Small-quantity lipid-based nutrient supplements (SQ-LNS) were developed to prevent malnutrition among vulnerable populations. These fortified food-based products (typically including vegetable oil, peanut paste and milk powder) deliver vitamins and minerals as well as essential fatty acids and small amounts of energy (∼100–120 kcal/d) and protein(Reference Arimond, Zeilani and Jungjohann1). Since the development of SQ-LNS ∼15 years ago, a strong evidence base has been established demonstrating that SQ-LNS for children 6–24 months of age reduces child mortality, stunting, wasting and anaemia and promotes healthy development in efficacy trials as well as effectiveness trials in programmatic settings(Reference Dewey, Stewart and Wessells2–Reference Wessells, Arnold and Stewart6). As a result, SQ-LNS was listed in the 2021 Lancet Series on Maternal and Child Undernutrition as an intervention with a strong evidence base supporting implementation(Reference Keats, Das and Salam7).
Alongside robust evidence of effectiveness, informed policy decisions made by governments, aid organisations and other agencies regarding their portfolios of nutrition interventions also require information about the cost and cost-effectiveness of various options. There is very limited evidence on the cost, and no evidence on cost-effectiveness, of providing SQ-LNS to young children; this study is a first step towards filling that gap. Our goal was to develop a modelling framework to estimate the potential effects, costs and cost-effectiveness of SQ-LNS. We estimated costs from a societal perspective rather than a narrow perspective (e.g. the perspective of programme implementers or the government) so that our estimates of cost-effectiveness would reflect not only the resources required to finance a programme to provide SQ-LNS but also the opportunity cost of caregivers’ time to participate in such a programme. We selected rural Uganda for this first modelling exercise because detailed data were available on the costs of delivering a similar product, micronutrient powder (MNP), to young children in that setting(Reference Schott, Richardson and Baker8). Our specific objectives were to (1) develop a cost model, adapted from the MNP study, to estimate the annual total societal cost and cost per child of providing SQ-LNS, (2) develop an effectiveness model to estimate the effects of SQ-LNS on child mortality, anaemia, developmental disability, stunting and wasting and (3) translate those effects into cost per disability-adjusted life years (DALY) averted, per death averted and per case of anaemia, developmental disability, stunting and wasting averted in rural Uganda.
Methods
We developed models to estimate the cost, cost-efficiency (cost per child) and cost-effectiveness of SQ-LNS for young children. The modelling was based on providing 12 months of daily SQ-LNS (20 g/d), beginning at 6 months of age and ending at 18 months of age, to all young children residing in rural districts of Uganda. The models were based on SQ-LNS being delivered via the existing Village Health Team (VHT) community health worker (CHW) programme. The VHTs in Uganda rely on volunteer health workers to deliver basic health and nutrition products and services, provide education and make referrals(Reference Mays, O’Neil and Mworozi9). Our modelling assumed that current VHTs could, with additional monetary incentives (described below), take on the delivery of SQ-LNS to households in addition to their current activities. We modelled costs and effects over the period 2021–2031, assuming 1 year of start-up and 10 years of SQ-LNS provision.
Costs
The cost model was developed based on an actual costing study conducted in one rural district in Uganda (Namutumba District) to estimate the cost of providing MNP to children 6–24 months of age(Reference Schott, Richardson and Baker8). The MNP costing study was designed to compare the cost and cost-efficiency of delivering MNP via community- v. facility-based platforms. The study found that the community-based platform (i.e. via VHTs) was more cost-efficient (lower cost per child reached), so we adopted the community-based platform for our SQ-LNS cost model.
We estimated economic costs from a societal perspective, meaning all costs were accounted for, regardless of who incurred them and including both the costs needed to finance the programme and opportunity costs (e.g. the opportunity cost of caregivers’ time to participate in the intervention and volunteer health worker time). Following the activity-based approach used in Schott et al.(Reference Schott, Richardson and Baker8), we estimated the cost of 1 year of start-up activities (primarily social and behaviour change communication and capacity building) and recurring activities over a 10-year time horizon, which included caregiver opportunity costs, social and behaviour change communication, logistics, capacity building, operational monitoring and evaluation costs, overhead and capital, VHT incentives, SQ-LNS product costs, SQ-LNS international shipping and handling and customs clearance and domestic SQ-LNS transport, storage and handling.
The product cost of SQ-LNS was based on the price of a carton (546 20-g sachets) of SQ-LNS published in the UNICEF Supply Catalog (https://supply.unicef.org/s0000245.html) in March of 2022 and adjusted to 2020 US dollars. Although local production is feasible in several African countries, we assumed international production because cost information was more reliable. The cost of SQ-LNS, which was applied to each of the 10 years of intervention, was $33·30/carton (2020 US dollars), or $3·06/kg, which is ∼$0·061 per 20-g sachet. International shipping and handling costs were estimated at US$0·31/kg based on the default estimate in the FACET tool for shipping and handling of SQ-LNS on the South East Africa trade route (http://facet4snf.org/). Customs clearance costs were estimated at 18 % for a value added tax + 33 % withholdings tax(Reference Ferris10), or ∼ US$1·01/kg. Domestic transport, storage and handling were estimated to cost US$0·17/kg, based on the default estimate for Uganda in the FACET tool.
Given documented challenges faced by the volunteer VHT system that underlies the community-based distribution platform in rural Uganda, such as inadequate support and high rates of attrition(Reference Mays, O’Neil and Mworozi9,Reference Turinawe, Rwemisisi and Musinguzi11) , we assumed that the successful addition of SQ-LNS to the products and services delivered by VHT would require a strengthening of the VHT system. Informed by a Uganda pilot performance-based incentive system(Reference Zheng, Musominali and Chaw12), we modelled an additional performance-based monetary incentive to VHT health workers of US$0·44 per delivery of SQ-LNS, assuming SQ-LNS was delivered to households every 3 months.
All other cost estimates were adapted from the MNP costing study(Reference Schott, Richardson and Baker8) by first identifying the base cost of each input (personnel, transport, materials, in-kind incentives) for each activity (plus overhead and capital costs) based on the costs actually incurred in Namutumba. We converted all base costs to 2020 US dollars using the Bureau of Economic Analysis implicit price deflators for gross domestic product (online Supplementary Table 1).
We then extrapolated those base costs to all other rural districts in Uganda via a set of extrapolation indices defined relative to Namutumba. The extrapolation indices were: (1) a spatial index based on the area of each district relative to Namutumba, (2) a child population index based on the number of eligible children per district, (3) a VHT population index based on the number of VHTs per district and (4) a VHT-child population index based on the ratio of VHTs to eligible children per district compared to Namutumba (online Supplementary Figure 1). We used these indices to generate district-specific costs for each rural district that reflected differences in each district relative to Namutumba that would likely impact costs. For example, we assumed the cost of transport required for capacity building activities would vary based on the area of each district, so if the area of Namutumba was approximately 820 km2 and the area of another rural district was approximately 1585 km2, the index for that district was defined as (1585/820) = 1·93 and the cost for transport associated with capacity building in that district was 1·93 times higher than in Namutumba District.
Effectiveness
The effectiveness model was designed to estimate annual deaths averted and annual cases of moderate or severe anaemia, developmental disability, stunting and wasting averted attributable to SQ-LNS over a 10-year time horizon (Table 1). The model was also used to estimate DALYs averted, as described below. We applied the effect of SQ-LNS on each of these outcomes to specific age bands depending on the expected timing of effects relative to supplementation. We applied mortality, stunting and wasting reductions through the full period of supplementation (6–18 months of age). We applied reductions in moderate and severe anaemia from 9 to 24 months of age, assuming that changes in anaemia status would become evident after ∼3 months of supplementation and would persist for 6 months post-supplementation. Finally, we modelled the onset in reductions in developmental disability during the 18- to 24-month age range. We used the Lives Saved Tool (LiST) population projections and the UN World Urbanisation Prospects to estimate rural child populations in 2022–2031 (online Supplementary Table 2).
Table 1 Effectiveness modelling parameter values and data sources
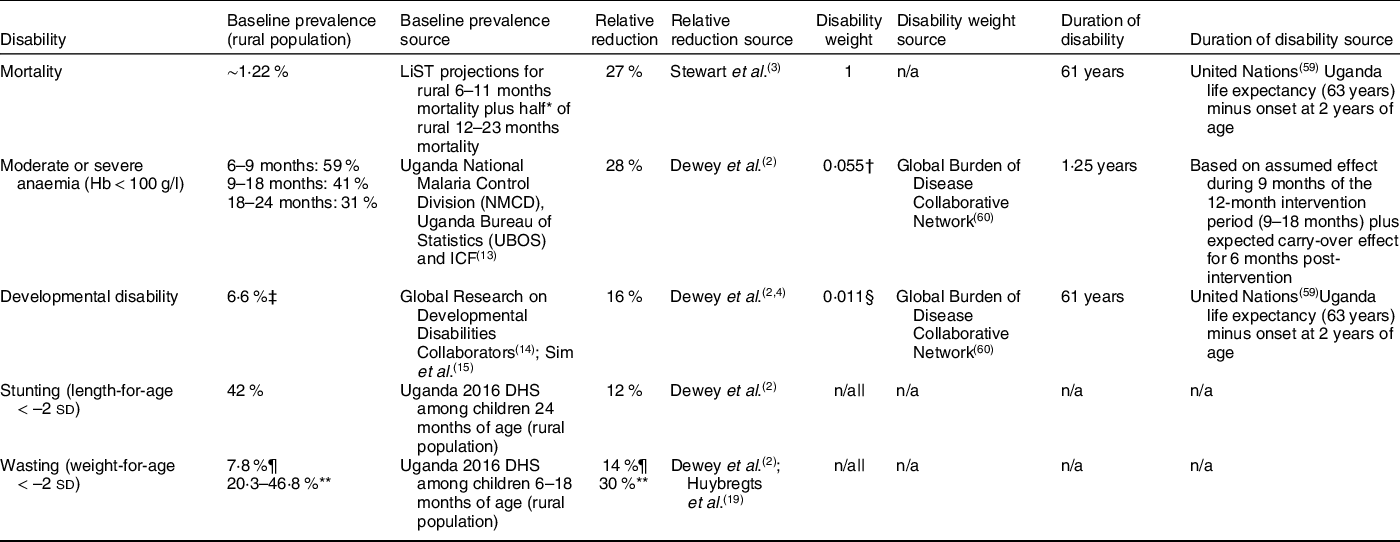
DHS, Demographic and Health Survey; GBDS, Global Burden of Disease; LiST, Lives Saved Tool; MIS, Malaria Indicators Survey.
* Assumes mortality uniformly distributed among children 12–23 months.
† Calculated as a weighted average of the disability weights for moderate (0·052) and severe (0·149) anaemia weighted by the prevalence of moderate and severe anaemia in the target population.
‡ Calculated as developmental disability for children under 5 years of age (10 %) × 65·6 % predictive validity.
§ Disability weight for borderline idiopathic developmental intellectual disability.
|| Stunting and wasting excluded from DALY calculation (stunting not included as a disability in the 2019 GBD study; disability weight for moderate wasting with or without oedema = zero).
¶ Cross-sectional prevalence; relative reduction of 14 % based on Dewey et al.(Reference Dewey, Stewart and Wessells2).
** Lower and upper bound longitudinal baseline values estimated by applying correction factors of 2·6 (calculated following UNICEF(17)) and 6 (from Barba, Huybregts and Leroy(Reference Barba, Huybregts and Leroy18)), respectively, to cross-sectional prevalence; relative reduction of 30 % based on data for Mali(Reference Huybregts, Le Port and Becquey19).
For each outcome, modelled effectiveness of the intervention was calculated as the baseline prevalence of the adverse outcome, multiplied by the relative reduction as a result of SQ-LNS, multiplied by the relevant population size. The baseline mortality rate (∼1·22 %) was based on LiST subnational projections of mortality among rural Ugandan children age 6–11 months plus half of projected mortality among rural children 12–23 months (Table 1). We used data from the most recent Malaria Indicator Survey (2018–2019) to estimate the baseline prevalence of moderate and severe anaemia among rural children 6–9 months (59 %), 9–18 months (41 %) and 18–24 months (31 %)(13). The baseline prevalence of developmental disability of 6·6 % was based on the estimate of 10 % prevalence of developmental disability among children under 5 years of age in low- and middle-income countries(14) multiplied by the predictive validity of 65·6 % for a score in the lowest decile of the early childhood MacArthur–Bates Communicative Development Inventories to predict later language delay(Reference Sim, Thompson and Marryat15) (the Communicative Development Inventory was the most commonly used tool to assess the effect of SQ-LNS on language development among the studies in the most recent meta-analysis of SQ-LNS)(Reference Prado, Arnold and Wessells4). We used data from the 2016 Uganda Demographic and Health Survey to estimate the baseline prevalence of stunting of 42 %(16). For wasting, we used a cross-sectional prevalence of 7·8 % based on data from the 2016 Demographic and Health Survey(16). We also estimated a lower and upper bound for the longitudinal prevalence of wasting by applying correction factors of 2·6 (calculated following UNICEF(17)) and 6 (from Barba, Huybregts and Leroy(Reference Barba, Huybregts and Leroy18)), respectively, to the cross-sectional baseline prevalence.
We modelled a 27 % relative reduction in mortality based on a meta-analysis of randomised controlled trials assessing the impact of SQ-LNS on all-cause mortality in children 6–24 months(Reference Stewart, Wessells and Arnold3). Relative reductions in moderate-to-severe anaemia (28 %), developmental delay, i.e. scoring in the lowest decile for language (16 %), stunting (12 %) and cross-sectional prevalence of wasting at endline (14 %) were based on individual participant data meta-analyses(Reference Dewey, Stewart and Wessells2). The SQ-LNS meta-analyses also demonstrated relative reductions in developmental delay for motor (16 %) and socio-emotional development (19 %), but to be conservative we chose to use only one of these domains (language) because there is overlap among these three outcomes and there is evidence for predictive validity of the language development assessment tool most commonly used in the SQ-LNS trials, as explained above. We modelled a 30 % relative reduction in longitudinal prevalence of wasting based on findings from a cluster randomised controlled trial in Mali(Reference Huybregts, Le Port and Becquey19), assuming that the impacts of SQ-LNS would be similar in Uganda.
We estimated DALYs as the sum of years of life lost due to mortality and years lived in disability due to moderate or severe anaemia and developmental disability. Years of life lost and years lived in disability were calculated as the product of cases of disability, the duration of disability and the disability weight (Table 1). We did not apply age weighting. DALYs averted were calculated as the difference in DALYs with and without the SQ-LNS intervention. We did not include stunting or wasting in our DALY estimates because stunting is not included as a disability in the 2019 GBD study, and the disability weight for moderate wasting without oedema is zero. Mild anaemia was not included because estimates of the impact of SQ-LNS on reductions in the prevalence of mild anaemia alone were not available from the meta-analyses, and the disability weight for mild anaemia was close to zero (0·004).
Discounting
We calculated costs, effectiveness and cost-effectiveness under two scenarios. The first assumed a discount rate of zero following the recommendation in Murray et al.(Reference Murray, Vos and Lozano20) and the second a discount rate of 3 % as recommended in WHO(21) and Sanders et al.(Reference Sanders, Neumann and Basu22). A discount rate greater than zero implies that costs and effects incurred in the future have a lower value than those same costs and effects had they been incurred today. Discounted DALY averted were calculated following Fox-Rushby and Hanson(Reference Fox-Rushby and Hanson23) without age weighting.
Sensitivity analyses
We conducted several analyses to evaluate the sensitivity of our results to key assumptions and influential parameter values. First, based on the result of a sensitivity analysis scenario in Stewart et al.(Reference Stewart, Wessells and Arnold3) in which passive control arms were excluded from the analysis, we modelled a scenario in which the relative reduction in mortality was 18 % rather than 27 %. We also modelled several different cost scenarios that included (1) elimination of the customs clearance costs for SQ-LNS, (2) a 10 % decrease in the price of SQ-LNS, (3) an increase in the price of SQ-LNS from $0·06 per sachet to $0·09 per sachet (2020 USD) to reflect the current, updated price of SQ-LNS according to the UNICEF supply catalog, (4) lower VHT monetary incentives (reduced from $0·44 to $0·29 per delivery of SQ-LNS) or (5) higher VHT monetary incentives (increased from $0·44 to $0·87 per delivery of SQ-LNS). We also modelled ‘best’ and ‘worst’ case scenarios in which sensitivity analyses parameter values that would improve the cost-effectiveness of SQ-LNS were modelled simultaneously and those that would reduce cost-effectiveness were modelled simultaneously.
Patient and public involvement
Because our study was based on secondary data, there was no patient or public involvement in this research.
Results
Costs
The estimated annual average cost of providing SQ-LNS for 12 months to all children starting at age 6 months in rural districts of Uganda via the VHT system was ∼$58·7 million (2020 US dollars) or $52 per child (Table 2; online Supplementary Table 3 for costs by year). Approximately $22 of the total cost per child was the cost of the product, ∼$11 was international shipping and handling, customs clearance and domestic transport, storage and handling, and ∼$19 was non-product programmatic costs. The cost of the product represented the highest share of the total annual average cost (42·5 %), followed by international shipping and handling and customs clearance for SQ-LNS (18·7 %), capacity building (15·3 %) and logistics (6 %) (Figure 1).
Table 2 Estimated cost and cost-efficiency of providing daily SQ-LNS to all children in rural Uganda (2020 US dollars)

SQ-LNS, small-quantity lipid-based nutrient supplement.
* Product shipping, taxes and domestic transport include the cost of international shipping and handling, customs clearance and domestic transport, storage and handling.
† Assuming daily supplementation for 12 months.
‡ Based on providing SQ-LNS to an average of 1 118 340 children per year.
§ Average cost per year on the basis of non-product programmatic cost and total cost include annualised start-up costs.
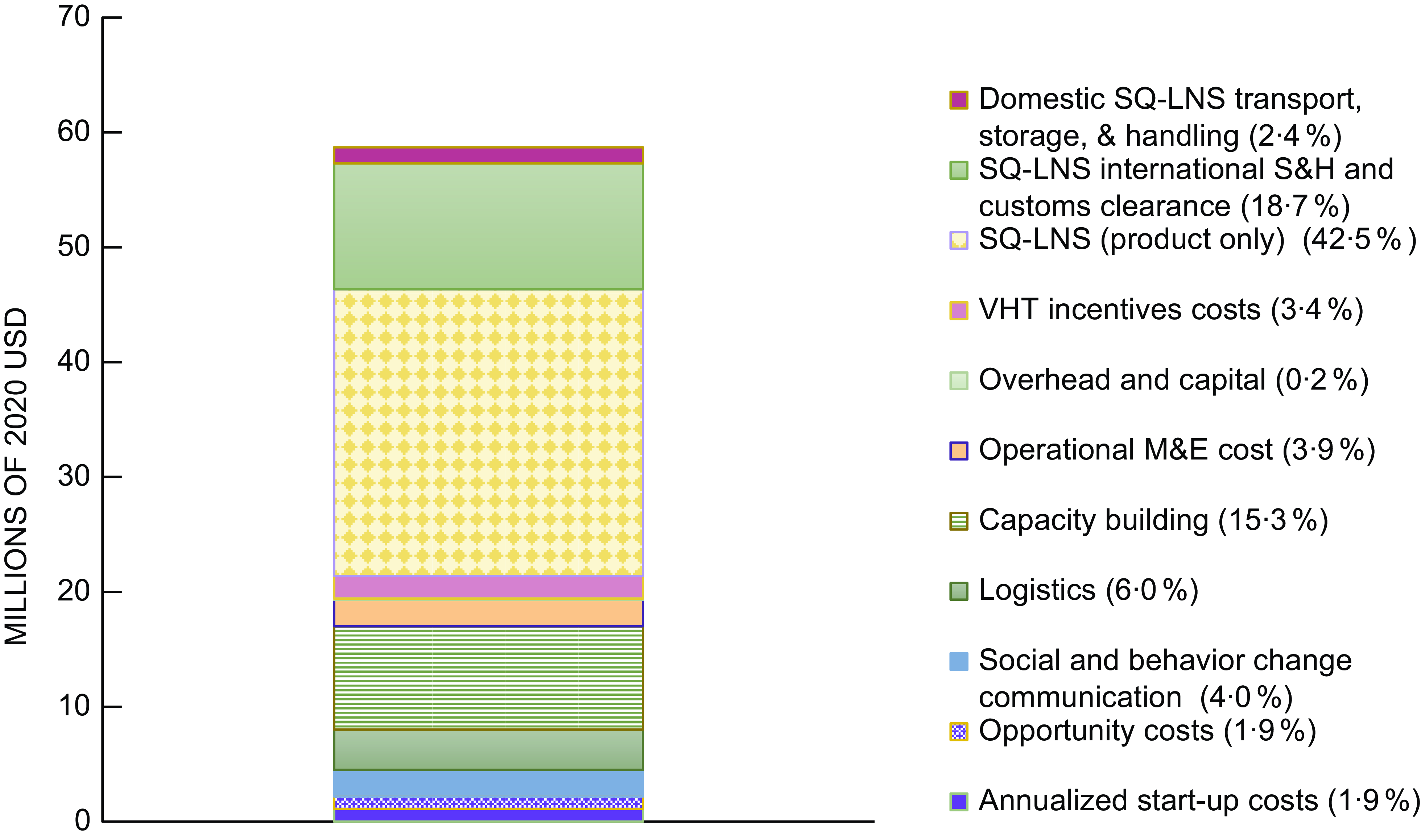
Fig. 1 Estimated average annual cost, by activity, of daily provision of SQ-LNS to all children aged 6–18 months in rural Uganda delivered via village health teams. Costs in millions of 2020 US dollars. M&E, monitoring and evaluation; SQ-LNS, small-quantity lipid-based nutrient supplements; S&H, shipping and handling; VHT, village health team
Effects and cost-effectiveness
We estimated that the provision of SQ-LNS in rural Uganda could avert an annual average of 242 292 DALYs as a result of 3689 annual child deaths averted, over 160 000 cases of moderate or severe anaemia averted and almost 6000 cases of developmental disability averted (Table 3). In addition, we estimated that SQ-LNS could annually avert an average of 55 858 cases of stunting, over 12 000 cases of wasting based on cross-sectional estimates and between 68 040 and 157 015 cases of wasting based on longitudinal estimates.
Table 3 Estimated effectiveness and cost-effectiveness of providing daily SQ-LNS to all children in rural Uganda
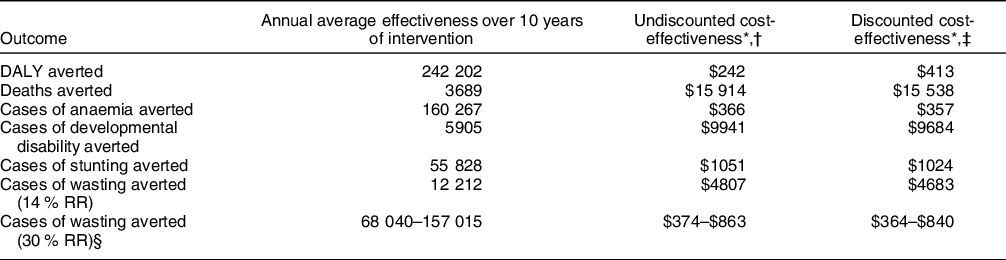
SQ-LNS, small-quantity lipid-based nutrient supplement; DALY, disability-adjusted life year; RR, relative reduction.
* All estimates of cost-effectiveness presented in 2020 US dollars.
† Cost-effectiveness (cost per unit of effectiveness) over modelled intervention period of 2021–2031.
‡ Discounted cost-effectiveness estimates based on discounting both costs and effects at 3 %.
§ Based on lower and upper bound longitudinal prevalence of wasting estimates of 20·3 % and 46·8 %
We estimated that the undiscounted cost-effectiveness of SQ-LNS over the 10-year intervention time horizon was $242 per DALY averted or $413 per DALY averted with costs and effects discounted at 3 % (Table 3). For individual outcomes, undiscounted cost-effectiveness ranged from $366 per case of moderate-to-severe anaemia averted to $15 914 per death averted.
In the sensitivity analyses (Figure 2), the undiscounted cost per DALY averted ranged from $195–$448 under the best- and worst-case scenarios, and the cost per death averted ranged from $12 823 to $30 472. The cost per DALY averted and per death averted was most sensitive to changing the assumed mortality reduction from 27 % to 18 %.
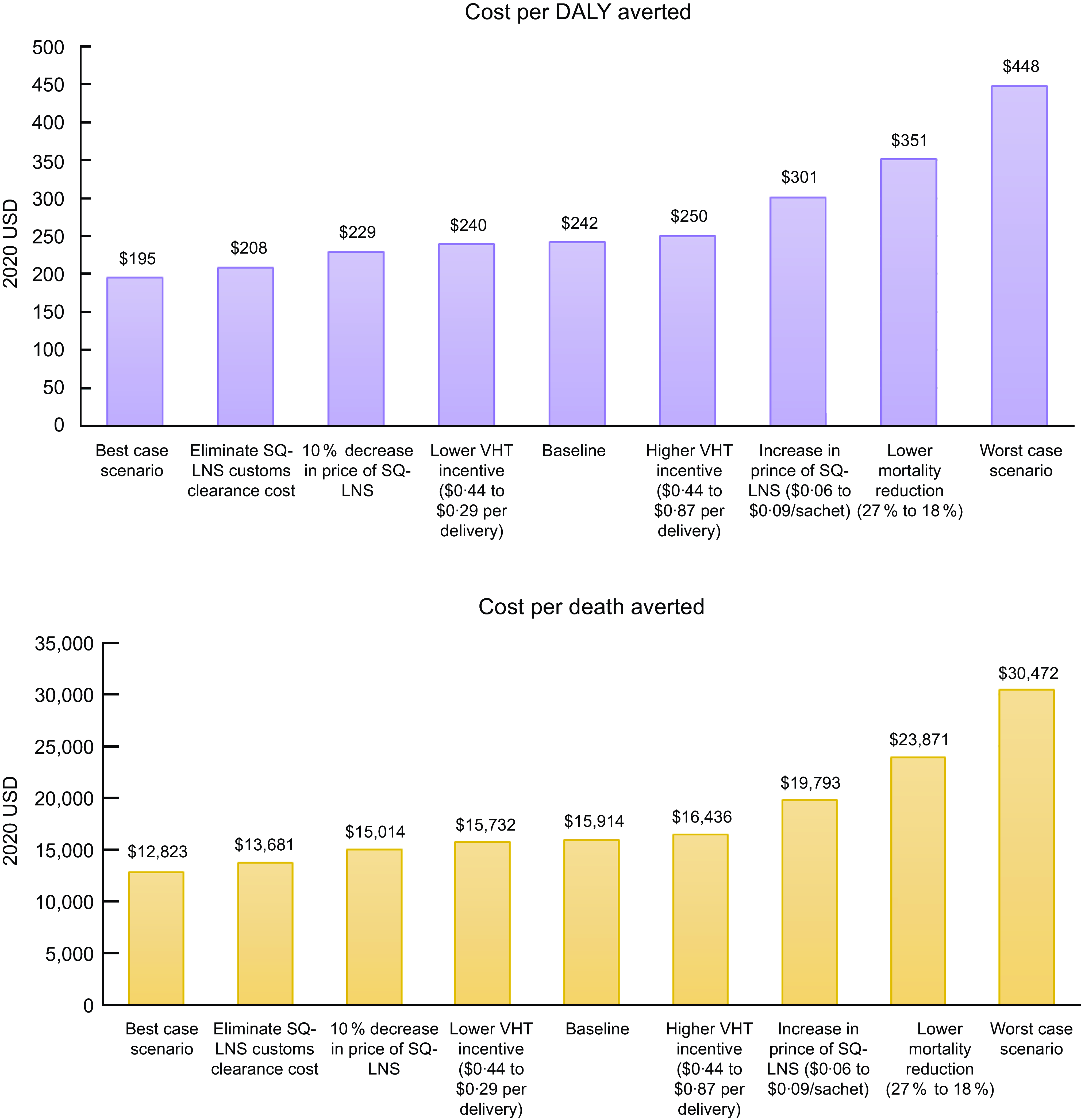
Fig. 2 Cost per disability-adjusted life year (DALY) averted (top panel) and cost per death averted (bottom panel) under each sensitivity analysis scenario. The best-case scenario simultaneously models the elimination of customs clearance costs, a 10 % decrease in price of SQ-LNS, and lower VHT monetary incentives. The worst-case scenario simultaneously models an 18 % mortality reduction, an increase in price of SQ-LNS ($0·06 to $0·09/sachet) and higher VHT monetary incentives. SQ-LNS, small-quantity lipid-based nutrient supplements; VHT, village health team
Discussion
In this study, we developed a framework for modelling the cost and cost-effectiveness of providing SQ-LNS to young children in rural Uganda, a framework that can be adapted and used to make similar sets of estimates in other countries. We estimated that the economic cost of providing SQ-LNS to all children for 12 months, from 6 to 18 months of age, in rural Uganda would be ∼$52 per child (2020 US dollars). Cost-effectiveness estimates were $15 914 per death averted and $242 per DALY averted (undiscounted) or $413 per DALY averted with costs and effects discounted at 3 %. Although there is no consensus on a specific cost-effectiveness threshold, the WHO has suggested that interventions with a cost per DALY averted less than gross domestic product per capita may be considered very cost-effective(24). Per capita gross domestic product was $822 in Uganda in 2020(25), so SQ-LNS would be classified as very cost effective. It is important to note, however, that this classification of cost-effectiveness does not imply affordability or policy feasibility. To this point, the estimated average annual cost of the programme (∼58·7 million US dollars, Table 2) represents a substantial share of Uganda’s total health budget, which was UGX2,718 billion in 2021/2022 or ∼$75 million (2020 USD based on the average 2020 exchange rate)(26). We discuss possible strategies for sharing and/or reducing costs below.
These results should be interpreted in the context of study strengths and limitations. One strength was the ability to model costs based on cost estimates adapted from a comprehensive costing study of MNP in Uganda. This sets our study apart from many other efforts to model the cost and cost-effectiveness of nutrition interventions that rely on generic unit cost estimates. In addition, our estimates of effects were based on direct evidence of the impact of SQ-LNS on each outcome from recent meta-analyses that included 14–18 randomised controlled trials (mostly in Sub-Saharan Africa) that included a total of > 37 000 children. About half of these trials were conducted within existing community-based or clinic-based programmes, so the results are relevant to real-world settings. Finally, we used the CHEERS 2022 checklist to ensure we met each relevant criterion for high-quality reporting of our methods and results(Reference Husereau, Drummond and Augustovski27). Because Uganda was not one of the sites in which the trials of SQ-LNS were conducted, one study limitation is that we had to assume that the findings of the meta-analyses would apply to Uganda. Another limitation is that we were not able to estimate costs and cost-effectiveness in urban settings due to the lack of information on the cost of delivering a product like SQ-LNS via platforms that are prevalent in urban areas such as routine health clinic services. As a result, there is uncertainty around the extent to which these results would apply to urban settings or to settings in other countries in which a delivery platform like Uganda’s VHT system does not exist. There is also some uncertainty regarding the estimates for developmental disability because we based this outcome on persistent language difficulty, which contributes to but is not a direct measure of intellectual disability. However, we may also have underestimated the impact on developmental delay because we based our estimates only on language delay even though the SQ-LNS meta-analyses also documented reductions in delayed motor and socio-emotional development. Finally, there is some evidence from low- and middle-income countries that interventions providing Fe to children can heighten the risk of malaria(Reference Sazawal, Black and Ramsan28) and diarrhoea(Reference Soofi, Cousens and Iqbal29,Reference Jaeggi, Kortman and Moretti30) . While evidence from randomised controlled trials from multiple low- and middle-income countries has shown that SQ-LNS does not increase the risk of fever or suspected malaria mortality(Reference Adu-Afarwuah, Lartey and Brown31–Reference Prendergast, Chasekwa and Evans35), and trials in Bangladesh have shown that SQ-LNS reduces the prevalence of diarrhoea(Reference Luby, Rahman and Arnold36) and the duration of diarrhoea, pneumonia and dysentery(Reference Christian, Shaikh and Shamim37), it is theoretically possible that consuming SQ-LNS could have negative side-effects, including increased risk of infection. Any costs to caregivers associated with such side-effects, including medical expenses and the opportunity cost of caregivers’ time caring for sick children, were not accounted for in our analysis.
We are aware of only one other published study that estimated the cost of providing SQ-LNS to children. Hiebert et al.(Reference Hiebert, Phelan and Kinda38) estimated the cost of including daily SQ-LNS for children 6–23 months of age as part of an integrated package of maternal and child interventions in rural Niger. They found that the incremental cost of adding 18 months of SQ-LNS delivery per child to the standard of care (interventions that were already being delivered) was $44 per child ($29/year) (2019 US dollars), though this cost was based only on the cost of the product plus freight and is therefore not comparable to our total cost estimates.
In addition to the cost study of MNP from which our cost estimates were derived(Reference Schott, Richardson and Baker8), several studies have modelled the cost and cost-effectiveness of providing various nutrition interventions to young children in Uganda, including Pasricha et al.(Reference Pasricha, Gheorghe and Sakr-Ashour39) who estimated the cost-effectiveness of MNP supplementation, and Shekar et al.(Reference Shekar, Hyder and Subandoro40) who estimated the cost-effectiveness of providing complementary food. Several key methodological choices complicate the direct comparison of results across studies. For estimating costs, the chosen perspective and type of costing can significantly influence cost estimates. Cost studies conducted from a societal perspective, as is the case for our study, account for the economic value of all resources used in providing and accessing an intervention for all stakeholders. Cost studies conducted from a narrower perspective, for example, from the perspective of programme implementers or the government, are limited to the costs incurred only by those stakeholders. Similarly, cost studies using economic costing are comprehensive in that all inputs used in providing and accessing an intervention are included, even inputs that are not paid for such as volunteer labour or household time to participate in an intervention, in which case the economic cost of the input is the input’s value in its next best use (i.e. the input’s opportunity cost)(21). This can lead to very different estimates of cost than is the case in studies based only on financial costs, which exclude opportunity costs. These and other intervention characteristics that can influence costs estimates are summarised, by study, in Table 4. After adjusting for differences in study characteristics where possible, estimates from other studies ranged from $22 per child to deliver MNP to $72 per child for the provision of complementary food. Our estimate of $52 per child for SQ-LNS falls within this range. There was wide variation in the percentage of the total cost per child attributed to the cost of the product itself, ranging from 18 % for MNP based on the Schott et al.(Reference Schott, Richardson and Baker8) study to 77 % for complementary food (note that, for the provision of complementary food, although the cost of the food itself is high, it is also possible that the programme would generate local economic benefits if locally produced foods are chosen, although smaller-scale production may lead to higher food prices, potentially offsetting some local economic benefits). Our model indicated that product costs represent 42 % of the cost of delivering SQ-LNS. If non-product costs of nutrition interventions are underestimated, cost-effectiveness estimates may be overly optimistic.
Table 4 Study-specific costing characteristics and cost, adjusted* cost and cost-effectiveness estimates
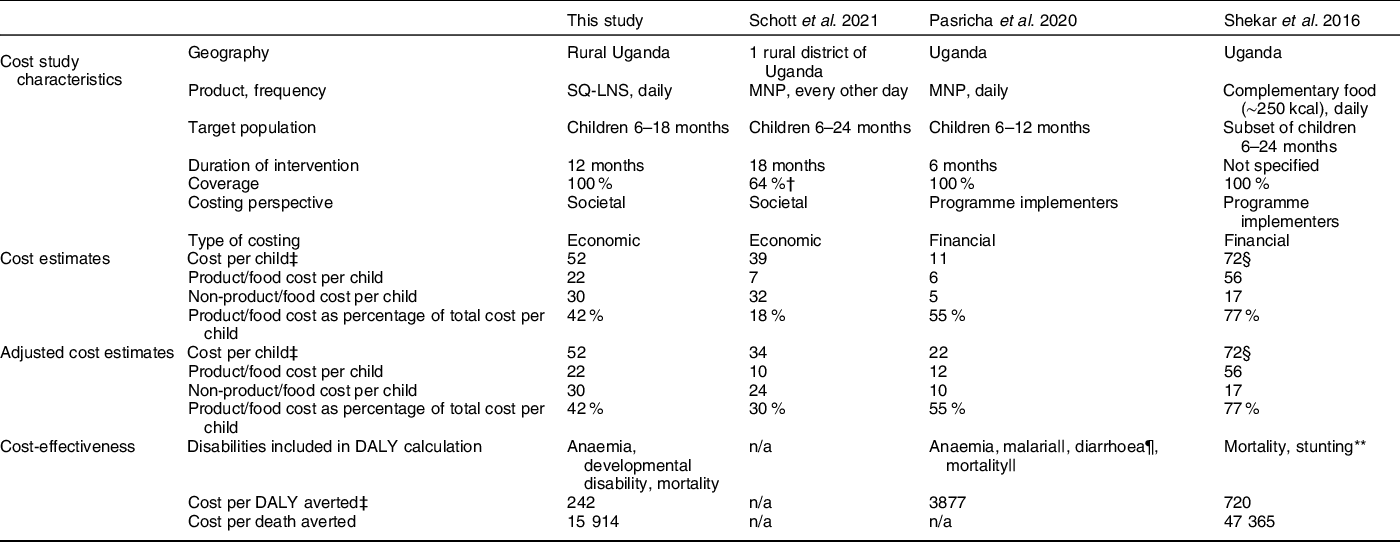
DALY, disability-adjusted life year; MNP, micronutrient powder; SQ-LNS, small-quantity lipid-based nutrient supplements.
* Costs adjusted to estimate the cost per child of daily supplementation for 12 months at 100 % coverage. For Schott et al., product/food costs adjusted by multiplying reported unit cost per packet (30 sachets) of MNP by 12 months, and adjusted non-product costs estimated based on (1) increased ‘last mile’ VHT/HW and household opportunity costs to account for additional MNP deliveries and (2) higher total product shipping and import duty costs. For Pasricha et al., product/food costs adjusted by multiplying reported unit cost per sachet by 365 d, and non-product costs calculated by assuming the ratio of food to non-food costs would remain the same. Because unit costs in Shekar et al. were already defined based on daily supplementation for 12 months, no adjustment was necessary.
† Based on reach, defined as consumption of MNP in past 7 d. Costs per child based on the subset of reached children. If instead costs per child were on the basis of all targeted children, the cost per child would be $25, the product cost per child would be $4, the non-product cost per child would be $20 and the product cost as a percentage of the total cost would be 17 %.
‡ All costs in undiscounted 2020 US dollars.
§ The cost per child is based on the annual cost of supplementation (supplementation for 12 months), not supplementation from 6 to 24 months.
|| Potential increase or decrease in malaria and mortality attributable to MNP.
¶ Potential increase in diarrhoea attributable to MNP.
** The projected number of lives saved and cases of childhood stunting averted were calculated using the Lives Saved Tool (LiST).
Differences in the methodology used to model the effects of an intervention can also make it difficult to directly compare estimates of cost-effectiveness. The approach we took, which was also taken by Pasricha et al. (Reference Pasricha, Gheorghe and Sakr-Ashour39), was to use direct effects of a specific intervention for all modelling based on systematic reviews of randomised controlled trials of that intervention. This is in contrast to the approach employed by the Investments Framework for Nutrition work(Reference Shekar, Kakietek and Eberwein41), including the Shekar et al. (Reference Shekar, Hyder and Subandoro40) Uganda study, in which estimated effects of interventions on diseases (morbidity), anaemia, wasting and stunting based on systematic reviews of similar interventions were inserted into the LiST model to estimate lives saved and other outcomes. The LiST pathways for mortality are defined via cause-specific mortality, which means that the impacts of complementary feeding interventions on child mortality are estimated based on effects on specific diseases, stunting and wasting(Reference Scott, Delport and Hainsworth42). Our model used direct effects of SQ-LNS on all-cause mortality; we did not have data on cause-specific mortality. Our estimated undiscounted cost per DALY averted was $242 for SQ-LNS, considerably lower than the estimates for MNP ($3877) and provision of complementary food ($720) in Uganda (Table 4). Our estimated undiscounted cost per death averted was $15 914 for SQ-LNS, much lower than the estimate of $47 365 for provision of complementary food; there is no estimate for MNP because no effect of MNP on mortality has been documented(Reference Suchdev, Jefferds and Ota43). However, it is important to bear in mind that variation in the approaches to cost and effectiveness modelling across these studies could account for some of these differences.
As mentioned, our models assumed that all children would be provided with SQ-LNS for 12 months starting at 6 months of age. We made this assumption because most of the randomised trials in the meta-analyses started SQ-LNS at 6 months and provided the supplements for at least 12 months, so our estimates of effects are mostly relevant to this scenario. In real-world programmes, children may begin receiving SQ-LNS later than 6 months, and duration of supplementation might vary. These variables would affect both costs and effectiveness. There is currently insufficient evidence to estimate effectiveness for scenarios in which the age at beginning of supplementation and duration of access to SQ-LNS vary. This is an important gap and is a high priority for further research.
In real-world programmes, adherence to the recommended daily consumption of SQ-LNS can also be highly variable. The trials in the SQ-LNS meta-analysis defined adherence in very different ways, so no overall estimate is available. In the programme-based trials, the lowest estimate was 37 %(Reference Becquey, Huybregts and Zongrone44), based on caregiver receipt of SQ-LNS in the previous month, and the highest estimate was 97·4 %(Reference Dewey, Mridha and Matias45), based on the percentage of caregivers reporting ‘high adherence’ (child consumption > 4 d/week). In three other programme-based trials, average adherence was 47 %(Reference Huybregts, Le Port and Becquey19), 73·5 %(Reference Humphrey, Mbuya and Ntozini32) and 97 %(Reference Iannotti, Dulience and Green46). Thus, our effectiveness estimates do not assume 100 % adherence. Moreover, average study-level adherence generally did not modify the effect of SQ-LNS in the meta-analysis(Reference Dewey, Stewart and Wessells2). We thus believe that our effectiveness estimates are robust with regard to variation in adherence.
Our model was based on a community-based platform in which CHWs would deliver SQ-LNS every 3 months. More frequent delivery (e.g. every month) would increase costs somewhat, probably similar to the cost increment we modelled for providing a higher VHT incentive in the sensitivity analyses. There is wide variability across and within countries in the performance of CHW/VHT systems, which will influence cost-effectiveness. Some programmes may need strengthening to adequately deliver SQ-LNS. On the other hand, the addition of a supplement to be provided by CHW to caregivers may enhance CHW performance. For example, in Bangladesh(Reference Dewey, Mridha and Matias45), CHWs were more likely to conduct the intended monthly home visits when they had a supplement to deliver. Additional implementation science research to evaluate the programmatic impact of adding delivery of supplements such as SQ-LNS to the usual activities of CHWs would be useful.
There are other potential platforms for distribution of SQ-LNS that warrant cost-effectiveness modelling, including provision via health clinics. The integration of SQ-LNS provision into existing programmes for wasting prevention and treatment has been evaluated in a health system platform in Burkina Faso(Reference Becquey, Huybregts and Zongrone44) and a CHW platform in Mali(Reference Huybregts, Le Port and Becquey19). In both sites, provision of SQ-LNS was a powerful incentive for caregivers to attend monthly screenings for acute malnutrition. If there are cost savings associated with reduced need for treatment for moderate or severe acute malnutrition and for hospitalisation, this can be factored into economic analyses of SQ-LNS. Further work is also needed to evaluate the impact of providing SQ-LNS as an incentive to attend health clinics and/or social and behaviour change communication sessions, with regard to potentially increased uptake of non-nutrition services such as immunisations.
Although our results suggest that provision of SQ-LNS is highly cost-effective, the total cost for a programme aimed at all age-eligible children in a given country is high. This is inherent to a food-based intervention that is designed for prevention (rather than treatment) of malnutrition, such as blanket provision of complementary foods. Cost-sharing arrangements in which households or other stakeholders have been called upon to pay some of the cost of goods and services provided to children are not uncommon. Previous work to estimate willingness-to-pay for SQ-LNS from several countries in Sub-Saharan Africa suggests that, across countries and methods of eliciting willingness-to-pay (including hypothetical (money did not change hands) and experimental (money changed hands)), average willingness-to-pay for SQ-LNS is above the product price of $0·06 per sachet(Reference Adams, Vosti and Ayifah47–Reference Tripp, Perrine and de Campos49). If some rural Ugandan households were willing to cover the cost of SQ-LNS, financing needs for those children would drop to ∼$30 per child, although shifting costs from implementing agencies to households does not change the total societal cost of the intervention.
Although financing needs could be greatly reduced if households covered some or all of the cost of the product, SQ-LNS cost-sharing strategies would need to account for the fact that, in many settings, most households cannot afford to pay the full price of the product. For example, evidence from a market trial in Burkina Faso suggests that, in that context, household persistent demand for SQ-LNS was very limited(Reference Lybbert, Vosti and Adams50). In addition, expecting households to cover the cost of the product could substantially limit uptake and/or consistent use over time, which has been observed in other settings for preventative health products(Reference Cohen51) and would likely have unquantified negative implications for the effectiveness of SQ-LNS on our outcomes of interest. Developing a cost-sharing distribution strategy in which households with the ability to pay are expected to pay while poorer households are provided SQ-LNS free of charge through a voucher system, for example, might be a possibility. This would require research into the cost and feasibility of mechanisms for identifying households who could and could not pay, complemented by research on demand for SQ-LNS, uptake and consistency of use and benefits under a targeted voucher system. This would provide an empirical basis upon which the cost-effectiveness of such a strategy could be modelled.
Another option for reducing costs would be to target the intervention to the most vulnerable communities, such as those with high levels of stunting, wasting, child mortality or food insecurity. For instance, in Uganda, SQ-LNS could be targeted to rural districts in sub-regions with the highest rates of child mortality. According to the 2016 Demographic and Health Survey, of the fifteen sub-regions represented in the survey, the worst-off sub-regions in terms of child mortality were West Nile, Busoga, Tooro, Ankole and Karamoja(52). By matching rural districts to these five sub-regions, we estimated that, if targeting were based on child mortality rates, approximately 35 % of children 6–18 months of age in rural Uganda would receive SQ-LNS (396 808 annual average children compared to 1 118 340 children under the untargeted scenario). While the cost per child would still be ∼$52, assuming that the targeting could be accomplished with existing, regularly collected data (if community-level targeting required new data collection, the cost per child would increase), the annual average cost of the programme would drop by ∼65 % (from ∼$58·7 million to ∼$20·8 million per year). In addition to reducing costs, targeting SQ-LNS to children in the most vulnerable communities could enhance effectiveness for certain outcomes. In the SQ-LNS meta-analyses, the impacts of SQ-LNS on development and Fe status were greater among children with a higher burden of undernutrition or lower socio-economic status(Reference Prado, Arnold and Wessells4,Reference Wessells, Arnold and Stewart6) . Note that the cost of individual-level targeting, which would involve screening children, could be substantial, so targeting at the community-level rather than the individual-level is generally more feasible.
Conclusion
Despite increased focus on early childhood malnutrition over the past decade, millions of children in low- and middle-income countries remain vulnerable to undernutrition(53–Reference Black, Victora and Walker55). Strategies that include provision of supplements such as SQ-LNS have been shown to be effective in helping reduce this burden. However, making informed policy decisions aimed at developing a portfolio of effective, cost-effective and financially feasible interventions requires that robust estimates of intervention programme costs and cost-effectiveness be available to policymakers and programme planners alongside evidence of effectiveness.
Investing in SQ-LNS has the potential to cost-effectively save child lives and to avert cases of anaemia, developmental disability, stunting and wasting, but the total cost of programmes that include SQ-LNS can be substantial. Financing strategies may require innovative financing mechanisms based on stakeholders’ recognition that such programmes will enhance child welfare and make fundamental contributions to reducing poverty and promoting overall economic development over the long term. This approach has been used for other types of investments, such as early childhood education programmes, which contribute to an array of development objectives(Reference Psacharopoulos and Woodhall56) but are expensive to establish and to maintain; indeed, multiple stakeholders are generally called upon to fund early childhood education programmes(Reference Belfield57). For their part, households recognise these benefits, some of which accrue to them, and are willing to pay for them(Reference Gertler and Glewwe58), but requiring resource-poor households to do so can raise equity issues. Similarly, for programmes that include SQ-LNS, households may be willing to cover some of the costs(Reference Adams, Vosti and Ayifah47), though more research is needed on household demand persistence(Reference Lybbert, Vosti and Adams50). If policymakers choose to invest in SQ-LNS, sustainably financing its incorporation into portfolios of child nutrition interventions may require targeting and/or other strategies to reduce and spread costs among multiple stakeholder groups.
Acknowledgements
The authors gratefully acknowledge Robert Ackatia-Armah for his guidance on the development of the cost model and for providing important Uganda-specific context. We also thank Belinda Richardson and Emily Baker for guiding us through the Uganda MNP costing study data. We also gratefully acknowledge the coordinating support of Caroline Joyce.
Financial support
This work was supported, in whole or in part, by a grant from the Bill & Melinda Gates Foundation (OPP49817) to the University of California, Davis. Under the grant conditions of the Foundation, a Creative Commons Attribution 4.0 Generic License has already been assigned to the Author Accepted Manuscript version that might arise from this submission.
Conflicts of interest
All authors declare they have no conflicts of interest.
Authorship
K.G.D. conceptualised the study. All authors designed the study. K.P.A. and S.A.V. developed the cost model. K.P.A. and C.D.A. developed the effectiveness model. K.P.A. and K.G.D. wrote the first draft of the manuscript. All authors contributed to data interpretation and revisions of the manuscript, and read and approved the final manuscript.
Ethics of human subject participation
Not applicable.
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S1368980023001805









