summer and autumn seasons and that in JPFHS-09 was executed from October to December 2009( 27 ) during the autumn and winter seasons, which were 16–20 months and 34–36 months, respectively, after fortification of Mowahad wheat flour with multiple micronutrients was initiated (March 2006) in Jordan( 24 ). This 4-month difference in Hb testing for the two time survey points is accompanied with diversity in temperature and seasonal food items. In the summer season, the relatively hot season, there are seasonal local fruits and vegetables such as tomatoes, melons and watermelons that are rich in water, whereas in the winter season, the relatively cold season, there is an increase in the production of leafy vegetables that are rich in vitamin A, vitamin B6, folic acid, Cu and Fe.
We chose to analyse data from the two JPFH surveys conducted in 2007 and 2009. The 2002 JPFHS was conducted 4–5 months after the start of the flour fortification programme with Fe and folic acid. The last JPFHS was conducted in 2012 after a reported stoppage and fluctuation in supplying 84·6 % of wheat flour mills with micronutrient premix( 24 ).
The JPFHS was a nationally representative survey and part of the international Demographic and Health Surveys carried out to provide reliable estimates and up-to-date information about the demographic parameters and nutritional status of non-institutionalized women aged 15–49 years and children aged 6–59 months. The JPFHS followed a stratified two-stage cluster sampling design. In each survey, a representative sample of households was drawn from each of the twelve governorates independently. First, clusters were selected systematically as the primary sampling units with a probability proportional to the size of the primary sampling unit in each cluster. Second, household selection was carried out as an equal probability systematic selection of a fixed number of sixteen households from each primary sampling unit. More details of the sampling methodology are described elsewhere( 27 ).
Data were collected through face-to-face interviews carried out by trained interviewers using standardized questionnaires and methodologies. A total of 14 564 and 14 470 households, and a total of 10 426 and 9650 children aged 0–59 months, were successfully surveyed in JPFHS-07 and JPFHS-09, respectively.
Subjects
The Hb concentrations in children aged 6–59 months and women aged 15–49 years were measured in a randomly selected sub-sample of the interviewed households in each of the two surveys. The subjects of the present study were restricted to children with valid Hb measurements, with a final unweighted sample size of 4124 and 3746 children from JPFHS-07 and JPFHS-09 (weighted sample size of 3789 and 3447), respectively.
Dependent variable
Blood drops collected by finger-prick were tested immediately for Hb concentration (HemoCue AB, Sweden). Before classifying children by level of anaemia, an adjustment to sea-level equivalents was made by JPFHS using the Centers for Disease Control and Prevention formula( 28 ), given that Hb requirements differ substantially depending on altitude( Reference Dirren, Logman and Barclay 29 ). Children with a Hb level below 7·0 g/dl are classified as having severe anaemia, those with a level of 7·0–9·9 g/dl are classified as having moderate anaemia and those with a Hb level of 10·0–10·9 g/dl are classified as having mild anaemia, according to criteria developed by the WHO( 4 ). The outcome variable was presence/absence of anaemia. Subjects were dichotomized based on a Hb cut-off level of 11 g/dl (Hb<11 g/dl, anaemia present; Hb ≥11 g/dl, anaemia absent)( 4 ).
Independent variables
Specific socio-economic and demographic variables of the children and mothers were used for the analyses. Data on the following characteristics were collected: age of the child in months (≤24, 25–48 or >48 months), sex (male or female), birth weight (≤2500 or >2500 g), place of residence (urban or rural), geographic location of residence (north, central or south) and duration of breast-feeding (≥6 months, <6 months or never breast-fed). Children who were, at the time of the survey, on breast-feeding or breast-fed for >6 months were regarded as breast-fed for ≥6 months. The household wealth index as used an indicator of the family wealth status (very poor, relatively poor, middle-income household, relatively rich and very rich)( Reference Rutstein and Johnson 30 ).
The children’s nutritional status was assessed by measuring their height-for-age. A poor height-for-age index reflects the long-term, cumulative effects of inadequate nutrition, poor health or both. For children aged >24 months, their standing height was measured, whereas for those aged <24 months, their recumbent length was measured using the Shorr height board. As compared with the median of the reference population, children with a height-for-age index below −2 sd were categorized as stunted/chronically malnourished, those with an index of −2 to −2·99 sd were categorized as showing moderately stunted growth, and those below −3 sd as showing severely stunted growth( 31 ).
Mother’s age (15–24, 25–34 or 35–49 years) and level of education (primary and below, secondary or higher education) were classified into three groups. To be comparable with respect to sea level( Reference Dirren, Logman and Barclay 29 ) and smoking status( Reference Nordenberg, Yip and Binkin 32 ), the measured maternal Hb concentration was adjusted by the JPFHS using the Centers for Disease Control and Prevention formulas( 28 , 33 ). According to WHO criteria, non-pregnant and pregnant mothers with a Hb level of <11·9 g/dl and <10·9 g/dl, respectively, were defined as anaemic( 4 ). BMI was measured to evaluate the mothers’ nutritional status, and the mothers were categorized based on the standard WHO classification into underweight (BMI<18·5 kg/m2), normal weight (BMI=18·5–24·9 kg/m2) or overweight (BMI≥25·0 kg/m2). Tobacco smoking was recorded as yes or no, and the mothers were also classified based on the type of tobacco smoked into either exclusive waterpipe or exclusive cigarette smokers.
Statistical analyses
Descriptive statistics are presented as weighted percentages for the measured variables and the differences in the percentage of children for each measured covariate between the JPFHS-07 and JPFHS-09 were assessed by the χ 2 test. The difference in the weighted mean Hb level in the surveyed children between JPFHS-07 and JPFHS-09 was assessed by the Student’s t test. We estimated the absolute difference in the weighted prevalence of anaemia for each measured covariate between the JPFHS-07 and JPFHS-09 by the χ 2 test. Differences in the percentage of weighted anaemia between sub-categories of each measured covariate were assessed by the χ 2 test after pooling the two data sets. A sampling weight was developed by JPFHS according to the sample allocation. Weighted analysis was conducted to adjust for the complex household survey design including potential over-sampling or under-sampling and to ensure the actual representativeness of the survey results at the national level as well as at the cluster level.
After excluding 268 children with missing information on height and forty-five children with missing information on Hb concentration of the mother in both surveys, multivariate models including only children for whom full information about the measured characteristics was available were performed to estimate the prevalence of childhood anaemia after adjustments for the effects of the survey phase, children’s characteristics and mothers’ characteristics in the overall population of children and by the survey phase. To adjust for potential confounders, all variables that showed a significant relationship with anaemia at P≤0·05 in the bivariate analysis were included in the multivariate models, simultaneously. Absence of multicollinearity between independent variables was confirmed using the variance inflation factor. Results were reported in terms of crude and adjusted odds ratios to assess the strength of the association between the predictors and anaemia, and the 95 % confidence intervals were calculated for significance testing. Analyses were performed using the statistical software package IBM SPSS Statistics version 18·0. A two-tailed P value of ≤0·05 was considered as denoting statistical significance.
Results
In both surveys, the most represented age group was children aged 25–48 months (44·9 %); about 83 % of the children were born with a birth weight of >2500 g and were from urban settings, without any significant differences in these three characteristics between the two surveys. The percentage of children breast-fed for <6 months increased from 16·6 % in JPFHS-07 to 19·3 % in JPFHS-09. The percentage of children living in very poor households declined from 27·5 % in JPFHS-07 to 24·2 % in JPFHS-09, with a corresponding increase in the percentage of children living in relatively rich households from 16·2 % to 19·1 %. The percentage of moderately or severely stunted children declined by more than a half, from 14·7 % in JPFHS-07 to 8·2 % in JPFHS-09 (Table 2).
Table 2 Descriptive data and prevalence of anaemia among children of pre-school age in Jordan by the children’s characteristics, stratified by the survey phase
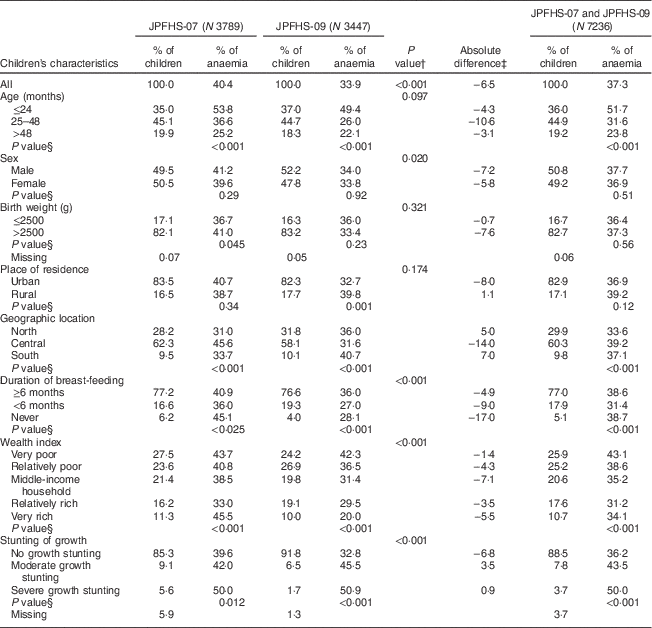
JPFHS, Jordan Population and Family Health Survey; N, weighted number of children.
† P value from χ 2 test for the difference in the percentage of children according to children’s characteristics by the survey phase.
‡ Absolute difference in the occurrence of anaemia between the two surveys (prevalence in 2007 subtracted from prevalence in 2009).
§ P value from χ 2 test for the occurrence of anaemia among children according to children’s characteristics in each survey phase.
On average, the children’s mean Hb level was 11·37 g/dl (range: 8·0–15·0 g/dl), with a significant increase in the overall mean by 2·08 g/dl from JPFHS-07 to JPFHS-09 (P<0·001). Of the total sample, 37·3 %, 15·5 % and 21·7 % of the children were severely anaemic, moderately anaemic and mildly anaemic, respectively. The most significant decline in the prevalence of anaemia was found in children with mild anaemia (from 24·3 % in JPFHS-07 to 18·9 % in JPFHS-09; P<0·001) during the study period (data not shown). The decline was across all the measured characteristics of the children, except among children who lived in rural areas, in the north and south regions of the country, and those with moderate and severe growth stunting. The decline was higher among children aged 25–48 months (−10·6 %), males (−7·2 %), children with a birth weight of >2500 g (−7·6 %), children from urban areas (−8·0 %), children from the central regions (−14·0 %), children belonging to middle-income households (−7·1 %) and children who had never been breast-fed (−17·0 %). The prevalence of anaemia was not associated with sex, birth weight or place of residence in either survey (Table 2).
Table 3 shows a comparison of the mother’s characteristics and the prevalence of anaemia among the children in JPFHS-07 and JPFHS-09. The percentage of mothers with a higher level of education increased from 27·5 % in JPFHS-07 to 30·2 % in JPFHS-09. Maternal anaemia declined by 5·8 %, while the percentage of overweight women increased by 6·2 % from JPFHS-07 to JPFHS-09 (P<0·001). Anaemia among children declined across all mothers’ ages and education groups. The anaemia prevalence declined by 7·4 % among children living with mothers aged 15–24 years and by 8·8 % among children born to mothers with a higher level of education. The anaemia prevalence also declined by 21·9 % among children born to tobacco-smoking mothers.
Table 3 Descriptive data and prevalence of anaemia among children of pre-school age in Jordan by the mothers’ characteristics, stratified by the survey phase
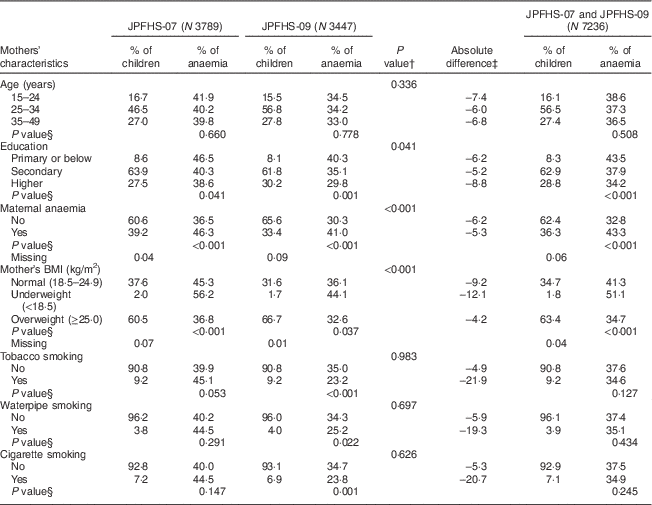
JPFHS, Jordan Population and Family Health Survey; N, weighted number of total children.
† P value from χ 2 test assessing the difference in the percentage of children according to mothers' characteristics by the survey phase.
‡ Absolute difference in the occurrence of anaemia between the two surveys (prevalence in 2007 subtracted from prevalence in 2009).
§ P value from χ 2 test assessing difference in the occurrence of anaemia among children according to mothers' characteristics in each survey phase.
After pooling the two data sets, children tested in JPFHS-09 were 25 % less likely to show a Hb level indicative of anaemia (adjusted OR=0·75; 95 % CI 0·68, 0·83; P<0·001) than those tested in JPFHS-07 (data not shown). Table 4 shows the adjusted OR of childhood anaemia by the children’s characteristics. In both surveys, childhood anaemia was positively associated with age of the child ≤24 months, living in the central region of the country, breast-fed for ≥6 months, living in a family of relatively lower wealth status and malnutrition. Chronic malnutrition was the characteristic that showed the greatest positive correlation with the presence of anaemia.
Table 4 Adjusted odds ratiosFootnote † of childhood anaemia among children of pre-school age in Jordan by the children’s characteristics and by the survey phase
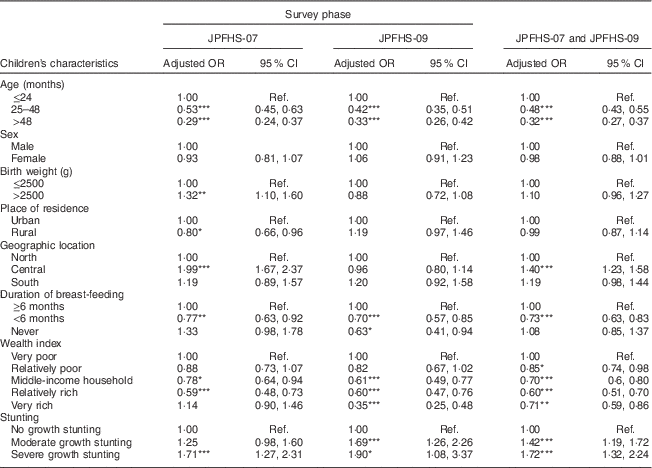
JPFHS, Jordan Population and Family Health Survey; Ref., reference category.
*P<0·05, **P<0·01, ***P<0·001.
† Adjusted OR include 6924 children with full information about all variables included in the multivariate model (in 2007, n 3538; in 2009, n 3386).
Table 5 shows the adjusted effect of the mothers’ characteristics on childhood anaemia. Anaemia among children was positively associated with increasing mother’s age, maternal anaemia and below-normal body weight of the mothers. Mother’s exposure to tobacco smoke was not associated with the prevalence of childhood anaemia. Living with an anaemic (adjusted OR=1·69) or an underweight mother (adjusted OR=1·75) showed the strongest positive association with the prevalence of childhood anaemia (P<0·01).
Table 5 Adjusted odds ratiosFootnote † of childhood anaemia among children of pre-school age in Jordan by the mothers’ characteristics and by the survey phase
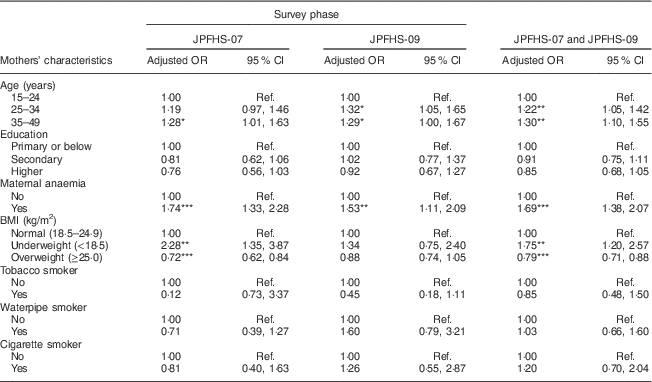
JPFHS, Jordan Population and Family Health Survey; Ref., reference category.
*P<0·05, **P<0·01, ***P<0·001.
† Adjusted OR include 6924 children with full information about all variables included in the multivariate model (in 2007, n 3538; in 2009, n 3386).
Discussion
The prevalence of anaemia in pre-school children aged 6–59 months declined significantly within the 2-year study period in Jordan, from 40·4 % in 2007 to 33·9 % in 2009, after the implementation of fortification of wheat flour with multiple micronutrients. The decline was more pronounced in children with better socio-economic and nutritional status. Furthermore, the decline was apparent across all of the measured characteristics of the children, except for residence in a rural area, residence in the north and south regions of the country, and moderate or severe malnutrition. The most pronounced decline was seen in children aged 25–48 months, children living in urban areas or the central region of the country, children who had never been breast-fed and children who did not show stunted growth.
The positive impact of food fortification on the prevention and control of micronutrient deficiencies has been widely discussed( Reference Kotecha 6 , Reference Demaeyer and Adiels-Tegman 15 ). A recent systematic review of 201 studies conducted on children and women (including 125 randomized controlled trials and seventy-six quasi-experimental studies) reported that fortification of foods with multiple micronutrients was associated with increased serum micronutrient and Hb concentrations among children( Reference Jai, Rehana and Salam 21 ). Another systematic review carried out in Brazil of the data from studies conducted pre- and post-flour fortification with folic acid showed that the serum folate concentrations increased by 57 % in healthy children and 174 % in adults( Reference Britto, Cançado and Guerra-Shinohara 34 ). In Costa Rica, the prevalence of anaemia in children decreased from 19·3 % to 4·0 % and that of Fe deficiency declined from 26·9 % to 6·8 % after mass fortification of staple foods and condiments with multiple micronutrients( Reference Martorell, Ascencio and Tacsan 35 ). Among pregnant women assisted in primary health-care services in Maringá municipality in southern Brazil, as one of the vulnerable groups for anaemia, a study found that women tested after flour fortification had a higher Hb level than those tested before flour fortification with Fe, after women who were receiving Fe supplementation were excluded( Reference Araújo, Uchimura and Fujimori 36 ). Another large-scale population-based study using repeated cross-sectional samples of 12 119 pregnant women in five Brazilian regions showed that the prevalence of anaemia declined from 25 % pre-fortification to 20 % (P<0·001) among tested pregnant women at least 1 year after the mandatory fortification of wheat and corn flour with Fe( Reference Fujimori, Sato and Szarfarc 37 ). In the present study, the observed improvement of nutritional status over time was in parallel with a decline in the severity of the public health problem of anaemia in children of pre-school age from severe to moderate. An expanded fortification of Mowahad wheat flour with multiple micronutrients initiated in 2006 was consistent with reduction of the prevalence of anaemia.
Different countries in the Middle East and North Africa region have implemented wheat flour fortification programmes. In the 1990s, Oman, Bahrain, Iraq, Libya and Iran began fortifying wheat flour with only Fe and folic acid( 38 ). In the present study, although a decline was observed in the prevalence of childhood anaemia in Jordan over the 2-year study period, the prevalence in the last survey carried out in 2009 was still higher than that among pre-school children in many other countries in the region, including Bahrain (24·7 %), Cyprus (18·6 %), Egypt (29·9 %), Lebanon (28·3 %) and Qatar (26·2 %)( 2 ). Nevertheless, findings from the current study encourage continuation of fortifying wheat flour with multiple micronutrients in addition to Fe and folic acid in Jordan.
Different factors were associated with the low Hb concentrations found among pre-school children. The severity of anaemia varied among different subgroups of age: younger children were at a higher risk of developing anaemia, with children aged ≤24 months at twofold higher risk of anaemia than children aged 25–48 months. This is consistent with the findings of numerous previous studies( Reference Ewusie, Ahiadeke and Beyene 39 – Reference Osório, Lira and Batista-Filho 42 ). The prevalence of anaemia among children tends to decrease linearly from 2 years of age( Reference Assis, Santos and Martins 40 , Reference Miglioli, Brito and Lira 41 ). In the first 2 years of life, growth and development occur at an accelerated pace in children and they need higher amounts of Fe( Reference Stekel 43 , 44 ). After the sixth month, Fe stores are depleted and nutrition plays a crucial role in the supply of this mineral to the infant. Older children, as a result of growth and development, obtain adequate amounts of Fe quite easily from the consumption of different foods( Reference Osório, Lira and Batista-Filho 42 – 44 ).
The decline in the prevalence of childhood anaemia was associated with the place of residence and region. There was a decline over time in the prevalence of anaemia in urban areas, while the prevalence actually increased slightly in rural areas. The anaemia prevalence declined in children living in the central region of the country, but increased in those living in the northern and southern regions. In the central region, the public health problem of anaemia declined from severe to moderate; in the southern region, it increased from moderate to severe; and in the northern region, it remained moderate, although the prevalence increased. These geographical variations in the prevalence of anaemia can be linked to two factors: (i) most of the areas in the central region of the country are urban areas with low levels of poverty and illiteracy; and (ii) there is a possibility of variation in the distribution of fortified flour. A recent nationally representative survey in Jordan reported that only 44·1 % of bread samples collected from households tested positive for the presence of Fe, indicating that only some of the bread was fortified, and the percentage of adequately iodized salt samples was higher in urban areas than in rural areas( 24 ). The mill monitoring reports in Jordan for the sixteen months prior to 2010 found that of the thirteen mills that were supposed to produce fortified flour in Jordan, 84·6 % did not fortify flour for five of the sixteen months, because they did not receive the premix( 24 ). This fluctuation in adding premix to the flour creates inequality in the access to the supplemented micronutrients across different regions in the country and also raises concern about increasing micronutrient deficiencies and prevalence of anaemia in the future. In Sweden, after 50 years of flour fortification with Fe, withdrawal of the general Fe fortification resulted in a decrease of the total Fe intake by 39 % and a substantial increase in the prevalence of Fe deficiency in girls( Reference Sjöberg and Hulthén 45 ).
Blood Hb concentration is affected by tobacco smoke. Over the 2-year period, there was an observable decline in the prevalence of anaemia among children living with tobacco-smoking mothers. Passively and actively inhaled tobacco smoke increases the blood level of carboxyhaemoglobin, which is a type of Hb formed by the binding of carbon monoxide to Hb( Reference Maziak, Ward and Afifi Soweid 46 ). In smokers, the reduced oxyhaemoglobin levels due to the formation of carboxyhaemoglobin stimulate the production of erythropoietin, which increases the Hb concentration( Reference Milman and Pedersen 47 ). However, a positive relationship between low Hb concentration and passive exposure to tobacco smoke has been documented previously among infants( Reference Hong, Betancourt and Ruiz-Beltran 11 , Reference Gavalov, Soboleva and Deriagina 48 ). In the present study we failed to document any positive relationship between passive exposure to mothers’ tobacco smoke and childhood anaemia, even after stratifying the children according to the type of tobacco smoked by the mothers. This is in line with a previous report from Jordan which reported that while smoking by both parents was positively associated with anaemia in children, smoking by one of the parents was not associated with the development of anaemia in the children( Reference Hong, Betancourt and Ruiz-Beltran 11 ). However, in the present study, only information about tobacco smoking by the mothers was available.
Breast-feeding for ≥6 months was independently associated with an increased risk of childhood anaemia compared with breast-feeding for <6 months. Breast-feeding for ≥6 months is associated with an increased risk of anaemia( Reference Maziak, Ward and Afifi Soweid 46 , Reference Dewey, Cohen and Rivera 49 ). By 6 months, complementary foods are required to provide the Fe and other nutrients necessary for the development of the infant( Reference Dewey, Cohen and Rivera 49 , Reference Picciano 50 ). In Jordan it is a usual practice for mothers to introduce solid foods, including bread, to their babies by the fourth month of life. More than two-thirds of children under 6 months old in Jordan have already been introduced to complementary foods( 27 ). The WHO recommends exclusive breast-feeding for the first 6 months of life based on a review of different studies carried out prior to 2001( 51 ). However, because of the recent growing evidence of the association between exclusive breast-feeding and childhood anaemia, particularly in developing countries where anaemia is prevalent, there is a growing debate about whether solid foods should be introduced to babies before 6 months of age( Reference Meinzen-Derr, Guerrero and Altaye 52 ).
In the current study we chose a cut-off of Hb <11·0 g/dl to indicate anaemia because it is the WHO recommended and the most common cut-off point for pre-school children aged 6 months to 5 years( 4 ). However, use of different cut-offs such as <12·0 g/dl used by the WHO for all children in the global burden of Fe-deficiency anaemia( Reference Rastogi and Mathers 53 ) or 10·5 g/dl used by another study( Reference Domellöf, Dewey and Lönnerdal 54 ) revealed a significant decline in anaemia prevalence among children tested in 2009 compared with those tested in 2007. Thus, anaemia trends over this study period declined significantly and should not have been affected by the different thresholds used.
The high prevalence of anaemia among children in Jordan compared with other countries in the region( 2 ) could be explained by different potential contributors, beyond factors revealed in our study. These factors include, but are not limited to: the decrease in consumption of white flour and fruits and vegetables from 2002 to 2008; in 2010 less than half of households were consuming fortified bread( 24 ); economic crisis and increase in the cost of living, where the real growth in gross domestic product declined from 8·1 % in 2005 to 2·6 % in 2011( 55 ); in addition to the huge influx of refugees into the country who live under dire life circumstances and place extra burden on the available resources and food prices( 56 ). To achieve further decline in the prevalence of childhood anaemia in Jordan, more concerted efforts are needed. Anaemia-related health education and a focus on parents (particularly mothers), rural areas and expansion of the fortification programme to other food items besides flour and salt, such as milk( 51 , Reference Daly, MacDonald and Aukett 57 , Reference Torres, Sato and Queiroz 58 ) and rice( 59 ), are necessary. In high rice-consuming countries, such as Jordan, the WHO recommends rice fortification programmes for supplementing more essential minerals and vitamins to the general population( 59 ). Fortifying multiple food items should be preceded with assessment of intakes in the population, degree of small-scale and home production, and industry consolidation. As well, in food fortification, it is preferable to use food vehicles that are centrally processed( 60 ). Fortifying centrally processed food has the potential to be an effective public health strategy for the elimination of micronutrient deficiencies and reducing the burden of anaemia. Promoting dietary diversity is another preferred strategy to improve the nutrition of a population( 60 ). A comprehensive combination of these interventions would be expected to result in a rapid and significant further decline in the prevalence of childhood anaemia in Jordan.
The current findings should be interpreted bearing the following limitations in mind. First, the cross-sectional analysis limits establishment of a cause-and-effect relationship. Second, due to cultural barriers and beliefs, interviewing women in front of their family members may reduce the likelihood of obtaining correct answers about behavioural variables, such as smoking, and could result in response bias. In addition, due to the unavailability of information about the father’s smoking status, we could not measure the association between exposure to both parents’ smoking and the development of anaemia. Third, the decline in anaemia prevalence during the 2-year period could be also attributed to other changes independent of the fortification programme. For example, the difference in Hb level between the two survey phases could be subject to the seasonal vegetable and fruit supply in summer and winter, as well as by the seasonal difference in temperature. A large cross-sectional study reported significantly lower values of Hb in August( Reference Kristal-Boneh, Froom and Harari 61 ), which was attributed to increased plasma volume expansion( Reference Borgna-Pignatti, Ventola and Friedman 62 – Reference Sawka, Convertino and Eichner 65 ). Plasma volume during the summer was reported to be higher by 5·5 % in healthy non-smokers( Reference Kristal-Boneh, Froom and Harari 66 ). One study found that seasonal change in Hb level was attributable to changes in the length of the day rather than changes in temperature( Reference Sjostrand 67 ). On the other hand, the intake of vitamin A, vitamin B6, folic acid, Cu and Fe, which is considered to contribute to blood formation, is influenced by the availability of seasonal foods; the JPFHS-09 that showed higher Hb level was conducted in winter where more green leafy vegetables that contain vitamin A, vitamin B6, folic acid, Cu and Fe are available compared with summer, which might influence the anaemia status. A study showed that the frequency of consumption of leafy vegetables that are rich in vitamins, Cu and Fe was higher during winter compared with summer, alongside higher mean Hb concentration during winter and the rainy season( Reference Deepa, Bharati and Kasturiba 68 ). Fourth, the relative bioavailability and stability of micronutrients during processing, manufacturing, baking and storage were not assessed in the present study( 60 ). Finally, owing to the use of pre-existing data and defining anaemia based on the blood level of Hb alone, without testing for other indicators such as the serum ferritin, transferrin saturation and erythrocyte protoporphyrin levels, we could not identify the type of anaemia in individual subjects: whether it was related to nutritional, genetic, parasitic, or other physiological or infectious factors. However, the current findings are very important to fine-tune the development of national strategies to combat anaemia in Jordan.
Conclusions
The current study provides confirmatory evidence for the shift of the public health problem of anaemia in Jordanian children of pre-school age from severe to moderate. This decline has occurred following the initiation of a fortification programme of wheat flour and suggests the improvement in micronutrient deficiencies in these children as a result of nutritional strengthening of wheat flour with multiple micronutrients. However, anaemia in children still remains a public health issue affecting one-third of pre-school children in Jordan. Children aged ≤24 months, children living in low socio-economic strata such as in rural areas, children living with anaemic mothers and children who are exclusively breast-fed for ≥6 months are groups that are at increased risk for the development of anaemia.
Acknowledgements
Acknowledgements: The authors are greatly thankful to the Department of Statistics, Jordan and ICF Macro for the 2007 and 2009 JPFHS through the MEASURE DHS programme and to the individuals in Jordan who contributed to the 2007 and 2009 JPFHS. Financial support: This study was partly supported by a Grant-in-aid for Scientific Research from the Japan Society for the Promotion of Science (JSPS; grant number 24390164). The JPFHS-07 and JPFHS-09 were funded primarily by the Government of Jordan with additional funding from the US Agency for International Development (USAID) and the United Nations Population Fund (UNFPA). The funders had no role in the design, analysis or writing of this article. Conflict of interest: None. Authorship: R.A.R. and K.N. conceptualized and designed the study. R.A.R. obtained the data and R.A.R. and K.N. analysed the data. R.A.R. wrote the manuscript and K.N. and K.S. critically reviewed the manuscript. All authors approved the final manuscript. Ethics of human subject participation: This study was conducted according to the principles laid down in the Declaration of Helsinki and all procedures involving human subjects were undertaken with the approval of the Ethics Review Committee for scientific researches in Jordan. Verbal informed consent was obtained from the children’s mothers or caregivers prior to their participation in the study and the blood testing. Verbal consent was witnessed and formally recorded.








