Undernutrition impairs growth. Multiple sources of historic evidence of child hunger during and after World War I and World War II were summarised by Keys et al.(Reference Keys, Brozek and Henschel1). More recent historic literature refers to the work of Gomez and colleagues(Reference Gomez, Galvan and Cravioto2) who distinguished between first-degree malnutrition with ‘actual body weight between 85 and 75 % of the average theoretic weight for age’; second-degree malnutrition,Footnote * with ‘actual body weight between 75 and 60 % of the average theoretic weight for age’ and third-degree malnutrition, with ‘actual body weight <60 % of the average theoretic weight for age’. Gomez and colleagues studied third-degree malnutrition in 584 children aged 6 months to 5 years, referred to the Hospital Infantil de Mexico, in Mexico City. These children exhibited classic clinical symptoms of severe food shortage, and they were short. On average, they reached 84·2 % of their theoretic height for age. This study was repeatedly cited in the subsequent decades. When in the early 1970s, Waterlow classified protein–energy malnutrition and introduced the term ‘stunting’(Reference Waterlow3,Reference Waterlow4) , he referred to the Gomez et al. article as justification for choosing 85 % of the average theoretic height-for-age as the cut-off for stunting. A similar cut-off was also chosen by Pelletier(Reference Pelletier5) when discussing the relationship between child mortality and height-for-age. Death rates per 1000 children rose markedly at average population height below 85 % of theoretic height-for-age. Both Gomez et al. and Pelletier referred to average population height, but not to individual children. In their papers, it remained unclear whether a particular child below the 15 % deficit in height showed more, or more severe, clinical signs of undernutrition, or was at greater risk of death than children above this cut-off.
The historic phrase ‘percent of theoretic height for age’ has been replaced by z-scores for height or sd score for height (hSDS). Children are defined as stunted if their height-for-age is more than two sd below the WHO Child Growth Standards median (https://www.who.int/nutrition/healthygrowthproj_stunted_videos/en/). The WHO describes stunting as ‘…the result of chronic or recurrent undernutrition, usually associated with poor socioeconomic conditions, poor maternal health and nutrition, frequent illness, and/or inappropriate infant and young child feeding and care in early life’ and particularly states that ‘stunting holds children back from reaching their physical and cognitive potential’ (https://www.who.int/news-room/fact-sheets/detail/malnutrition). This may certainly be debated as it is not stunting per s e that holds children back from reaching their physical and cognitive potential, but rather the food shortage or their disadvantageous socio-economic status (SES) environment.
The Indonesian 2013 National Basic Health Survey(6) reported a 37·2 % prevalence of stunted children under the age of 5 years. This survey also reported a 12·1 % prevalence of wasted children under the age of 5 years (below minus 2 sd from median weight-for-height of reference population), with only 2·5 % of the children being both wasted and stunted. Many Indonesian children were either short with normal weight (27·4 %) or short and overweight (6·8 %).
In order to better understand the link between the historic nomenclature of ‘percent of theoretic height and weight for age’ and modern terminology using z-scores, Figure 1 relates percentage of theoretic height-for-age and weight-for-age and height and weight z- or sd scores (based on WHO reference). Height deficits of some 15 % (85 % of theoretic height-for-age) reported by Gomez et al. and Pelletier correspond to height z-scores between −3·2 and −4·8 hSDS depending on age. These children were truly short. Assuming a usual sd of approximately 1 hSDS, these historic samples consisted of up to more than 99 % stunted children.

Fig. 1 Percentage of theoretic height-for-age and weight-for-age and height and weight sd scores (based on WHO reference). ![]() , Weight 95 %;
, Weight 95 %; ![]() , height 95 %;
, height 95 %; ![]() , weight 85 %;
, weight 85 %; ![]() , height 90 %;
, height 90 %; ![]() , weight 70 %;
, weight 70 %; ![]() , height 85 %
, height 85 %
Children in many developing countries are short(Reference Lartey7–Reference de Onis and Branca10). The estimate of the global prevalence of stunting for under 5-year-olds is close to 155 million(Reference de Onis and Branca10). Most published interpretations of this number interpret it to mean that the majority of these children are undernourished and in greater need for nutritional support than those whose height-for-age z-scores are above the critical cut-off of hSDS < 2. We do not question that all of these children are short – they are short when referred to WHO reference – but we question that all of these children are undernourished(Reference Scheffler, Hermanussen and Bogin11).
Here, we describe Indonesian and Guatemalan schoolchildren with prevalence of stunting of up to 53·3 % in Indonesia and up to 56·5 % in Guatemala. These populations are short; many of these children were even below −3·2 hSDS. The question arises as to how many of these very short children are undernourished. We considered the possibility of a critical threshold (breakpoint) in the height distribution of stunted children below which body height might become a valid and reliable indicator of nutritional status. Can we distinguish between ‘mildly stunted’ children who are just short and well-fed, and ‘severely stunted’ children below some critical threshold with measurable signs of undernutrition? Considering thinness as an easily measurable sign of energetic undernutrition, we formulated the following hypothesis:
Among groups of stunted children, subgroups of very short children exist that show a relevant association between body height and thinness, defined by low BMI, low mid-upper arm circumference or low skinfold thickness.
Samples and methods
For testing our hypothesis, we used data obtained in Indonesian and Guatemalan, two nations with a high prevalence of stunting for children <5 years old. In 2010, 35·6 % of Indonesian children were stunted and this increased to 37·2 % in 2013 (https://en.wikipedia.org/wiki/List_of_Indonesian_provinces_by_GRP_per_capita). Based on the conventional perception that stunting indicates undernutrition, Indonesian children are officially considered ‘seriously’ affected by starvation, with a Global Hunger Index of 22 (http://www.globalhungerindex.org/pdf/en/2017/posters.pdf). In contrast, Indonesia is not a poor country and ranks seventh out of 190 countries in the World Bank list of GDP (https://en.wikipedia.org/wiki/List_of_countries_by_GDP_(PPP)), which makes it difficult to understand how more than one-third of its youngest children are seriously hungry. It becomes more understandable by noting that the Global Hunger Index includes stunting as one of its four components (https://www.globalhungerindex.org/about.html). Thus, the assumption that ‘stunting = malnutrition’ is implicit.
Guatemala has a prevalence of stunting of 49·8 %, the highest in the region the Americas and the sixth highest prevalence of stunting for children <5 years old in the world. In the Maya group, the prevalence reaches almost 70 % of children <5 years old (https://www.usaid.gov/sites/default/files/documents/1864/USAID-Guatemala_NCP.pdf). Guatemala is a middle-income country and ranks 75th out of 190 countries in the World Bank list of GDP. With a global hunger index of 20·7, also Guatemala ranks within the group of nations that are considered ‘seriously’ affected by starvation (http://www.globalhungerindex.org/pdf/en/2017/posters.pdf).
Anthropometric measurements were taken from 1716 healthy Indonesian schoolchildren aged between 6·0 and 13·2 years, from three geographical regions: the urban regions Ubud (Bali) and Kupang (capital of West Timor, East Nusa Tenggara), and the in rural regions the village Soe (West Timor, East Nusa Tenggara) and Marbau (North Sumatra) in February and March 2018. The measurements were performed in the presence of the children’s teachers and supervised and accompanied by local physicians, paediatricians and medical residents(Reference Scheffler, Hermanussen and Bogin11). We measured body height, weight, mid-upper arm circumference (MUAC) and triceps and subscapular skinfolds on the right site of the body. The children were lightly dressed and measured without shoes. Weight of the school uniforms was found to be close to 200 g in children below age 10 years, and about 300 g in children above age 10 years, and was subtracted from the weight measurements. Body height was determined by digital laser rangefinder GLM Professional® Bosch 250 VF(Reference Schrade and Scheffler12) to the nearest mm, weight by digital scales (Soehnle Style Sense Compact 100) to the nearest 100 g, and skinfold thickness by caliper (Holtain, Ltd) to the nearest 0·2 mm and MUAC by non-stretchable metric tape. All measurements were taken under standardised conditions(Reference Knussmann13).
Data of 3838 Guatemalan school students were obtained from the Longitudinal Study of Child and Adolescent Development conducted by the Universidad del Valle de Guatemala(Reference Bogin, Camacho de Paz and MacVean14). Particularly, the schoolchildren of low socio-economic status showed high prevalence of stunting. The participants were born 1963–1974, aged 4·00–18·99 years at the time of measurement and were from three SES and the two major ethnic groups of Guatemala, Ladinos and Maya. Very low SES Maya children came from a state-run primary school with grades kindergarten to sixth year, and from a private, low-cost secondary school that operated three grades. Both schools are in a village situated about 25 km from Guatemala City. The Ladino samples included an expensive private school of very high SES and a state-run school (low SES) in Guatemala City.
Statistical analyses
Standard deviation scores for body height (hSDS) and BMI (BMI SDS) were calculated according to WHO references (https://www.who.int/growthref/en/). In the Indonesian sample, from three measures of triceps and three measures of subscapular skinfolds, the average values were calculated as final value. In the Guatemala, data set values for mean skinfold thickness (SF) were obtained from two measures of triceps and subscapular skinfolds and if within 3 mm agreement, the average of these was the final value.
As MUAC depends on height(Reference Frisancho15), we used the ratio MUAC divided by height (MUAC/h = MUAC (cm) × 10/height (cm)) for our analyses (Fig. 2). The figure illustrates the case of the Guatemalan children and shows that this ratio is almost independent of height and very similar to MUAC/h of American children(Reference Frisancho15). Average MUAC/h is 1·52 (sd = 0·13) with third centile of 1·3, fifth centile of 1·32 and 10th centile of 1·36. We chose the 10th centile as a plausible cut-off according to the work of Fiorentino in Cambodian children(Reference Fiorentino, Sophonneary and Laillou16). Upper-arm muscle area was calculated according to Frisancho(Reference Frisancho15), as Frisancho recommends this as a nutrition indicator.
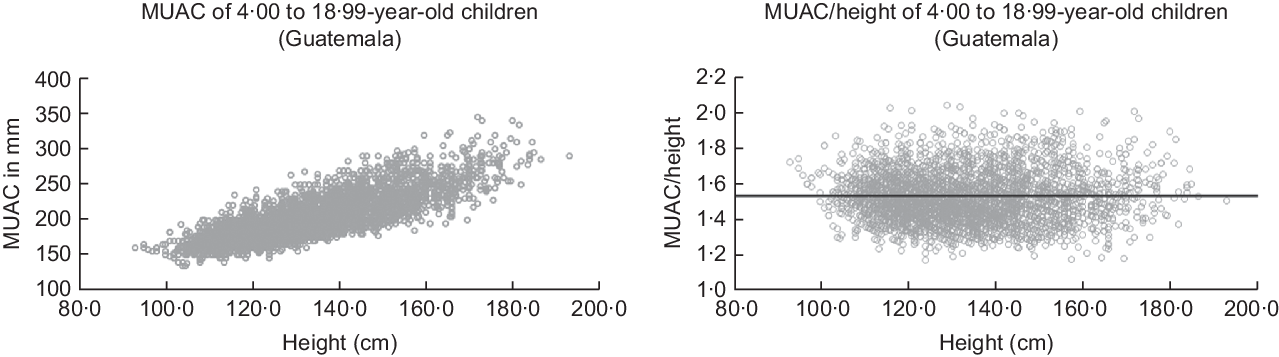
Fig. 2 MUAC (mm) and relative MUAC to height (MUAC/h) of 4·00 to 18·99-year-old children and adolescents of our Guatemalan sample. Dark line is corresponding to the mean of MUAC/h = 1·56 by American children (after Frisancho(Reference Frisancho15))
To test our hypothesis, we searched for a threshold that might separate children who are just short, and very short children with measurable thinness for whom the assumption of a relevant association between height and thinness holds true. We used breakpoint analysis(Reference Muggeo17). Breakpoint analysis segregates two segments of a linear regression. The breakpoint quantifies an abrupt change of the response function of a varying influential factor. The breakpoint can be interpreted as a critical threshold value beyond or below which a particular effect occurs. We defined the critical threshold for thinness by three criteria: (1) BMI SDS < −2, the cut-off recommended by the WHO (https://www.who.int/nutrition/healthygrowthproj_stunted_videos/en/), (2) by MUAC/h < 1·36 corresponding to the 10th centile of Guatemalan children (see above) and (3) by SF < 7 mm corresponding to the 10th centile of well-nourished German schoolchildren(Reference Schilitz18). Figure 3 shows three idealised graphs illustrating associations between hSDS, with BMI SDS, MUAC/h and SF. The graphs depict no association between nutritional status and body height above, and positive associations below, the critical thresholds of thinness.
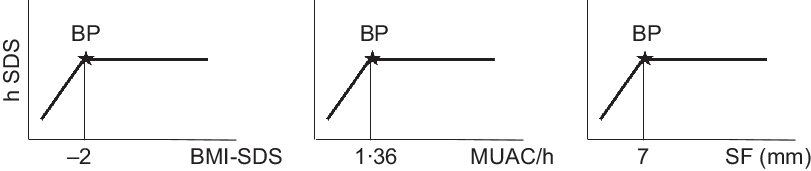
Fig. 3 Theoretical graphs of our hypotheses, that within stunted child populations, at least subgroups might exist with measurable signs of undernutrition, that is, with BMI, mid-upper arm circumference (MUAC) and skinfold thickness (SF) below critical limits, for whom the assumption of an association between nutritional status and body height holds true. We considered the critical limit for BMI at BMI-SDS < −2 (UNICEF) the critical limit for MUAC to height at MUAC/h < 1·36 corresponding to the 10th centile of Guatemalan children (see Statistical analyses) and the critical limit for SF at SF < 7 mm corresponding to the 10th centile of well-nourished German schoolchildren (Schilitz(Reference Schilitz18)). BP, Breakpoint
R package segmented(Reference Muggeo17) was used to fit linear multiphase regressions to estimate the association between the three anthropometric indicators of the nutritional status and hSDS.
Results
Figure 4 exemplifies the association between mean skinfold thickness and hSDS in the urban children from Kupang. The black line represents the Distance Weighted Least Square Regression for hSDS at different SF. The figure illustrates the prima facie impression that the thinnest children, those with an SF <~8·5 mm, are shorter.
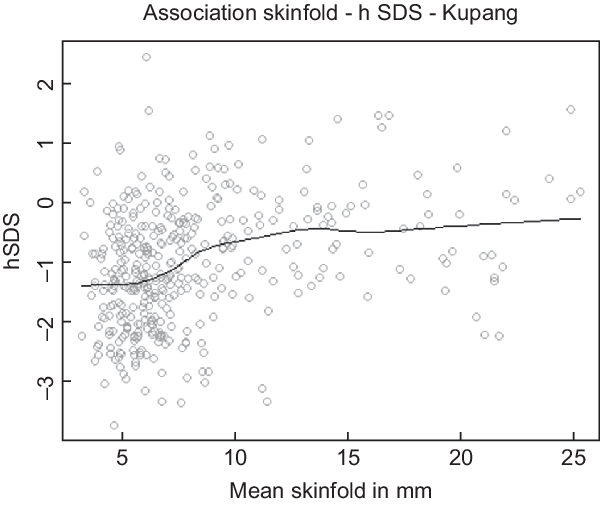
Fig. 4 Association between mean skinfold thickness (triceps and subscapular) and height SDS (WHO reference) of children from Kupang, Indonesia. The black line represents the moving average
Yet, this impression is deceptive. The breakpoint analysis distinguished between Kupang children with skinfold thickness above and below 10·16 mm (Table 1). This threshold is significantly larger than the critical threshold for thinness at SF < 7 mm and close to the 50th centile of healthy German children(Reference Schilitz18). It indicates that the majority of these Indonesian children cannot be undernourished, at least by this criterion.
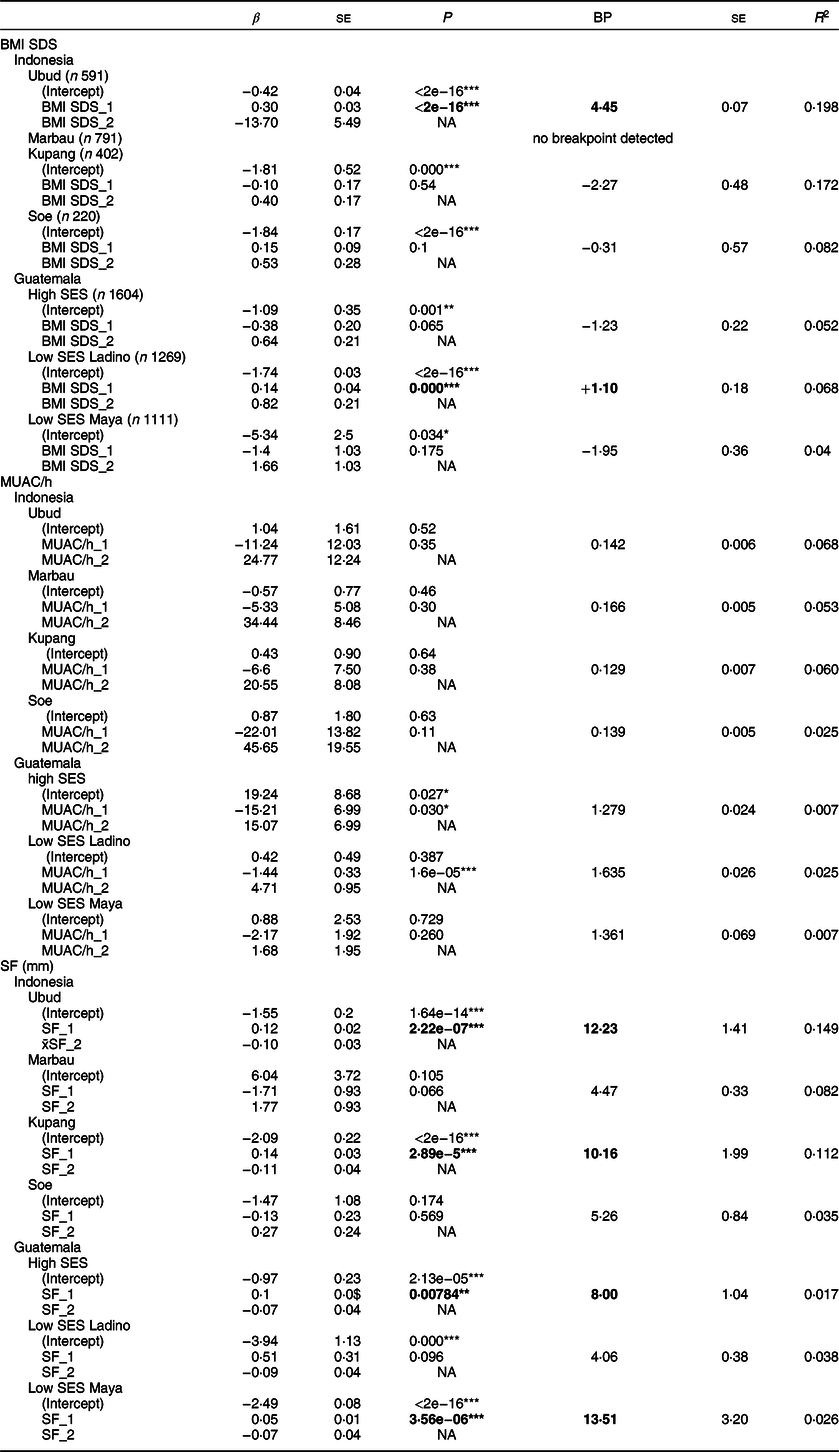
Table 1 Breakpoint (BP) analyses of the association between BMI SDS (WHO reference), upper arm circumference related to height (MUAC/h) and mean skinfold thickness (SF; mean of triceps and subscapular skinfold) and height-SDS (hSDS) (WHO reference) of children from Indonesia (Ubud, Marbau, Kupang, Soe) and Guatemala (high SES, low SES Ladino, low SES Maya) †
NA, not available.
Significance: * low, ** middle, *** high.
† Values in the column BP indicate the localisation of the BP; β indicates the slope of the linear regression lines before (*_1) and after the BP (*_2). Bold numbers indicate significant BP separating associations between indicators of nutritional status and hSDS below and above the BP. R 2 refers to the entire model.
For BMI, we either found no breakpoints (Marbau) or breakpoints that failed to reach statistical significance (Kupang, Soe and Guatemala in high SES Ladinos and low SES Maya, respectively). We found a significant breakpoint in the schoolchildren of Ubud and Guatemalan low SES Ladinos. Yet, the breakpoints were above the critical threshold of thinness (BMI SDS < −2), at +4·45 and +1·10 BMI SDS, respectively. Also the analysis of MUAC/h failed to identify stunted individuals. Breakpoints were detected in the children of Guatemala (high SES and low SES Ladino), but suggested negative associations between mid-upper arm circumference and height, that is, below the breakpoint, the shorter individuals had larger MUAC. In other words, neither BMI SDS nor mid-upper arm circumference supported the hypothesis of height growth impairment in exceptionally thin children. There were no breakpoints in the association between upper arm muscle area(Reference Frisancho15) and hSDS (results not shown in detail). In the case of skinfold thickness, we similarly failed to identify stunted individuals. We detect statistically significant breakpoints in the schoolchildren of urban Ubud, Kupang and high SES as well low SES Maya-children of Guatemala. Yet, none of these breakpoints appeared plausible from a nutrition and public health perspective. It was found that Ubud, Kupang and low SES Maya-children increased in height with increasing skinfold thickness, but this trend occurred well above the threshold of thinness. The trend likely reflects the general stimulation of overweight and obesity on height(Reference Shalitin and Kiess19).
In order to further evaluate the putative association between nutritional status and height, we determined the coefficients of regression (R 2) below all significant breakpoints for each row of Table 1 separately. Yet, the coefficients remained statistically insignificant or had negligible effect size (Ubud – BMI SDS: P < 0·001, R 2 = 0·064; x̄SF: P = 0·74, R 2 = 0·0003; Kupang – x̄SF: P = 0·431, R 2 = 0·0019; high SES – x̄SF: P = 0·743, R 2 = 0·0001; low SES Maya – x̄SF: P < 0·001, R 2 = 0·023; low SES Ladino – BMI SDS: P = 0·737, R 2 = 0·009). The R 2 for x̄SF with height of the high SES Guatemalan children at 0·017 showed that any putative association between indicators of nutritional status and height was negligible.
We considered that chronological age of the participants might influence the prevalence of stunting. One reviewer of this articles suggested that participants <10 years old might be more stunted than older participants. We found that the percentage of stunted children did not vary with age. The association between height SDS and age was negligible with r = 0·12 (P < 0·01) in the Indonesian children, and insignificant in the Guatemalan children.
Discussion
The breakpoint analysis failed to support our hypothesis that subgroups of very short children and youth exist with a relevant association between thinness and body height. There is no apparent relation between BMI, MUAC and skinfold thickness as indicators of the nutritional status, and body height in the Indonesia and Guatemala samples, both with high prevalence of stunting.
It is not new and has never been questioned that undernutrition is terrible and impairs growth. Yet, short stature on its own is neither a synonym of undernutrition(Reference Scheffler, Hermanussen and Bogin11) nor an appropriate indicator of undernutrition. Already in 1916, the German pediatrician Meinhard von Pfaundler(Reference Pfaundler20) summarised body mass studies in children before World War I. Even though he considered food as a potentially influencing factor, he stated: ‘that the undernourishment of the children of the poor, with the exception of the fact that it certainly does not occur in the assumed extent, is probably over-estimated in its importance for the growth of body length’. Shortly after World War I, Schlesinger(Reference Schlesinger21) wrote: ‘The child’s longitudinal growth is largely independent of the extent and nature of the diet… Even during severe dietary restrictions, impairments of infant growth are markedly small, and occur slowly and delayed. Only during severe infectious nutritional disorders of the infant… Stolte and others(22) observed a temporary growth inhibition… Malnourished infants show an inhibition of longitudinal growth only, and especially during periods of reparation, when food supplies, e.g. breast milk, was low in protein and minerals, but they quickly recovered when given protein rich milk’. Screening for undernutrition has always been an important issue. After World War I, the American Quaker Children’s Aid Mission offered additional meals to undernourished German schoolchildren and requested from the German paediatricians to better define undernutrition and to develop criteria for classifying the degree of undernutrition of a given child.
This was not a trivial task. Simply taking height and weight was considered inappropriate, and instead the paediatricians discussed indices. Pfaundler(Reference Pfaundler20) originally introduced Livi’s (ponderal) index. Livi’s index – 100 (√3 weight)/body height) – relates the cube root of weight to height and was considered to better mirror the nutritional state than body weight alone, with arguments similar to those used today to recommend the BMI. Later, Pfaundler suggested an index that had recently been introduced by Pirquet. The index ignored leg length and thus appeared to better mirror the nutritional status. This ‘Pelidisi’ index – Pondus dEcies LInear DIviso SedentIs altitudo = 100 (√3 weight)/sitting height) – was then thoroughly discussed and appreciated by Wagner in his work on the numerical assessment of the nutritional status in 1921(Reference Wagner23). He discussed practical aspects of the necessity to determine the nutritional status of children for detecting the poor, and those who are in need of ‘warm food’. Wagner discussed possibilities to better define a state of undernutrition. He wrote that ‘…the precondition for the usability of a body fullness index is that it represents an unnamed ratio derived from the division of equidimensional quantities’. He rejected indices such as weight-for-height because they divide a three-dimensional size, the weight, by a one-dimensional length, which results in an area. Yet, in spite of these thoughts, the pelidisi was never widely accepted and eventually disappeared from the literature presumably due to arithmetic clumsiness at a time when computers did not exist. In 2015, Burton(Reference Burton24) presented similar thoughts recommending that BMI be replaced by an index of body build that is less dependent on relative leg length and age in children and adults than are the BMI and the Rohrer Index and proposed Weight/Sitting Height3.
Half a century after Pirquet, Pfaundler and Wagner, and apparently unswayed by their considerations, Jelliffe(Reference Jelliffe25) introduced mid-upper arm circumference (in those days called arm girth) as a new practical, useful and sensitive index for assessing protein–energy malnutrition (PEM) in early childhood. The rationale was that the arm girth depicts a heavily muscled area with good quantity of fat. In PEM, all musculature is presumed to be uniformly affected(Reference Jelliffe and Jelliffe26). McKay(Reference McKay27) reported positive correlation between arm girth and weight in Malaysian children in 1969. In contrast to this historic observation, the stunted children of modern Indonesia and Guatemala appear well-nourished, some of them significantly obese, and thus lack convincing evidence of undernutrition. We failed to detect meaningful associations between weight, BMI, skinfold thickness and mid-upper arm circumference with body height in these children. Even using the more elaborate statistical tool of breakpoint analyses, we failed to detect subpopulations among these children for whom the common perception of an existing association between thinness and short stature might hold true.
Meanwhile, the conventional wisdom of nutritional epidemiology seems to change gradually. A slowly increasing number of articles in the current literature start to question if stunting is the single indicator to reliably detect poor child nutrition. In 2014, Becker et al.(Reference Becker, Nieman Carney and Corkins28) and later Bouma(Reference Bouma29) stated that cut-offs at −2 height SDS and −1 height SDS for diagnosing moderate and mild undernutrition are not useful and should be replaced either by combinations of indicators, such as loss in lean body mass, infections, delayed wound healing and others.
Undernutrition needs intervention. But, how to define undernutrition by anthropometry? At the beginning of the 20th century, it was obvious to the paediatric community that neither height nor weight contained that specific information. The indices of Quetelet, Livi and Pelidisi were soon dismissed. Re-inventing stunting as a measure of nutrition deficit in the later part of the 20th century was neither new nor original and is showing signs of its shortcomings. It appears that better indicators for undernutrition than some trivial SDS of < −2·0 sd in height are needed. Systematic reviews show that existing anthropometric cut-offs for detecting nutrition deficits and for planning nutrition interventions have little to no public health impact(Reference Hermanussen, Bilogub and Lindl30–Reference Goudet, Griffiths and Bogin32).
It is well-known that emotional deprivation and psychological stress can cause growth failure and short stature. These non-nutritional causes of stunting are known as ‘hospitalism’(Reference Spitz33) and psychosocial short stature(Reference Powell, Brasel and Blizzard34,Reference Powell, Brasel and Raiti35) and are associated with impaired growth hormone secretion. Growth failure due to emotional deprivation may be followed by catch-up after appropriate intervention and treatment as described more than half a century ago, by Widdowson, Talbot et al. and Bakwin(Reference Widdowson36–Reference Bakwin38).
Building on these fundamental clinical observation, we have proposed that much of the current short stature/stunting observed in infants and children from impoverished families of low- and middle-income countries has causes due more to emotional/psychological stress and insecurity rather than undernutrition per se (Reference Bogin, Hermanussen and Scheffler39–Reference Hermanussen, Bogin and Scheffler41).
A relatively large study of growth failure related to emotional factors was published more than a century ago. Igl(42) published rich data on catch-up growth in height of 4896 school boys and 4612 school girls from a boarding school from Brno, school year 1903/1904. He compared height increments during 10 months of school attendance with increments during interposed 2 months of summer holiday (Table 2). He documented remarkable catch-up in height with up to 3·5 cm during holiday, corresponding to a height velocity of up to 21 cm/year. These growth rates by far outranged average growth of schoolchildren at that time and surpassed the growth rate during the school term by almost a factor of six. As urban Austrian school children before World War I have neither been reported chronically undernourished, nor chronically ill, we interpret the annual school-related period of growth depression followed by catch-up as an expression of significant familial emotional deprivation temporarily interrupted by emotional support during summer holidays.
Table 2 Height increments of children of a boarding school, Brno, school year 1903/1904, during 10 months of school attendance and during interposed 2 months of summer holiday at the end of each school year*
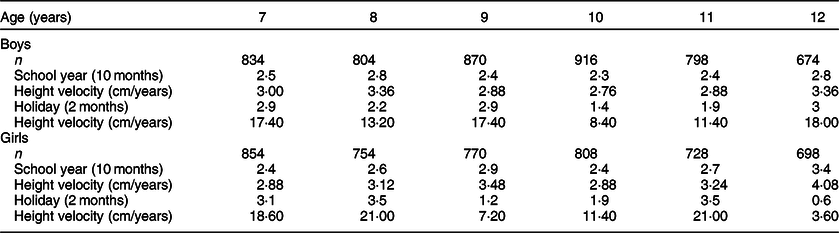
* Catch-up in height with up to 3·5 cm during holiday, corresponding to a height velocity of up to 21 cm/year, by far outranged growth velocity during the 10 months of school attendance.
A way forward in nutritional epidemiology
We suggest changing the focus of nutritional epidemiology. Anthropometric variables and ratios of these variables are stationary descriptions of a particular condition. Such variables provide a momentary glance. We instead suggest taking a more dynamic view, focusing on responsiveness. Catch-up growth is well known and occurs when successfully recovering from food shortage, illness or other forms of developmental impairment. Children catch-up both in weight and in height. Catch-up growth is cause-specific. It follows periods of growth inhibition, and it is characterised by weight and height velocity above the limits of normal for age(Reference Wit and Boersma43).
Studies published shortly after World War I showed catch-up growth of 3–5 cm in height within 6–8 weeks on re-feeding, in starved German children after being transferred to care families or communal lodges in Switzerland(Reference Hermanussen, Bilogub and Lindl30,Reference Hermanussen, Bogin and Scheffler31) . Catch-up growth after re-feeding and after recovering from major illness has later extensively been presented in the clinical paper of Prader et al.(Reference Prader, Tanner and von Harnack44). Catch-up growth occurs in migrant children when moved into more prosperous conditions(Reference Bogin, Camacho de Paz and MacVean14). In 1994, Golden(Reference Golden45) reviewed the possibilities of catch-up growth in stunted malnourished children and concluded that ‘it seems that most malnourished children retain their capacity for full catch-up’.
Catch-up growth is an immediate and sensitive indicator of a variety of both biological and psychosocial factors influencing child growth. A catch-up growth spurt may follow improvements of previous growth impairment within a few days and manifests itself by either one single large or serial mini growth spurts(Reference Golden45). Catch-up growth characterises the dynamic physical response to preceding developmental malfunction. Using this approach for diagnosing developmental impairment fundamentally differs from any stationary measure of anthropometric variables and ratios.
Many modern nutrition interventions in the stunted child populations of low- and middle-income countries lack relevant catch-up in height and thus strongly suggest that it is not nutrition that keeps these children short(Reference Goudet, Griffiths and Bogin32,Reference Bogin46) . We consider education and social-economic-political-emotional factors being most significantly involved in the high prevalence of child stunting in these countries. More and better-quality education and a higher quality of a broad range of social-economic-political-emotional factors have long been associated with better maternal health, higher birth weight, healthier physical growth, more successful schooling and greater earnings in adult employment(Reference Bogin, Hermanussen and Scheffler39–Reference Hermanussen, Bogin and Scheffler41,Reference Bogin46) . In view of the ease to perform repetitive daily or short-term (e.g. 4–6 weeks) measurements of height and weight in children(Reference Hermanussen and Hermanussen47), we suggest to abstain from single measures of height, weight and related ratios and instead utilise the dynamic physical response of length/height catch-up to preceding developmental malfunction as a more appropriate indicator of successful growth promotion in the individual short child.
In summary, the catch-up growth spurt following a nutrition intervention is a better indicator of undernutrition than static thresholds for stunting. Absence of catch-up growth following re-feeding strongly indicates non-nutritional causes of stunting.
Acknowledgements
Acknowledgements: The authors gratefully thank Melanie Dammhahn from University of Potsdam – Animal Ecology Group for her intensive discussion and suggestions for statistical analysis. Financial support: No. Conflict of interest: No. Authorship: C.S. and M.H. designed the study. C.S., M.H. and B.B. directed implementation and data collection. C.S. and B.B. analysed the data. C.S. and M.H. drafted the manuscript. B.B. edited the manuscript for intellectual content and provided critical comments on the manuscript. Ethics of human subject participation: This study was conducted according to the guidelines laid down in the Declaration of Helsinki. Ethical approval was provided by the Medical and Health Research Ethics Committee, Faculty of Medicine, Gadjah Mada University, Yogyakarta, Ref. no. KE/FK/0175/EC/2018. Written informed consent was obtained from all parents of children. The Guatemala Longitudinal Study was approved by the Guatemalan Ministry of Education, and the secondary analysis of these anonymised data has been approved by Universidad del Valle de Guatemala and Loughborough University Human Participants Sub-Committee (Ref No: G13-P2).









