Iodine deficiency is one of the leading causes of preventable brain damage worldwide(1). The spectrum of iodine deficiency-related psychomotor disturbances varies from discrete neurological or cognitive deficits, through poor performance at school and formal psychomotor tests, to the most worrisome presentation in the form of endemic cretinism, and is at least partially dependent on iodine deficiency severity (although it may be modified by many factors such as other nutrient deficits, a goitrogenic diet or the socioeconomic environment)(Reference Pearce, Lazarus and Moreno-Reyes2–Reference Melse-Boonstra and Jaiswal4). For example, children living in areas of severe iodine deficiency in China scored 12·5 IQ points less than children residing in iodine-sufficient regions. Moreover, the introduction of iodine prophylaxis overcame that difference in children born afterwards(Reference Qian, Wang and Watkins5). Even mild to moderate iodine deficiency in pregnancy may impact the verbal IQ of children, as it was recently demonstrated in a meta-analysis of results of three prospective European birth cohorts (Generation R, INMA, ALSPAC)(Reference Levie, Korevaar and Bath6). Adequate iodine nutrition during the first 1000 days of life (including fetal period, infancy and early childhood) is of utmost importance, as during that period a child is almost exclusively dependent on iodine and thyroid hormones supply from his or her mother(Reference Velasco, Bath and Rayman7). However, even an improvement in iodine nutrition later in life may result in better cognitive function, as was demonstrated in Albanian school children(Reference Zimmermann, Connolly and Bozo8). Iodine deficiency, as well as prophylactic measures against it, influences also the epidemiology of thyroid disorders(Reference Zimmermann and Boelaert9).
Therefore, the elimination of iodine deficiency became part of the goals of the World Health Assembly already in 1991(1). Since that time, much progress has been made, and preventive measures undertaken in many countries have led to a substantial improvement or optimisation of iodine nutrition in the general population(Reference Pearce, Andersson and Zimmermann10–12).
Poland is one of the countries that pioneered iodine prophylaxis, with the first attempts of introducing iodised salt undertaken in 1935(Reference Szybiński13). After World War II, household salt iodisation was reintroduced as a voluntary model, with iodine (KI) content reaching 12 mg/kg of salt. However, the unstable economic and political situation led to the interruption of the prophylaxis in the first half of the 1980s(Reference Szybiński13). The voluntary model of prophylaxis with a KI concentration of 20 mg/kg of household salt was started again in 1986(Reference Szybiński13). However, the results of a nationwide study conducted among school children in 1992/1993 proved it being unsuccessful. Most of the Polish territory was recognised as moderately iodine-deficient, with >80 % of children having UIC <100 µg/l(Reference Szybiński13,Reference Szybiński and Żarnecki14) . The low iodine status of Polish school children was confirmed by the results of the project ‘Standardized Evaluation of Iodine Deficiency in Europe’ conducted in 1994/1995(Reference Delange, Benker and Caron15,Reference Szybiński, Delange and Lewiński16) . Also, the iodine nutrition of pregnant women was poor, with only 8·1 % of them having UIC >150 µg/l in the first trimester of pregnancy(Reference Gołkowski, Bałdys-Waligórska and Huszno17).
Therefore, in January 1997, the Polish Ministry of Health and Social Welfare introduced obligatory iodine prophylaxis with 30 ± 10 mg of KI per kilogram of salt, which corresponds to approximately 23 mg of iodine in 1 kg of salt(Reference Szybiński13). It needs to be stressed that the Polish model differs from the universal salt iodisation recommended by the WHO, which also includes the fortification of salt used in the food industry(1).
However, iodine deficiency once eliminated might return, as it is susceptible to, among others, changes in salt industry policies, import practices, law regulations and consumer groups’ attitudes and awareness, including dietary habits and preventive measures against other diseases(1,Reference Pearce, Andersson and Zimmermann10,Reference Herrick, Perrine and Aoki18–Reference Rohner, Zimmermann and Jooste20) . Changes in salt consumption may be of particular importance for iodine prophylaxis efficacy(Reference Szybiński, Jarosz and Hubalewska-Dydejczyk19). Salt intake in Poland in 2010 was estimated at 11·5 g/d (both household salt and salt from processed foods)(Reference Sekula, Oltarzewski and Ciskowska21). In a study conducted between 2012 and 2015, salt consumption in the elderly exceeded 8 g daily, and covered the recommended daily iodine intake (median 134 µg/d)(Reference Guligowska, Pigłowska and Śmigielski22). In Poland, a public campaign for the reduction of dietary sodium consumption as a preventive measure in CVD is gaining momentum under the auspices of the National Food and Nutrition Institute and the Ministry of Health. If successful, it might result in inappropriately low dietary iodine intake. There is some evidence proving this possibility. In the abovementioned study, only 16 % of elderly patients with a history of myocardial infarction met daily nutritional iodine requirements as a result of diminished salt consumption(Reference Guligowska, Pigłowska and Śmigielski22).
Excessive iodine intake as a result of uncontrolled food iodisation or high natural content of iodine in soil and drinking water is also possible(Reference Huang, Peng and Huang23–Reference Farebrother, Zimmermann and Abdallah25). Iodine deficiency elimination in the general population does not exclude its persistence in groups requiring increased amounts of this micronutrient(Reference Pearce, Andersson and Zimmermann10,Reference Wong, Sullivan and Perrine26) .
According to the WHO and UNICEF guidelines, monitoring the impact and sustainability of preventive measures is essential for effective elimination of iodine deficiency disorders(1,27) . The issue of iodine intake monitoring as a tool of successful iodine prophylaxis in Europe was also stressed lately by the EU Horizon 2020 EUthyroid Consortium(Reference Völzke, Erlund and Hubalewska-Dydejczyk28,Reference Völzke, Caron and Dahl29) .
The current study aimed to evaluate the effectiveness and sustainability of the WHO mandatory iodine prophylaxis adopted in Poland on vulnerable Polish populations (school children, pregnant and lactating women).
Materials and methods
The study was performed between October and December 2017 within the framework of the National Health Programme funded by the Polish Ministry of Health. The study was approved by the local ethical board. Written informed consent was obtained from every participant (adult women) or the participants’ (school children) legal guardians, usually parents.
Study group and area of investigation
Seven out of sixteen administrative areas in Poland (voivodships) were randomly selected, then grouped into five regions: central (Świętokrzyskie), north (Kujawsko-Pomorskie), north-east (Podlaskie), south (Opolskie and Śląskie) and south-east (Małopolskie and Podkarpackie). In each of the voivodships, one to two towns or cities with a maternity/obstetrics ward were randomly chosen, and cooperation of each selected ward was asked for. If the collaboration of such a department was assured, a primary school with at least 100 pupils aged 6–12 years, localised within 20 km of the ward location, was randomly drawn. The school was included in the survey if the approval of the head and parental council was obtained.
Participant recruitment
Since the General Data Protection Regulation does not allow to search for and address study subjects directly in Poland (which also excludes stratification of the study population), pregnant and lactating women volunteered to the study. The survey was advertised in selected maternity wards and cooperating outpatient obstetrics clinics; social media were also used.
In the case of school children, information about the survey was distributed in advance among eligible 6–12-years-old children’s parents by the school authorities. A child was included in the study if, on the day of examination, he or she provided a written consent from their legal guardian.
Pregnant and lactating women
The study included 300 pregnant women (aged 18–46 years, median age 30 years) at a median 29 weeks of gestation. Forty-four (14·7 %) of them were in the first trimester of pregnancy, 63 (21 %) in the second trimester and the remaining 193 (64·7 %) in the third trimester. The survey also comprised 100 lactating women aged 15–44 years (median age 31 years) up to 1 year after delivery; the median duration of lactation at the moment of testing was 7 weeks. The study was conducted in the following regions of Poland: northern (two locations), north-eastern (one location), southern (two locations), south-eastern (three locations) and central (one location). The exact number of women assessed in each region is presented in Table 1.
Table 1 Number of women assessed according to the place of residence

School children
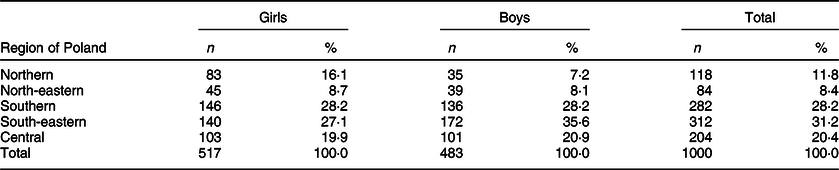
The survey included 1000 school children aged 6–12 years (517 (51·7 %) girls and 483 (48·3 %) boys). The investigation was conducted in the following regions of Poland: northern (two locations), north-eastern (one location), southern (two locations), south-eastern (three locations) and central (one location). The exact number of children investigated in each region is presented in Table 2.
Table 2 Number of school children investigated according to the place of residence and gender
Methods
Urinary iodine concentration
Urinary iodine concentration (UIC) measurements were performed in the Medical Diagnostics Unit of the Faculty of Pharmacy of the Jagiellonian University Medical College in Krakow and were handled according to the WHO(1) and the EUthyroid Consortium(Reference Erlund, Arohonka and Raman30) recommendations. UIC was measured in the spot morning urine sample provided by each participant. The study subjects were instructed to avoid the first morning void. Two millilitres of the urine was transferred to urine tubes, frozen and stored at –20°C until measurement. UIC was measured after sample defrosting by Sandell–Kolthoff reaction(Reference Sandell and Kolthoff31). The laboratory was participating in the UIC accuracy testing programme led by the National Institute of Health and Welfare (THL), Helsinki, Finland, within the EUthyroid Consortium (unpublished data).
The results of UIC measurements were interpreted according to the WHO/UNICEF criteria(1,27,Reference Zimmermann, Jooste and Pandav32) . Iodine intake was considered adequate if median UIC of the studied population was in the range of 150–249 µg/l in pregnant women, ≥100 µg/l in lactating women and in the range of 100–299 µg/l in school children(1,27,Reference Zimmermann, Jooste and Pandav32) . In the case of school children, an additional criterion of iodine sufficiency of the studied population was <20 % of urine samples with UIC <50 µg/l(1,27) .
Thyroglobulin
Serum thyroglobulin was estimated in pregnant and lactating women. The samples were handled according to the WHO and EUthyroid Consortium criteria(1,Reference Erlund, Arohonka and Raman30) . Samples of whole blood were collected to standard no-additive tubes, centrifuged at room temperature, and serum aliquots were pipetted to storage vials, frozen and stored at –20°C till measurements.
All blood samples were analysed in the Clinical Biochemistry Department of the University Hospital in Krakow with ROCHE Cobas® 6000/8000 platform by electrochemiluminescence (Roche Eclesys Tg II assay; Roche Diagnostics). The Clinical Biochemistry Department was certified by the Randox International Quality Assessment Scheme, the Unity Interlaboratory Comparison Program, the Sysmex International Quality Assurance System and the StandLAB IQS.
The WHO proposed 4–40 ng/ml as the normal range of thyroglobulin in iodine-sufficient school children (preferably in dried blood spots (DBS))(1,Reference Zimmermann, de Benoist and Corigliano33) . Based on the results of a large cohort study, iodine sufficiency in school children was defined as a median thyroglobulin level in the studied population <13 ng/ml and <3 % of studied subjects with thyroglobulin values >40 ng/ml(Reference Zimmermann, de Benoist and Corigliano33). These values were adopted as an iodine nutrition marker for pregnant and lactating women in our survey. Additionally, in pregnant women, the reference values proposed by Stinca et al. (Reference Stinca, Andersson and Weibel34) in 2017 (DBS thyroglobulin reference range 0·3–43·5 ng/ml; iodine sufficiency indicated by a median thyroglobulin level <10 ng/ml and <3 % of thyroglobulin values ≥44 ng/ml) were applied.
Statistical analysis
The database was double-checked independently by two members of the study team to assure the correctness of data entry. The statistical analysis was performed with Microsoft Excel, IBM SPSS 25 and Statistica 13 (StatSoft) software. Due to non-gaussian distribution of the data, median and interquartile range (IQR = quartile 3, quartile 1) were used for descriptive statistics. According to UNICEF guideline(27), 95 % bootstrapped CI (95 % BCI) for median UIC was calculated. Kruskal–Wallis ANOVA was applied to compare the medians.
Results
Pregnant women
Median UIC was 111·6 µg/l (IQR 134·3, 95 % BCI 97·8, 124·2). There was no significant inter-regional difference in median UIC (P = 0·3388) (Table 3). Median thyroglobulin in pregnant women was 13·3 ng/ml (IQR 17·0), with no significant inter-regional difference (P = 0·1250) (Table 4). In 8 % (24/300) of investigated pregnant women, thyroglobulin was >40 ng/ml, and in 7·3 %, it was >44 ng/ml. According to the WHO criteria, the investigated sample of pregnant women was iodine-deficient.
Table 3 Median urinary iodine concentration (UIC) (µg/l) in pregnant and breastfeeding women according to the place of residence

BCI, bootstrapped CI.
Table 4 Median serum thyroglobulin (ng/ml) in pregnant and breastfeeding women according to the place of residence

IQR, interquartile range.
Lactating women
Median UIC in breastfeeding women was 68·0 µg/l (IQR 74·7, 95 % BCI 50·9, 74·9), with significant inter-regional difference in median UIC (P = 0·0143) (Table 3). Median thyroglobulin in this group was 18·5 ng/ml (IQR 20·7); no significant difference in median thyroglobulin was noted between the regions (P = 0·1315) (Table 4). In 18 % (18/100) of investigated breastfeeding women, thyroglobulin was >40 ng/ml. According to the WHO and IGN criteria, the surveyed lactating women were iodine-deficient.
School children
Median UIC in the investigated sample of school children was 119·8 µg/l (IQR 92·4), which is consistent with iodine sufficiency in this population. The difference in median UIC between the regions was statistically significant (P = 0·0000), with the highest values observed in south and south-east regions of Poland (Table 5). Ninety-four children (9·4 %) had UIC <50 µg/l. Only in the northern region the percentage of children with UIC <50 µg/l was >20 % (Table 5).
Table 5 Median urinary iodine concentration (UIC) (µg/l) and the percentage of UIC <50 µg/l according to the place of school children’s residence area

BCI, bootstrapped CI.
Discussion
Choice of iodine nutrition markers
The WHO recommends the following indicators to assess the severity of iodine deficiency and the impact of disorder preventive programmes(1):
1. Median UIC in children, pregnant and non-pregnant women
2. Thyroid size: by palpation in school children, pregnant and lactating women (particularly useful for assessing iodine deficiency severity before introducing any intervention; less practical in assessing their impact) or by ultrasound in school children
3. Thyroid-stimulating hormone concentration in the blood of neonates (usually while screening for congenital hypothyroidism)
4. Serum thyroglobulin in 5–14-year-old children (preferably in DBS).
In our survey, we chose median UIC as the main marker of the current iodine status in the Polish population. UIC from spot samples was regarded as a reliable indicator of iodine nutrition at the population level(Reference Rohner, Zimmermann and Jooste20,Reference Pearce and Caldwell35) . The very high day-to-day variability in UIC, as a result of the daily variability of KI content in the diet, is compensated by a large number of samples in population studies. However, this marker might be useless in assessing the iodine status of an individual(Reference Pearce and Caldwell35). The correction for creatinine is not required(Reference Pearce and Caldwell35). Due to a good agreement between UIC in school children and women of child-bearing age, as well as between adult men and women, the results obtained in children may be used for assessing iodine nutrition in a non-pregnant, non-lactating adult population(Reference Liu, Su and Li36). It is still disputed which marker – UIC or thyroglobulin in DBS – may be best for iodine nutrition in pregnancy(Reference Stinca, Andersson and Weibel34). UIC measurements in pregnant women may be affected by the gestational age, although the reported trends of UIC according to pregnancy trimester are inconsistent. For example, a fall in UIC with advancing pregnancy was reported in iodine-deficient Turkish women(Reference Anaforoglu, Algun and Incecayir37), as well as in Bangalore, India, where a successful salt iodisation programme provided adequate iodine intake during pregnancy(Reference Jaiswal, Melse-Boonstra and Sharma38). On the other hand, an increase in UIC in the second and/or third trimesters of pregnancy was observed recently in iodine-deficient pregnant women in the UK(Reference Bath, Pop and Furmidge-Owen39), Israel(Reference Ovadia, Arbelle and Gefel40) and Latvia (where that trend was not explainable by an increased intake of iodine-containing supplements)(Reference Konrade, Kalere and Strele41). Therefore, the real impact of overrepresentation of women of certain gestation age in the studied population (such as in our report) cannot be easily interpreted. The greatest controversy is related to UIC as a marker of iodine nutrition during breastfeeding, as preferential excretion of iodine to breast milk may result in the underestimation of iodine nutrition if the WHO-recommended cut-off level of 100 µg/l is used(Reference Dold, Zimmermann and Aboussad42).
Since we were unable to measure thyroglobulin concentration in DBS, we could not use that biomarker to assess iodine status in the investigated school children. However, we decided to measure thyroglobulin in the sera of pregnant and lactating women. Although the application of this indicator is not recommended by the WHO yet, there is strong data supporting that thyroglobulin measurement in DBS is a good predictor of iodine sufficiency in pregnant women(Reference Stinca, Andersson and Weibel34). The results obtained with the assay used by Stinca et al. (Reference Stinca, Andersson and Weibel34) were independent of women’s anti-thyroglobulin antibodies (aTgA) status; however, it needs to be confirmed by other methods of thyroglobulin estimation. Also, serum thyroglobulin has been tested as a biomarker of iodine status, although data are available from smaller samples of pregnant women(Reference Bath, Pop and Furmidge-Owen39). Eltom et al. (Reference Eltom, Elnagar and Elbagir43) confirmed the negative correlation between UIC and serum thyroglobulin in Swedish (in the second and third trimesters) and Sudanese (in the third trimester) pregnant women. Bath et al. (Reference Bath, Pop and Furmidge-Owen39) have found using a linear mixed model, controlling for confounders, that serum thyroglobulin is significantly higher in pregnant women with urinary iodine-to-creatinine ratio <150 µg/g than in women with a ratio of at least 150 µg/g. The performance of thyroglobulin as a marker of iodine nutrition in lactating women needs to be tested.
It is generally accepted that the presence of aTgA in serum is a confounding factor in serum thyroglobulin measurements(Reference Baloch, Carayon and Conte-Devolx44). It has been proved that automated autoimmunoassays, including Roche Eclesys II Tg assay used in the present survey, are prone to this phenomenon resulting in thyroglobulin underestimation(Reference Netzel, Grebe and Carranza Leon45,Reference Rotteveel-de Groot, Ross and Janssen46) . The impact of aTgA positivity on the performance of serum thyroglobulin as a biomarker of iodine nutrition status in pregnancy needs to be elucidated. Surveys evaluating serum thyroglobulin as a marker of iodine nutrition in pregnancy either excluded aTgA-positive women from analysis(Reference Bath, Pop and Furmidge-Owen39) or did not include that factor into the analysis(Reference Eltom, Elnagar and Elbagir43,Reference Mioto, Monteiro and de Camargo47) , as was the case in our study. The effect of iodine status on thyroglobulin levels may also be influenced by other factors such as gestation week(Reference Bath, Pop and Furmidge-Owen39). Bath et al. (Reference Bath, Pop and Furmidge-Owen39) have noticed a rise in serum thyroglobulin with advancing pregnancy (which needs to be considered when analysing the presented results, as the majority of women were tested in the third trimester). Such a trend, however, was present only in women with urinary-to-creatinine ratio <150 µg/g; therefore, it may be interpreted as an additional marker of inadequate iodine nutrition.
There have been controversies regarding goitre assessment as a reliable marker of iodine nutrition in countries where universal salt iodisation was recently implemented(Reference Gorstein48). Thyroid volume estimation, even by ultrasound, is observer-dependent and may be systematically biased if not performed correctly(Reference Zimmermann, Molinari and Spehl49). Discrepancies between the frequency of goitre and UIC as markers of iodine status have already been reported. Sweden, with a salt iodisation programme fixed in 1966 and confirmed iodine sufficiency in school children (median UIC 125 µg/l in a 2007 survey(Reference Andersson, Berg and Eggertsen50)), had a high prevalence of goitre among school children if the 2004 international thyroid volume reference was applied (22·3 % for age-normative values, 15·7 % for body surface area reference)(Reference Filipsson Nyström, Andersson and Berg51,Reference Zimmermann, Hess and Molinari52) . Chinese data obtained in a representative sample of iodine-sufficient school children (median UIC 178·3 µg/l, with 12·7 % of children with UIC <100 µg/l), 19 years after the implementation of mandatory universal salt iodisation in 1994, showed that the age-specific 97th percentile of thyroid volume was on average 32·4 % higher in boys and 22·7 % higher in girls than the WHO-adopted international standard(Reference Mo, Lou and Mao53). In the UNICEF guideline published in 2016, it recommended stopping goitre assessment as part of routine monitoring of iodine status of populations(27). Therefore, we decided not to include the data from thyroid ultrasound, which was performed both in school children and adult women.
Breast milk iodine concentration (BMIC) has been suggested as a marker of iodine nutrition in lactating women in the early 2000s(Reference Semba and Delange54). Whether BMIC values reflect the optimal iodine status during breastfeeding is a matter of dispute. For example, Azizi & Smyth(Reference Azizi and Smyth55), based on a retrospective analysis of data from over thirty surveys, suggested in 2009 that a BMIC range of 100–150 µg/l reflects iodine sufficiency. The best evidence for BMIC as a biomarker of iodine nutrition during lactation comes from a study by Dold et al. (Reference Dold, Zimmermann and Aboussad42) Data obtained from three iodine-sufficient regions suggested a BMIC reference range of 60–465 µg/kg in exclusively breastfeeding women. The authors, moreover, stated that median UIC ≥100 µg/l is not a good marker of iodine sufficiency during lactation. For example, median UIC was equally low in mothers from Croatia (with an effective programme of iodine prophylaxis based on universal salt iodisation) and in women from Morocco (where mandatory salt iodisation has not been successfully implemented). In contrast, the BMIC in Croatian mothers was four times higher than in Moroccan mothers, covering the daily iodine requirement of their infants. The authors concluded that the cause of that phenomenon is preferential partitioning of iodine into breast milk at lower intakes in lactating women with sufficient iodine status, whereas in chronically iodine-deficient breastfeeding women, a constant proportion of iodine (approximately one-third) is excreted into breast milk and cannot be compensated because of obligatory renal iodine losses even at very low iodine intake(Reference Dold, Zimmermann and Aboussad42). Multicollector, inductively coupled plasma MS using isotope dilution analysis with 129I and tellurium for mass bias correction was applied for BMIC determination in the discussed study, a method that was proved to be highly precise and characterised by a lower limit of detection compared with the colorimetric Sandell–Kolthoff reaction(Reference Dold, Baumgartner and Zeder56). Limited availability of resources and access to the preferred method of BMIC estimation were the reasons for not including that biomarker in our survey.
Iodine nutrition monitoring in Poland
A nationwide study conducted among school children in 1992/1993 proved that voluntary salt iodisation with KI content of 20 mg/kg of salt was not able to provide adequate iodine nutrition for the Polish population; mean UIC in school children varied from 45·16 to 75·64 µg/l in the investigated regions(Reference Szybiński13,Reference Szybiński and Żarnecki14) . The low iodine status of Polish school children was confirmed in 1994/1995(Reference Delange, Benker and Caron15,Reference Szybiński, Delange and Lewiński16) , with a mean UIC of 32–93·1 µg/l depending on the study site. During the 1994/1995 survey, adequate UIC was found only in school children living in the Hel peninsula (mean 161·3 µg/l), which might be attributed to a higher seafood consumption in this group(Reference Szybiński, Delange and Lewiński16).
Monitoring of the effectiveness of the current obligatory model of iodine prophylaxis in Poland started in 1999. Results from 1999–2005 surveys showed a significant increase in median UIC in school children to 93 µg/l, still below the WHO-recommended level of 100 µg/l(Reference Szybinski, Golkowski and Buziak-Bereza57). Repeated evaluation of iodine status of school children in the town of Opoczno located in central Poland showed an increase of median UIC from 45·5 µg/l in 1994 to 100·6 µg/l in 2010(Reference Zygmunt, Adamczewski and Wojciechowska-Durczyńska58). Our results also proved that the model of iodine prophylaxis adopted in Poland can provide appropriate iodine nutrition to school children (median UIC 119 µg/l, median UIC >100 µg/l in all investigated areas). The improvement in the iodine status of school children investigated after 1997 may be explained by the better quality of salt iodisation (in 2000, 80 % of salt samples examined by the State Sanitary Inspection had an appropriate iodine content; in 2002, 79 % of samples; in 2005, 92 % of samples; and in 2006, 94 % of samples)(Reference Stos, Szponar and Glowala59).
Before the introduction of mandatory iodine prophylaxis in Poland, iodine deficiency existed also in pregnant women. In a small study conducted in 1992/1993, mean UIC in pregnant women was 34·9 µg/l(Reference Krzyczkowska-Sendrakowska, Zdebski and Kaim60). In a later study, only 8·1 % of them had UIC in the first trimester of pregnancy >150 µg/l(Reference Gołkowski, Bałdys-Waligórska and Huszno17).
However, our results from 2017 also prove that iodine prophylaxis based on the fortification of household salt failed to eradicate iodine deficiency among pregnant women in Poland (median UIC 114·3 µg/l), despite the fact that they should be encouraged to use supplements containing iodine. This is consistent with other reports. Gietka-Czernel et al.(Reference Gietka-Czernel, Dębska and Kretowicz61) have shown that 10 years after the introduction of obligatory iodine prophylaxis in Poland, median UIC in pregnant women was only 112·6 µg/l, and even in women receiving iodine supplements, it was <150 µg/l (146·9 µg/l). Zygmunt et al. (Reference Zygmunt, Adamczewski and Zygmunt62) obtained similar results, with median UIC of 79·6 µg/l in the whole group of 115 pregnant women, and 129·4 µg/l in those additionally supplemented with formulas containing KI. Also, a survey performed between 2007 and 2011 in a group of 911 pregnant women has shown low median UIC of 92·5 µg/l, with optimal iodine nutrition defined as UIC between 150 and 249 µg/l in 17·3 % of them(Reference Trofimiuk-Muldner, Sokołowski and Konopka63).
Failure to reach the required iodine nutrition status in pregnant women may be, at least partially, explained by a low adherence to the recommended use of iodine-containing supplements. In a study by Gietka-Czernel et al.(Reference Gietka-Czernel, Dębska and Kretowicz61), only 35 % of investigated pregnant women were taking iodine supplements. According to a survey published in 2011, only 59 % of investigated pregnant women residing in Krakow were using iodine-enriched vitamin formulas. Supplement use was more frequent in women with a university degree (71·2 %)(Reference Milewicz, Czyżewicz and Stochmal64).
Results of serum thyroglobulin screening also confirmed the inadequate iodine status of investigated pregnant women, although the median serum thyroglobulin of 13·3 ng/ml only marginally exceeded the cut-off level. This may be explained by the lack of correction of the thyroglobulin level for the aTgA status, as aTgA positivity may result in lower-than-actual serum thyroglobulin levels(Reference Netzel, Grebe and Carranza Leon45,Reference Rotteveel-de Groot, Ross and Janssen46) . However, such discrepancies between UIC and serum thyroglobulin have already been reported. In a Brazilian study by Mioto et al. (Reference Mioto, Monteiro and de Camargo47), pregnant women were found to be marginally iodine-deficient based on median UIC (146 µg/l), with median serum thyroglobulin 11·2 ng/ml and 3·3 % of investigated subjects with thyroglobulin >40 ng/ml (aTgA status was not assessed).
To our knowledge, this is the first report on iodine nutrition status in breastfeeding women in Poland, unfortunately also showing that the Polish prophylaxis model is not sufficient to reach adequate iodine nutrition in this group (median UIC 68·0 µg/l, median serum thyroglobulin 18·5 ng/ml). It may be argued that the result is influenced by the small sample size (which does not compensate for large inter- and intraindividual variations in IUC resulting from daily differences in iodine intake(Reference Rohner, Zimmermann and Jooste20)), sampling error, and uneven distribution of participants between the regions (which is probably the reason for inter-regional differences in UIC). It needs to be stressed that UIC is not an optimal marker of iodine status in breastfeeding women, especially as the WHO-recommended UIC cut-off level for lactating women is probably too high even in case of iodine-sufficient subjects(Reference Dold, Zimmermann and Aboussad42).
Iodine nutrition in vulnerable populations worldwide
Iodine status worldwide has been improving over the last decades. In 2017, <10 % of the world’s population lived in countries classified as iodine-deficient based on the general population data on iodine intakes(Reference Gizak, Rogers and Gorstein65). It was reported that adequate iodine intake was assured among school children in 111 countries out of 140 with available data in that year, with only nineteen countries being iodine-deficient(Reference Gizak, Rogers and Gorstein65). Optimal iodine intake in pregnant women was confirmed, however, in thirty-three out of seventy-two countries with available data(Reference Gizak, Rogers and Gorstein65).
The discrepancy between good iodine status of the general population (usually represented by school children) and iodine deficiency in pregnant women, stressed in the present survey, may emerge even in countries with iodine prophylaxis programmes. In 2009, Wong et al. (Reference Wong, Sullivan and Perrine26) presented the results of the analysis of the WHO Global Database on Iodine Deficiency, a part of the Vitamin and Mineral Nutrition Information System, in which forty-eight survey pairs presenting data for both school children and pregnant women were identified. In sixteen out of thirty-four studies, in which children were proven to have adequate or above-required iodine intake, co-assessed pregnant women were classified as iodine-deficient based on UIC results. In all six surveys revealing iodine deficiency in school children, inadequate iodine status was also found in pregnant women. A regression analysis showed that for the predicted median UIC of at least 150 µg/l in pregnant women, median UIC in school children would need to be at least 178 µg/l. If the predicted median UIC in school children was 100 µg/l, the predicted median UIC in pregnant women would have reached 104 µg/l. Therefore, it is not surprising that with a median UIC of 119·8 µg/l in Polish school children in 2017, co-investigated pregnant women failed to achieve adequate iodine nutrition represented by a median UIC of 150 µg/l. A survey conducted in 2014–2015 in rural Niger, a country with mandatory salt iodisation since 1996 and requiring iodine content of 30–60 ppm at importation and 20–60 ppm at retail, has shown optimal iodine nutrition in school children (median UIC 100·9 µg/l) and persistent iodine deficiency in pregnant women (median UIC 69·0 µg/l, median thyroglobulin in DBS 34·6 µg/l), which may be explained by the poor quality of iodised salt, with lesser-than-required iodine content in 98·1 % of investigated salt samples(Reference Hess, Ouédraogo and Young66). Iran implemented mandatory salt iodisation between 1994 and 1996, with a required iodine content in salt of 40 ± 10 µg/g (confirmed in >80 % of available salts)(Reference Shamsollahi, Nadarloo and Rastkari67). The programme ensured sufficient iodine nutrition for Iranian school children (median UIC 161 µg/l in 2013) but failed to do so for pregnant women (median UIC 87·3 µg/l in 2014)(Reference Delshad and Azizi68). This was also the case in Greece, generally considered iodine-sufficient(Reference Koutras, Alevizaki and Tsatsoulis69) with voluntary salt iodisation (iodine content of 50 mg/kg of salt), where median UIC in the pregnant population was 127·1 µg/l(Reference Koukkou, Ilias and Mamalis70). In India, mandatory iodisation of household salt was introduced in 1998 (iodine content of 25 mg/kg of salt). A study conducted in Uttarakhand, India, in 2013–2014 confirmed sufficient iodine intake in school children (median UIC 115–150 µg/l, depending on the location) and persistent iodine deficiency in pregnant women (median UIC 117·5–124 µg/l), which may be attributed to a low consumption of adequately iodised household salt (50·3–67 %)(Reference Sareen, Kapil and Nambiar71). In the UK, the main sources of iodine are dairy products, due to iodine supplements of the livestock(Reference Bath, Combet and Scully72), which is sufficient to provide adequate nutrition for children aged 8–10 years (median UIC 144 µg/l)(Reference Bath, Combet and Scully72). However, such iodine supplements did not ensure a proper iodine intake in pregnant women (median UIC 88 µg/l). It needs to be stressed that only 35 % of investigated women in the current study were taking iodine-containing vitamin supplements; however, no difference in UIC was noted between expecting mothers supplemented and not supplemented with iodine(Reference Knight, Shields and He73). In some countries generally considered long-term iodine-sufficient, such as the USA, the iodine status of pregnant women had worsened in recent years (median UIC in NHANES 2001–2006: 153 µg/l; NHANES 2006–2010: 129 µg/l)(Reference Perrine, Herrick and Gupta74–Reference Caldwell, Pan and Mortensen76). The suboptimal iodine nutrition during gestation may also result from poor adherence to iodine-containing supplements(Reference Zygmunt, Adamczewski and Zygmunt62,Reference Malek, Umberger and Makrides77,Reference Gupta, Gahche and Herrick78) .
Iodine intake in pregnant women calculated based on urinary iodine should be interpreted with caution due to increased glomerular filtration rate and, consequently, increased urine volume. A postulated increase in renal iodine clearance (and increased obligatory iodine loss with urine) may result in an underestimation of daily iodine intake(Reference Zimmermann79,Reference Glinoer80) . On the other hand, the extrapolation of daily iodine intake from the UIC in spot samples in subjects with a daily urine volume >1 l usually overestimates daily iodine consumption (e.g. with a daily urine volume of 1·5 l, UIC in spot samples constitutes about 60–65 % of iodine excreted during 24 h)(Reference Zimmermann and Andersson81). Therefore, a population of pregnant women with median UIC in spot samples nearly reaching 150 µg/l may, in fact, be iodine-sufficient.
Universal salt iodisation with iodine content at approximately 25 mg/kg, covering a large proportion of total consumed salt supplies, should be sufficient to provide adequate iodine nutrition during the first 1000 days of life (although iodine intake may be borderline in pregnancy), as suggested by a cross-sectional multicentre study published in 2018(Reference Dold, Zimmermann and Jukic82).
Although salt fortification programmes seem to provide optimal iodine intakes in the general population, they may fail in assuring adequate iodine intake when the demand for that nutrient increases, particularly during pregnancy. If, on the other hand, they result in adequate iodine nutrition during gestation, iodine intake in the general population may become excessive(Reference Jaiswal, Melse-Boonstra and Sharma38,Reference Dold, Zimmermann and Jukic82,Reference He, Su and Liu83) , particularly if iodine content in salt is too high. Therefore, the efficacy of iodine prophylaxis programmes should be monitored on a regular basis, and the iodine content in salt adjusted accordingly to the obtained results(Reference He, Su and Liu83,Reference Andersson, Hunziker and Fingerhut84) . It needs to be remembered that a profound decrease in iodine content in salt may trigger a return of iodine deficiency (as was observed in pregnant women in the Zhejiang province of China after a reduction of iodine content from 35 to 25 ppm)(Reference Wang, Xing and Zhu85).
Considering the obtained results, new ways to improve iodine intake during pregnancy should be searched for in Poland. The best option is to apply universal salt iodisation, including salt used in the food industry. A possible solution might be a diversification of dietary sources of iodine. Mandatory iodine fortification of bread, along with household salt iodisation, may be a solution for assuring appropriate iodine intake during pregnancy, which proved to be effective in Australia(Reference Condo, Huyhn and Anderson86).
There are some limitations in the present survey. One of them is a subnational representation; due to the random selection of investigated regions, western voivodships were not included. The outcomes may have been biased by the recruitment method (volunteers), lack of stratification (sampling error), the small number of lactating women included and poorer response in some regions, resulting in a disproportionate number of recruited subjects. Other limitations are the choice of serum thyroglobulin instead of DBS thyroglobulin (as reference ranges for that biomarker are missing), lack of correction for aTgA status and lack of assessment of iodine concentration in breast milk (both biomarkers should be ideally included in the next monitoring programme).
Conclusions
Mandatory iodisation of household salt has provided adequate iodine intake in the Polish general population, as represented by school children. As this group is currently marginally iodine-sufficient, a small decrease in iodised salt consumption without a concomitant increase in the concentration of iodine in salt might lead to a recurrence of iodine deficiency in Poland.
The current model of iodine prophylaxis has failed to assure adequate iodine nutrition in pregnant and lactating women. Ways to improve its effectiveness, particularly to diversify the dietary sources of iodine, should be searched for. Further monitoring of Poland’s iodine status should be ensured for a sustainable elimination of iodine deficiency-related disorders.
Acknowledgements
Acknowledgements: The authors would like to thank Dr. Anna Skalniak for support. Financial support: The current work has been supported by the National Health Programme of the Ministry of Health, Republic of Poland. Conflict of interest: There are no conflicts of interest. Authorship: Conception and design: M.T.-M., J.K., A.H.-D.; data acquisition: M.T.-M., J.K., G.S., A.D., M.K.-K., Ł.K., M.M., E.R., J.W., M.S., M.B.-B., J.T.; data analysis: M.T.-M., D.P.; drafting article: M.T.-M.; final approval of the version to be published: M.T.-M., A-H-D. Ethics of human subject participation: The current study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving study participants were approved by the Ethics Board of the Jagiellonian University. Written informed consent was obtained from all subjects or their legal guardians.







