The nutritional status of women during pregnancy influences physiological outcomes in the child, including birth size(Reference Mathews, Yudkin and Neil1, Reference Godfrey, Robinson, Barker, Osmond and Cox2), later risk of CVD and diabetes(Reference Gluckman and Hanson3, Reference Roseboom, van der Meulen, Osmond, Barker, Ravelli, Schroeder-Tanka, van Montfrans, Michels and Bleker4) and cognitive function(Reference Hurst5). Despite some concerns about exposure to methylmercury(Reference Debes, Budtz-Jorgensen, Weihe, White and Grandjean6), fish consumption during pregnancy provides an excellent source of dietary protein(Reference Roos, Wahab, Chamnan and Thilsted7) as well as a number of micronutrients essential for fetal development(Reference Gibson and Hotz8, Reference Clarkson and Strain9) such as Fe, iodine, Zn, Se, choline and long-chain PUFA.
Iodine and Fe deficiencies are two of the most common nutritional deficiencies in the world. An estimated 30 % of the world’s population inhabits areas of iodine deficiency(10) while Fe deficiency anaemia affects up to 50 % of pregnant women in developing(11) countries and up to 25 % of children under the age of 3 years, with higher rates observed in developing countries(10). Correction of iodine deficiency in pregnancy can be achieved with supplementation and is associated with improved psychomotor test scores in infants(Reference O’Connell, Rakeman, Zhi-Hong, Xue-Yi, Mei, DeLong, Brenner, Tai, Dong and DeLong12). Fe supplementation in children can improve mental and motor scores in standardised developmental assessments(Reference Idjradinata and Pollitt13). Maternal Zn status has been linked with infants’ early behaviour(Reference Mahomed, Bhutta and Middleton14), with Zn supplementation during pregnancy associated with increased motor activity in the offspring(Reference Kirksey, Wachs, Yunis, Srinath, Rahmanifar, McCabe, Galal, Harrison and Jerome15). Se is also a vital component of the maternal diet with essential roles in fetal development(Reference Merialdi, Caulfield, Zavaleta, Figueroa and DiPietro16). Se might influence fetal development directly by interacting with iodine in regulating thyroid function(Reference Holben and Smith17) and might also have a protective role in the prevention of methylmercury toxicity(Reference Watanabe18).
Long-chain PUFA play an important structural role in neural tissue, especially the brain and retina(Reference Sastry19). Fetal accretion is at its greatest in the third trimester of pregnancy(Reference Clandinin, Chappell, Leong, Heim, Swyer and Chance20) and supplementation with long-chain PUFA in pregnancy has been shown to improve cognitive function(Reference Hadders-Algra, Bouwstra, van Goor, Dijck-Brouwer and Muskiet21). Choline, a nutrient that can be synthesised de novo in the body, appears to be a conditionally essential dietary nutrient for optimal brain development both pre- and postnatally(Reference Zeisel22). Supplementation with choline in animal models has indicated a life-long enhancement in spatial memory(Reference Blusztajn, Cermak, Holler and Jackson23) and cognitive function(Reference Glenn, Gibson, Kirby, Mellott, Blusztajn and Williams24).
The Republic of Seychelles is a small tropical archipelagic state in the Indian Ocean with one of the highest per capita rates of fish consumption in the world(Reference Robinson and Shroff25). The population consumes a traditional diet based around high fish consumption in conjunction with a high intake of fruit and vegetables. Such a diet would be expected to provide optimal nutrient intake with respect to those micronutrients of importance in infant development. Evidence has indicated that fish consumption has decreased by up to one-third over the last two decades as the Seychellois population adopts a more Western-style diet and lifestyle(Reference Shamlaye, Shamlaye and Brewer26). This has led to increased concern that if these trends continue, micronutrient status may be compromised. However, no study to date has examined in detail dietary patterns during pregnancy in this population. The aims of the current project, therefore, were to characterise the diets of pregnant Seychellois women and to determine the role that fish play in promoting adequate intakes of nutrients important for fetal and neonatal development.
Materials and methods
Subjects
A total of 300 pregnant women were recruited in 2001 from all (n 9) antenatal clinics on Mahé in the Republic of Seychelles. All eligible women attending the antenatal clinics for their first antenatal visit within a 3-month period, who met the inclusion criteria, were invited to participate on the study. Inclusion criteria were aged over 16 years, resident on Mahé (main island of the Seychelles archipelago and where 90 % of the total population lives) and native-born Seychellois. The cohort of 300 represents one-fifth of total annual deliveries in Seychelles and 75 % of all women booking at antenatal clinics during the enrolment period, and was therefore considered to be a representative sample of the population. Women were excluded if they were vegetarian, or if they reported a serious medical illness such as insulin-dependent diabetes, toxaemia with seizures or a haematological disorder such as thalassaemia or sickle cell anaemia. The study was reviewed and approved by the Research Subjects Review Board in Seychelles and the appropriate Research Subjects Review Boards of the collaborating partners.
Dietary assessment
Detailed information on the issues involved in establishing the dietary survey methodology in Seychelles is documented elsewhere(Reference Robson, Choisy, Bonham, Duffy, Wallace, Esther, Strain and Livingstone27). Briefly, at 28 weeks’ gestation detailed dietary information was collected from each subject by means of a prospective 4 d semi-quantitative food diary (two consecutive weekdays and two weekend days). The diet diaries were available in both English and Kreol language, and detailed instructions on completion of the diet diary were given to each subject by trained investigators.
Nurses, trained by nutritionists from the University of Ulster, reviewed the diaries within one week of completion, and errors and omissions were clarified with subjects. Data in the diet diaries were then converted to gram weights for input into a dietary analysis package (WISP version 2·0; Tinuviel Software, Warrington, UK). Package weights of imported food, much of which was from the UK at that time, were obtained from UK standard food portion sizes(Reference Crawley28).
The dietary analysis package, WISP, was supplemented with food composition and recipe data for additional foods consumed in Seychelles. These data were obtained from a variety of food composition tables from around the world including The Composition of South African Foods (Reference Sayed, Frans and Schönfeldt29) and The Concise New Zealand Food Composition Tables (Reference Ather, McLaughlin and Taylor30). In addition, the energy and nutrient composition of ten of the most commonly consumed fish were chemically analysed (CCFRA Technology Ltd, Chipping Campden, UK) and nutrient values entered into the database. The WISP program was further augmented with data for the choline content of foods obtained from the US Department of Agriculture food composition database(31). Data were mapped to the most appropriate food codes in the UK database by a registered nutritionist. This process involved both matching for food name and nutrient profile.
Anthropometry
Maternal height and weight were measured according to standardised procedures by trained nurses at enrolment into the study and BMI was calculated as [weight (kg)]/[height (m)]2. Measuring equipment in each of the participating antenatal clinics was calibrated prior to initiation of the study, and regularly throughout the project, by the Seychelles Bureau of Standards.
Estimated BMR
BMR (MJ/d) was estimated for all subjects at enrolment into the study using the Schofield equations(Reference Schofield, Schofield and James32). Depending on the age of the subject at enrolment the following equations were used:
Diet of pregnant women in Seychelles and
where weight is in kilograms and height is in metres.
Under-reporting
The level of under-reporting (MJ/d) of energy intake (EI) was determined as follows. Cut-off limits for EI:BMR were calculated as described by Goldberg et al.(Reference Goldberg, Black, Jebb, Cole, Murgatroyd, Coward and Prentice33) using the following equation(Reference Black34):
where PAL (physical activity level) was assumed to be 1·4 × BMR as recommended by Prentice et al.(Reference Prentice, Spaaij, Goldberg, Poppitt, van Raaij, Totton, Swann and Black35) for the third trimester pregnancy; S is a factor that accounts for variation in BMR, EI and PAL; n is the number of subjects; and SDmin(or max) is −2 or +2sd for the 95% upper confidence limit.
Socio-economic status
Socio-economic status (SES) was assigned to each participant using the Hollingshead four-factor score based on education, occupation, sex and marital status. Occupational scores were based on a list of Seychellois employment codes, as previously reported(Reference Myers, Davidson and Cox36).
Statistical analysis
All data were analysed using the SPSS 12·0 for Windows statistical software package (SPSS Inc., Chicago, IL, USA). Data for all variables were tested for normality and adjusted where necessary. To reduce the inaccuracies associated with estimating the extent of nutritional inadequacy in this population based on short-term dietary data collection, statistical methods were used to estimate the usual distribution of intakes based on the observed intakes. Adjustment of observed intakes was carried out as follows. Dietary data were normalised, and within- and between-person variances were calculated. The mean intake of each subject was then adjusted as follows:
The resulting adjusted distributions were then used to compare reported nutrient intakes with dietary recommendations, using the cut-point method(37, Reference Morimoto, Marchioni and Fisberg38). In the Republic of Seychelles, nutritional guidelines are based on the UK Dietary Reference Values (DRV)(39).
To assess the potential impact of dietary misreporting on the extent of potential nutrient inadequacy, dietary intakes reported by the whole group were compared with those reported by subjects not classified as under-reporters using one-way ANOVA. Independent t tests were used to examine for differences between fish consumption in subjects meeting nutrient requirements compared with those who did not. A significance level of P < 0·05 was used to evaluate all statistical outcomes.
Results
Subject characteristics
Of the 300 women recruited to the study at their first visit to an antenatal clinic (mean gestational age 12·5 weeks) dietary data were available for 273 women. Dropouts were for a combination of reasons including miscarriage/abortion (n 12), not pregnant (n 4), illness (n 1), relocation (n 2) and non-compliance (n 8). On average, women who participated in the study had a mean (sd) age of 27·0 (6·1) years, were 1·60 (6·7) m tall, weighed 66·7 (16·6) kg and had a BMI of 25·9 (6·3) kg/m2 at enrolment. SES was assessed by the Hollingshead score (n 260). This score is divided into four groups: unskilled (13·5 %), semi-skilled (25·8 %), skilled (25·4 %) and business/professional (35·4 %). The highest percentage of pregnant women were in the business/professional category. SES had no influence on nutrient densities (nutrient intake/MJ energy intake; data not shown).
Estimated BMR was calculated at enrolment to the study. For the group as a whole, mean (sd) BMR was 5·98 (0·83) MJ/d. Mean EIrep:BMR was 1·33. Calculation of cut-off values was based on a PAL of 1·4 × BMR(Reference Prentice, Spaaij, Goldberg, Poppitt, van Raaij, Totton, Swann and Black35). The daily variance in energy intake was 22·07 % and the estimated BMR and physical activity levels were 8·5 % and 15 % respectively(Reference Black34), giving a cut-off for under-reporting of 1·37 × BMR. A total of 109 women (39·6 %) were classified as under-reporters.
Energy and macronutrient intakes
Table 1 presents mean (sd) and median (5th, 95th percentiles) dietary intakes of pregnant women in Seychelles for the group as a whole and after excluding under-reporters. For the whole group, median (5th, 95th percentiles) daily energy intake was 8·9 (5·0, 13·4) MJ and comprised 48·6 (37·8, 56·8) % carbohydrate, 36·3 (28·8, 44·0) % fat and 15·4 (12·0, 19·4) % protein. Comparisons between nutrient intakes of the whole group (n 273) and those who were classified as non under-reporters (n 164) indicated a significantly higher intake of all nutrients except vitamin A in those subjects who were not deemed to be under-reporters. Macronutrient intake expressed in relative terms (percentage of energy) was not significantly different between the group as a whole and after excluding under-reporting, suggesting that under-reporting was not macronutrient-specific.
Table 1 Observed nutrient intakes in pregnant Seychellois women including (n 273) and excluding under-reporters (n 164)
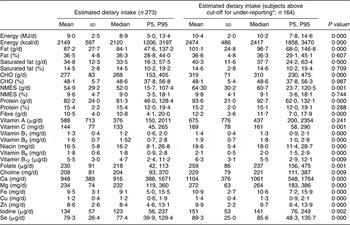
P5, 5th percentile; P95, 95th percentile; CHO, carbohydrate; NMES, non-milk extrinsic sugars.
*Cut-off points for under-reporting were calculated as described by Goldberg et al.Reference Goldberg, Black, Jebb, Cole, Murgatroyd, Coward and Prentice(33).
†P < 0·05 indicates a significant difference in dietary intakes between all subjects (n 273) and those who did not under-report (n 164).
Nutritional adequacy
The Estimated Average Requirement (EAR)(39) is advocated by the Institute of Medicine(40) as the most appropriate yardstick for assessing nutritional adequacy. Preferably, these comparisons should be made on data that have been statistically adjusted to estimate the distribution of usual intakes from the observed intakes(37). Table 2 presents a comparison of adjusted mean dietary intakes with the UK EAR for the group as a whole and after excluding under-reporters. Where nutrient recommendations for pregnancy are available (applicable to Reference Nutrient Intakes (RNI) only), comparisons have been made using the adjusted dietary intakes with the appropriate RNI (specifically protein, vitamins A, B1, B2, folate and vitamin C) and also for nutrients where only RNI are stated (Cu, iodine and Se). As there are recommendations for a number of nutrients during pregnancy, comparison with RNI was more frequent than with EAR.
Table 2 Comparison of adjusted mean intakes of energy and nutrients with UK DRV for the whole group (n 273) and after excluding under-reporters (n 164) in pregnant Seychellois women

DRV, Dietary Reference Value (a term used to cover all intakes, including RNI and EAR); RNI, Reference Nutrient Intake (appropriate for 97 % of the population); EAR, Estimated Average Requirement (appropriate for 50 % of the population); CHO, carbohydrate; NMES, non-milk extrinsic sugars.
*Available recommendations for pregnancy have been used; otherwise recommendations for women are for the age group specified.
†US Adequate Intake for pregnancy.
‡Last trimester only.
When fat and carbohydrate intakes (expressed as a percentage of energy) were compared with UK DRV, saturated fat intakes for >90 % of the study group were in excess of recommendations. Non-milk extrinsic sugar intakes, however, were below the recommended DRV of ≤10 % energy intake for 97 % of the population.
Vitamins B6 and B12 were the only nutrients that met recommended requirements (RNI) in the population as a whole. Intakes of protein and vitamin C when compared with the RNI were deemed to be adequate for >90 % of the population. When under-reporters were excluded, the percentage of the group attaining the appropriate DRV was higher for all nutrients except vitamin A (comparison with RNI). Intakes of nutrients specifically important in pregnancy such as Fe and Zn (comparison with EAR), folate and Se (comparison with RNI) were evaluated and low levels were observed in 80·6 %, 8·4 %, 91·2 % and 20·2 % of the population as a whole, respectively. The exclusion of under-reporters reduced these values to 69·5 %, 0 %, 84·8 % and 9·1 %, respectively.
Contribution of food groups to nutrient intakes
Average fish intake was 76 g/d and consumed by 98 % of all subjects. The most commonly consumed fish were karang (32 %), mackerel (12 %), spinefoot shoemaker (12 %), fresh tuna (6 %), tinned tuna (6 %) and barracuda (5 %). Meat and fish made similar contributions to protein intake and together accounted for 40 % of overall protein intake. Vegetables (excluding potatoes) were consumed by all subjects and fruit/fruit juice was consumed by 95·2 % of the subjects (data not shown).
Table 3 presents the contribution of the various food groups to the dietary intake of the nutrients of specific interest in pregnancy, i.e. Fe, Zn, Se, iodine and choline. The main food groups contributing to Fe intakes were vegetables, meat and bread and rolls. Egg consumption provided over one-third of choline intakes, with the second largest contribution coming from the fish and fish products food group. Although not the most nutrient-dense food in terms of Se content, bread and rolls were consumed in amounts that made this food group the most important source of dietary Se, followed by fish. Red meat was the best source of dietary Zn. In terms of overall dietary contribution of the aforementioned nutrients which have an important role in pregnancy, fish was the second most widespread source of iodine, Se and choline, and contributed to both Zn and Fe intakes.
Table 3 Contribution of food groups to nutrients important in cognitive development in pregnant Seychellois women

Separation of the subjects into those who met the appropriate recommendations for Fe, Zn, iodine and Se (n 35) and those who met recommendations for three or fewer of these nutrients indicated a significantly greater mean (sd) fish consumption of 98·6 (65·8) g/d compared with 73·2 (42·1) g/d (P < 0·05).
Discussion
The present paper reports dietary habits of pregnant women in the Republic of Seychelles, a small island developing state and the location for a number of long-term observational epidemiological studies examining the effect of fish consumption on infant neurodevelopment(Reference Myers, Davidson and Cox36, Reference Davidson, Myers, Cox, Wilding, Shamlaye, Huang, Cernichiari, Sloane-Reeves, Palumbo and Clarkson41). Mean (sd) weekly fish consumption was high at approximately 527 (327) g(Reference Bonham, Duffy, Wallace, Robson, Myers, Davidson, Clarkson, Shamlaye and Strain42) and therefore would be expected to contribute considerably to dietary intakes of micronutrients such as iodine, Se and Zn. Indeed, women meeting dietary recommendations for all of the aforementioned micronutrients had a significantly higher fish intake. In the group as a whole, however, comparison of micronutrient intakes (adjusted means) with UK EAR or RNI where appropriate indicated noticeable shortfalls. Dietary Fe requirements, for example, were not met by 80·6 % of the population (based on UK EAR). The Institute of Medicine have concluded that Fe is the only nutrient in pregnancy for which diet alone cannot meet requirements(43). Supplements are routinely supplied during pregnancy in Seychelles but uptake appears sporadic at best(Reference Wallace, Bonham and Strain44). Although some dietary intakes observed in our study were low, the findings are comparable to those in populations in the UK, Mexico and South Africa(Reference Mouratidou, Ford, Prountzou and Fraser45–Reference Kesa and Oldewage-Theron47).
Initial analysis of the current cohort suggested that iodine intakes did not meet recommendations in 62·9 % of the study group (based on UK RNI). Low intakes of dietary iodine have also been observed in women of childbearing age in Europe and are approximately 50 % of recommendations(Reference Zimmermann and Delange48). This apparent shortfall in iodine intake is of concern as low iodine status in pregnancy has adverse implications for fetal neurodevelopment(Reference Smallridge and Ladenson49). However, intakes are likely to have been underestimated as the iodine content of some fish species consumed in Seychelles is unknown and therefore was unaccounted for in the dietary analysis. Subsequent analysis, using an assumed average iodine concentration per 100 g fish, suggested that 36·3 % did not meet requirements. Dietary intakes of Se were adequate in 79·8 % of the whole group (based on UK RNI). Although not the richest source of Se, bread had high habitual consumption that ensured this food group was the highest contributor to dietary Se, followed by fish. Adequate Se status might be important in high fish-eating populations given its possible role in counteracting the toxicity of methylmercury, which is also present in fish. Although Zn deficiency has been estimated to be as high as 25 % in the world’s population(Reference Maret and Sandstead50), in our study group inadequate Zn intakes (based on UK EAR) were seen in only 8·4 % of the population. However, after excluding under-reporters, the levels of apparent nutritional inadequacy decreased by 13·8 %, 19·6 % and 55·0 % for Fe, iodine and Se, respectively, and the dietary recommendation for Zn intake of 5·5 mg/d was met by all subjects.
Since recommendations for choline intake were published, several studies have reported that choline intakes often do not meet recommendations in both pregnant(Reference Gossell-Williams, Fletcher, McFarlane-Anderson, Jacob, Patel and Zeisel51) and non-pregnant individuals(Reference Chiuve, Giovannucci, Hankinson, Zeisel, Dougherty, Willett and Rimm52). Currently no RNI or EAR has been defined for choline; however, in the USA an Adequate Intake (AI) for pregnant women of 450 mg/d has been established. In our study group choline intake was 198 mg/d. This intake is considerably lower than the AI, despite habitual consumption of eggs that in the current study supplied 33·4 % of the choline intake. However, as with the assessment of dietary iodine intake, dietary choline intakes are likely to have been underestimated owing to the incompleteness of the dietary database.
When interpreting the results of the present survey, the biases associated with conducting dietary surveys must be acknowledged; most notably, under-reporting and the use of standard portion sizes to estimate weights of food consumed. In our study the lack of data on pre-pregnancy weight, combined with the use of an estimated PAL(Reference Black53), will have influenced the determination of BMR and, subsequently, the calculated cut-off for under-reporting. Therefore, we might have underestimated under-reporting and it is not possible to state conclusively that subjects above the cut-off level determined for under-reporting were actually achieving their energy and/or nutrient requirements. It is also conceivable their levels of under-reporting could have been underestimated owing to reluctance among subjects to report foods accepted as inappropriate during pregnancy, such as alcohol. It is also impossible to state if some foods regarded as being ‘healthy’ were over-reported. Nevertheless, the levels of under-reporting observed are plausible based on one other study in pregnant women(Reference Okubo and Sasaki54) and within the range observed in other studies which used more objective measures to assess energy requirements(Reference Schoeller55, Reference Subar, Kipnis and Troiano56). Consequently, we believe the findings are likely to be an accurate reflection of misreporting in this group.
In the absence of biochemical indices of micronutrient status, adequacy of micronutrient intakes was judged against UK dietary recommendations. To reduce the inaccuracies associated with estimating nutritional inadequacy, statistical methods were used to estimate the usual distribution of intakes based on the observed intakes. The variety of foods generally available for consumption in the Seychelles is limited compared with Western societies. This lack of variability may have reduced reporting errors and improved accuracy in assessment of nutrient intake. In addition, piloting and feasibility studies were undertaken in Seychelles prior to initiation of the current dietary survey(Reference Robson, Choisy, Bonham, Duffy, Wallace, Esther, Strain and Livingstone27) and adjustments were made to reflect usual dietary intakes. However, while every effort was made to collect accurate records of food intake, the value of the intake data is currently constrained by the lack of comprehensive food composition data.
In conclusion, despite reports of a decline in fish consumption, the Seychellois population had a weekly average (sd) fish intake of 527 (327) g. This intake is almost four times greater than those observed in the UK(57). Fat intakes were higher than previously reported(Reference Shamlaye, Shamlaye and Brewer26) and in most subjects exceeded the UK DRV for fat as a percentage of energy intake for both total (<35 %) and saturated (<10 %) fat. Indeed, macronutrient intakes in pregnant women in Seychelles were similar to intakes reported among pregnant women in the UK(Reference Mouratidou, Ford, Prountzou and Fraser45). These findings are reflective of a move towards a more Western-type diet and the emergence of an increased prevalence or risk factors for CHD in the Seychelles(Reference Shamlaye, Shamlaye and Brewer26). Our observation that fish consumption was significantly higher in the subset of subjects who met nutrient recommendations for Fe, Zn, iodine and Se is an important finding and highlights the critical role of fish in ensuring optimal dietary intakes of key micronutrients during pregnancy. Furthermore, as a source of protein in the Seychellois diet, fish was equivalent to meat but without the associated higher energy and fat content. These findings are of vital public health importance to the Seychellois and emphasise the necessity in maintaining current levels of fish consumption in this population. However, the overall trend towards a lower consumption of fish could become problematic in the future. These findings suggest caution in establishing public health policies that promote limiting fish intake during pregnancy to reduce exposure to methylmercury. Such policies may result in concomitant decreases in important micronutrient intakes and increased energy and fat intakes. Emphasis on the benefits of fish consumption should, therefore, be prioritised.
Acknowledgements
The research was funded by the National Institute of Environmental Health Sciences (NIEHS) of the US National Institutes of Health (grant number RO1 ES010219). M.P.B. wrote the paper and analysed the dietary data. E.M.D. co-analysed the dietary data. T.W.C., G.J.M., P.W.D., C.F.S., J.M.W., P.J.R., J.J.S. and M.B.E.L. were responsible for study design. All authors read and contributed to finalisation of the manuscript. We thank Octavie Choisy, Anne-Marie Bibi and the maternity nurses for all their work with the subjects in the Seychelles. We also acknowledge Seychelles Bureau of Standards and CCFRA Technology Ltd, Chipping Campden, UK for their laboratory analyses of fish samples. There are no conflicts of interest.





