Gestational diabetes mellitus (GDM) is defined as any degree of hyperglycaemia or glucose intolerance with first recognition during pregnancy( Reference Prutsky, Domecq and Sundaresh 1 ). The worldwide prevalence of GDM varies considerably; some reports indicated that it occurs in up to 14 % of all pregnancies( Reference Karcaaltincaba, Kandemir and Yalvac 2 ). GDM and maternal obesity are independently associated with several adverse complications( Reference Owens, O’Sullivan and Kirwan 3 ). The Hyperglycemia and Adverse Pregnancy Outcome (HAPO) study demonstrated a positive linear relationship between fasting and post-load glucose concentrations and adverse perinatal outcomes including fetal size, adiposity and hyperinsulinism( 4 ). In addition, GDM is associated with increased risk of adverse maternal and fetal outcomes( Reference Coustan 5 ).
Medical nutrition therapy, including use of a low-glycaemic-index diet and the Dietary Approaches to Stop Hypertension (DASH) eating plan( Reference Asemi, Samimi and Tabassi 6 ), have been suggested for prevention and management of GDM( Reference Reader 7 ). Beyond lifestyle interventions, Ca or vitamin D supplementation has also been proposed as an approach that could improve pregnancy outcomes in GDM patients( Reference Asemi, Hashemi and Karamali 8 , Reference Park, Lee and Kim 9 ). Previous studies have shown that circulating Ca and vitamin D levels are strongly associated with fasting insulin and post-challenge glucose concentrations, GDM and pregnancy outcomes( Reference Whitelaw, Scally and Tuffnell 10 , Reference Bener, Al-Hamaq and Saleh 11 ). In a systematic review, Ca supplementation (≥1 g/d) was associated with a significant reduction in the risk of pre-eclampsia, preterm birth and occurrence of the composite outcome ‘maternal death or serious morbidity’( Reference Hofmeyr, Lawrie and Atallah 12 ). Furthermore, vitamin D deficiency during pregnancy was associated with increased primary caesarean section rate( Reference Merewood, Mehta and Chen 13 ). However, some reports have shown that Ca and vitamin D supplementation did not affect pregnancy outcomes in mothers without GDM( Reference Thorne-Lyman and Fawzi 14 , Reference Trumbo and Ellwood 15 ).
Vitamin D and Ca might affect pregnancy outcomes through influencing skeletal composition and smooth muscle strength( Reference Merewood, Mehta and Chen 13 ) as well as metabolic profiles( Reference Asemi, Karamali and Esmaillzadeh 16 ). Ca and vitamin D have been suggested to act jointly rather than independently. Previous reports have shown that joint supplementation is much more efficient in influencing metabolic profiles than single Ca or vitamin D supplementation( Reference Asemi, Foroozanfard and Hashemi 17 , Reference Tabesh, Azadbakht and Faghihimani 18 ). Considering the high prevalence of vitamin D deficiency and insufficient dietary intakes of Ca among Iranian pregnant women( Reference Asemi, Taghizadeh and Sarahroodi 19 , Reference Tabrizi and Pakdel 20 ), along with the beneficial effects of joint supplementation on metabolic profiles in GDM patients, we hypothesized that combined Ca and vitamin D supplementation might influence pregnancy outcomes in GDM patients. The current study was therefore conducted to investigate the effects of Ca+vitamin D supplementation on pregnancy outcomes among women with GDM.
Methods
Participants
We conducted a randomized, double-blind, placebo-controlled study in Arak, Iran, during March–July 2014 which was registered on the Iranian registry of clinical trials website (IRCT201407115623N23). For estimating sample size, we used a randomized clinical study sample size formula where type one (α) and type two errors (β) were 0·05 and 0·20 (power=80 %), respectively. Based on a previous study( Reference Roth, Perumal and Al Mahmud 21 ) and considering newborn weight at birth as a key variable, we considered 0·4 kg as standard deviation and 0·3 kg as the difference in the mean (d). According to this, we needed twenty-six subjects in each group to have 80 % study power. We included pregnant women aged 18–40 years who had been diagnosed with GDM by a ‘one-step’, 2 h oral glucose tolerance test with 75 g glucose load at 24–28 weeks’ gestation. Exclusion criteria were multiple gestation, major fetal anomalies, current illicit drug use, continuous daily Ca and/or vitamin D intake since last menstrual period, insulin-dependent diabetes, smoking and history of kidney stones. Pregnant women without a previous diagnosis of glucose intolerance were screened. Diagnosis of GDM was done based on the criteria of the American Diabetes Association( 22 ); those whose plasma glucose concentration met one of the following criteria were considered as having GDM: fasting ≥5·1 mmol/l, 1 h post-load ≥10 mmol/l or 2 h post-load ≥8·5 mmol/l. The present study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects were approved by the ethics committee of Arak University of Medical Sciences. Written informed consent was obtained from all participants.
Study design
At study baseline and after stratification for pre-intervention BMI and weeks of gestation, GDM women were randomized to one of two treatment arms using computer-generated random numbers in a ratio of 1:1. A trained midwife at the maternity clinic, who was not blinded to the intervention, did the randomized allocation sequence, enrolled participants and assigned participants to interventions. The supplementation was started when the participants were between weeks 24 and 28 of their pregnancy. Although all participants were at this point of gestational age, participants were stratified based on their gestational age (<26 weeks, >26 weeks) before randomization. The women allocated to the Ca+vitamin D arm were advised to take calcium carbonate 1000 mg/d for 6 weeks plus a pearl containing 1250 µg (50 000 IU) of cholecalciferol (vitamin D3) twice during the study: at study baseline and day 21 of the intervention. The women allocated to the placebo arm were advised to take separate placebos for Ca (daily for 6 weeks) and for vitamin D (twice during the study: at study baseline and day 21 of the intervention), which were identical to the Ca tablets and vitamin D pearls in shape, colour and size. Ca supplement and its placebo were manufactured by Tehran Shimi Pharmaceutical Company (Tehran, Iran). Vitamin D and its placebo were manufactured by Dana Pharmaceutical Company (Tabriz, Iran) and Barij Essence Pharmaceutical Company (Kashan, Iran). Although the duration of the intervention was 6 weeks, all women were followed up until delivery. We did not continue the supplementation until delivery because we wanted to determine the effects of co-supplementation on pregnancy outcomes and were concerned that a large number of women would drop out if they were asked to provide blood near the end of their pregnancies. Participants were asked not to consume any supplements other than the one provided to them by the investigators. All participants were also consuming folic acid 400 µg/d from the beginning of pregnancy and ferrous sulfate 60 mg/d from the second trimester. Compliance to the Ca+vitamin D supplementation was assessed through quantification of serum Ca and vitamin D levels. The use of Ca+vitamin D supplements and placebos throughout the study was also checked by asking participants to bring the medication containers. Dietary information was assessed by a trained dietitian using a 3 d diet record (two weekdays and one weekend day) that was completed at week 1, 3 and 5 of the intervention. The dietary records were based on estimated values in household measurements. To obtain nutrient intakes of participants based on these 3 d food diaries, we used Nutritionist IV software (First Databank, San Bruno, CA, USA) modified for Iranian foods.
Assessment of anthropometric measures
Anthropometric measurements were conducted at baseline and after 6 weeks of intervention. Maternal height was measured using a daily calibrated stadiometer (Seca, Hamburg, Germany) to the nearest 0·1 cm and weight was measured to the nearest 0·1 kg using digital scales (Seca). Maternal BMI was calculated as weight in kilograms divided by the square of height in metres. Weight and length of all babies were measured in the labour ward following birth by a trained midwife using standard methods (Seca 155 scale). Infants’ head circumference was measured to the nearest 1 mm with a Seca girth-measuring tape. We also determined infants’ 1- and 5-min Apgar scores as another measure of pregnancy outcome. Preterm delivery was defined when delivery occurred at <37 weeks of pregnancy and newborn macrosomia was defined as birth weight >4000 g( Reference Boulet, Alexander and Salihu 23 ).
Biochemical and polyhydramnios assessment
Ten millilitres of fasting blood were obtained from each participant after a 10–12 h overnight fast at study entry and at the end of the research at Arak reference laboratory. The blood samples were centrifuged and serum was stored at −70°C until further assays. Commercial kits were used to measure fasting plasma glucose and serum Ca concentrations (Pars Azmun, Tehran, Iran). Participants underwent a 2 h oral glucose tolerance test and blood samples were collected at time 60 and 120 min to measure plasma glucose levels. Serum 25-hydroxyvitamin D concentrations were measured using a commercial ELISA kit (IDS, Boldon, UK). The inter- and intra-assay CV for serum 25-hydroxyvitamin D assays ranged from 4·5 to 7·0 %. Hyperbilirubinaemia was considered when the total serum bilirubin level was at or above 15 mg/dl (257 mol/l) in infants 25 to 48 h old, 18 mg/dl (308 mol/l) in infants 49 to 72 h old, and 20 mg/dl (342 mol/l) in infants older than 72 h( Reference Porter and Dennis 24 ). Polyhydramnios was diagnosed with a sonographic method after second vitamin D or placebo administration. On the basis of this measurement, polyhydramnios was defined as an amniotic fluid index in excess of 25 cm( Reference Nobile de, Radaelli and Taricco 25 ).
Statistical analysis
To ensure the normal distribution of variables, histograms and the Kolmogorov–Smirnov test were applied. We used independent-samples Student’s t test to detect differences in baseline measures as well as in dietary intakes between the two groups. Pearson’s χ 2 test was used for comparison of categorical variables. To assess if the magnitude of change in dependent variables depended on the baseline maternal age, BMI and fasting plasma glucose, we controlled all analyses for baseline values of age, BMI and fasting plasma glucose to avoid potential bias. These analyses were done using two-factor repeated-measures ANOVA. P<0·05 was considered as statistically significant. All statistical analyses were done using the statistical software package SPSS 17.
Results
Nine hundred pregnant women attending maternity clinics affiliated to Arak University of Medical Sciences, Arak, Iran were screened for GDM and sixty patients were included in the clinical trial. Sixty GDM women in two groups were randomized to receive Ca+vitamin D or placebo. Finally, sixty participants (Ca+vitamin D (n 30) and placebo (n 30)) completed the trial (Fig. 1). On average, the compliance rate in the present study was high, such that 100 % of pearls and tablets were taken during the course of the study in both groups.
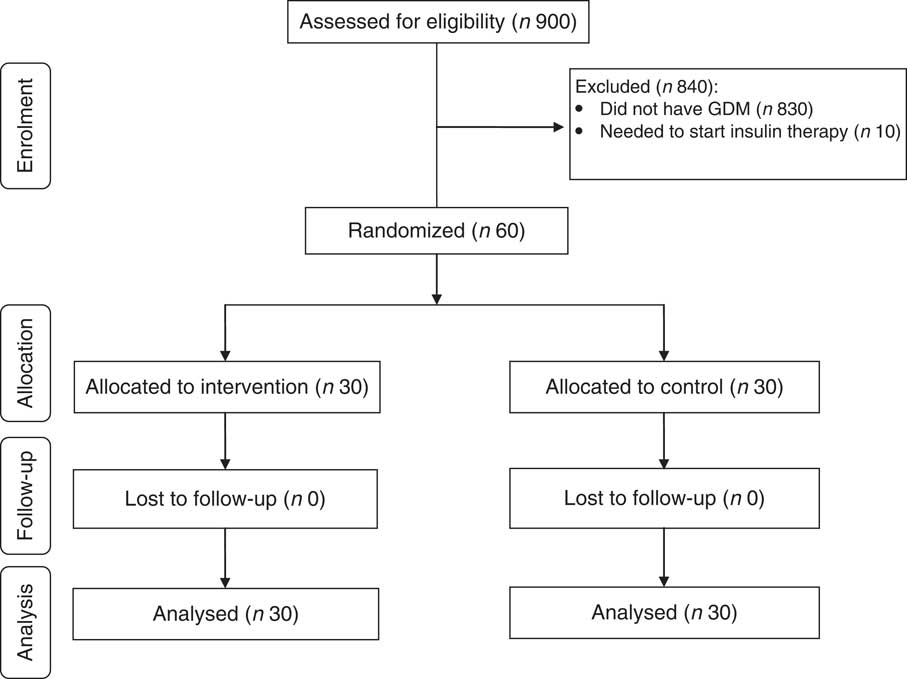
Fig. 1 Summary of patient flow in the present randomized, double-blind, placebo-controlled trial (GDM, gestational diabetes mellitus)
Mean age, pre-pregnancy weight and BMI was not statistically different between the two groups (Table 1). Baseline weight and BMI as well as post-intervention means of these variables were not significantly different between Ca+vitamin D and placebo groups. Participants who received Ca+vitamin D supplements had a significant rise in serum vitamin D level (19·0 v. 0·5 ng/ml, P<0·001) and a trend towards a significant increase in serum Ca level (0·6 v. −0·1 mg/dl, P=0·09) compared with the placebo group.
Table 1 General characteristics, serum calcium and vitamin D levels of the study participants: women with gestational diabetes mellitus and their newborns, Arak, Iran, March–July 2014
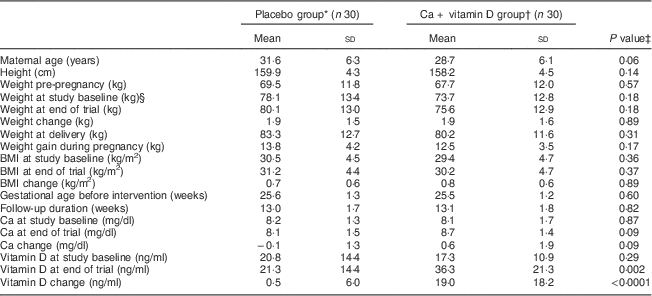
* Received placebo for Ca (daily for 6 weeks) and placebo for vitamin D twice during the study: at study baseline and day 21 of the intervention.
† Received calcium carbonate 1000 mg/d for 6 weeks plus a pearl containing 1250 µg (50 000 IU) of cholecalciferol (vitamin D3) twice during the study: at study baseline and day 21 of the intervention.
‡ Obtained from an independent-samples Student’s t test.
Based on the 3 d dietary records obtained throughout the intervention, no statistically significant difference was seen between the two groups in terms of dietary intakes of energy, fat, protein, carbohydrate, SFA, PUFA, MUFA, cholesterol, total dietary fibre, Ca and vitamin D (Table 2).
Table 2 Dietary intakes of study participants throughout the study: women with gestational diabetes mellitus, Arak, Iran, March–July 2014
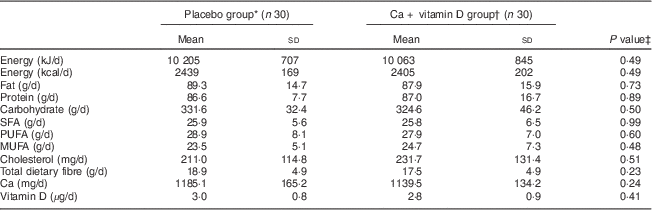
* Received placebo for Ca (daily for 6 weeks) and placebo for vitamin D twice during the study: at study baseline and day 21 of the intervention.
† Received calcium carbonate 1000 mg/d for 6 weeks plus a pearl containing 1250 µg (50 000 IU) of cholecalciferol (vitamin D3) twice during the study: at study baseline and day 21 of the intervention.
‡ Obtained from an independent-samples Student’s t test.
Women treated with Ca+vitamin D had a significant decrease in caesarean section rate (23·3 % v. 63·3 %, P=0·002) and maternal hospitalization (0 v. 13·3 %, P=0·03) compared with the placebo group (Table 3). In addition, newborns of GDM women randomized to Ca+vitamin D had no case of macrosomia, while the prevalence of macrosomia among those randomized to placebo was 13·3 % (P=0·03). Lower rates of hyperbilirubinaemia (20·0 % v. 56·7 %, P=0·03) and hospitalization (20·0 % v. 56·7 %, P=0·03) were also seen in the supplemented group of newborns than in the placebo group. We did not find a significant difference in pre-eclampsia, maternal polyhydramnios, needing to progress to insulin therapy after the intervention, newborns’ birth size and Apgar scores when comparing the two groups. Adjustment for baseline maternal age, BMI and fasting plasma glucose did not alter the findings.
Table 3 Effect of calcium plus vitamin D supplementation on pregnancy outcomes: women with gestational diabetes mellitus and their newborns, Arak, Iran, March–July 2014
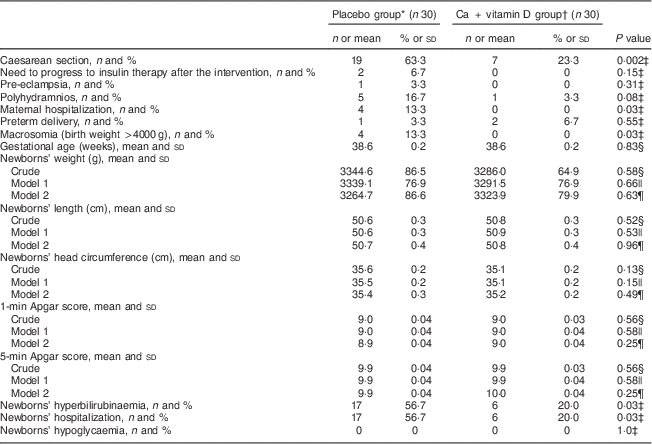
* Received placebo for Ca (daily for 6 weeks) and placebo for vitamin D twice during the study: at study baseline and day 21 of the intervention.
† Received calcium carbonate 1000 mg/d for 6 weeks plus a pearl containing 1250 µg (50 000 IU) of cholecalciferol (vitamin D3) twice during the study: at study baseline and day 21 of the intervention.
‡ Obtained from Pearson’s χ 2 test.
§ Obtained from an independent-samples Student’s t test.
|| Obtained from two-way repeated-measures ANOVA adjusted for baseline maternal BMI.
¶ Obtained from two-way repeated-measures ANOVA adjusted for baseline maternal age, BMI and fasting plasma glucose.
Discussion
Our study demonstrated that Ca+vitamin D supplementation for 6 weeks among pregnant women with GDM at 24–28 weeks of gestation led to decreased caesarean section and maternal hospitalization rates, and decreased macrosomia, hyperbilirubinaemia and hospitalization in newborns compared with placebo; however, we did not find any significant effect on other indicators of pregnancy outcome. To our knowledge, the present study is the first one reporting the effect of Ca and vitamin D supplementation on pregnancy outcomes of women with GDM.
Women with GDM are a high-risk group for several complications including development of type 2 diabetes mellitus, metabolic syndrome and CVD( Reference Noctor, Crowe and Carmody 26 ).
The findings of the present study showed that Ca+vitamin D supplementation among pregnant women with GDM resulted in decreased caesarean section rate and maternal hospitalization, but did not influence other pregnancy complications including pre-eclampsia, maternal polyhydramnios and needing to progress to insulin therapy after the intervention. In line with our study, vitamin D supplementation did not affect preterm delivery( Reference Thorne-Lyman and Fawzi 14 ) and pre-eclampsia among healthy nulliparous women( Reference Haugen, Brantsaeter and Trogstad 27 ). Furthermore, in another study, vitamin D deficiency during pregnancy was associated with almost four times greater odds of primary caesarean section( Reference Merewood, Mehta and Chen 13 ). Higher serum Ca levels were also reported in pregnant women at the time of vaginal delivery compared with term women not in labour or women who did not go into labour but delivered by scheduled caesarean( Reference Papandreou, Chasiotis and Seferiadis 28 ). Nevertheless, data on the effect of combined Ca and vitamin D supplementation on maternal, perinatal or infant health outcomes in GDM patients are rare. In a study by Zhou et al.( Reference Zhou, Su and Liu 29 ), no significant differences were found in terms of polyhydramnios, oligohydramnios, pre-eclampsia and caesarean section among pregnant women with different levels of vitamin D at 16–20-weeks’ gestation. Reduced intracellular Ca signals and expression of Ca entry channels in uteruses from diabetic patients may result in a reduction in muscle content( Reference Al-Qahtani, Heath and Quenby 30 ), which in turn could reduce force and increase the caesarean section rate. In addition, macrosomia has also been reported as a risk factor for caesarean section in women with diabetes( Reference Ehrenberg, Durnwald and Catalano 31 ). Furthermore, pure vitamin D deficiency, depending upon its chronicity, may induce hyperparathyroidism and consequent leaching of total bone Ca in order to maintain normocalcaemia, which might explain the initiation of early labour( Reference Merewood, Mehta and Chen 13 ).
The current study showed that taking Ca+vitamin D supplementation in GDM women led to decreased hyperbilirubinaemia and hospitalization in newborns. In agreement with our study, oral Ca supplementation resulted in a significant decrease in serum bilirubin in patients with Crigler–Najjar type I( Reference Van der Veere, Jansen and Sinaasappel 32 ). Furthermore, Miroliaee et al.( Reference Miroliaee, Nasiri-Toosi and Khalilzadeh 33 ) found that lower serum levels of vitamin D were associated with hyperbilirubinaemia in patients with non-cholestatic chronic liver disease. Fisher and Fisher( Reference Fisher and Fisher 34 ) have also shown that serum vitamin D levels of less than 25 nmol/l could be a reliable predictor of higher serum bilirubin. In addition, improved liver enzyme levels were seen in the cord blood of infants whose mothers received one 1500 µg dose of cholecalciferol in the second trimester or two 3000 µg doses of cholecalciferol each in the second and third trimesters( Reference Kalra, Das and Agarwal 35 ). However, some investigators observed no evidence of vitamin D insufficiency in cirrhosis( Reference Floreani, Zappala and Fries 36 ), non-cirrhotic viral liver disease( Reference Duarte, Farias and Coelho 37 ) and haemochromatosis( Reference Guggenbuhl, Deugnier and Boisdet 38 ). Several mechanisms can explain the effects of Ca and vitamin D supplementation on reducing newborns’ hyperbilirubinaemia. Ca may act as a trapping agent for bilirubin in the intestine, thereby preventing back-diffusion across the intestinal wall( Reference Van Der Veere, Schoemaker and Bakker 39 ). Furthermore, the strong relationship between both the prevalence and degree of vitamin D insufficiency and the severity of chronic liver disease may indicate specific impairments of vitamin D metabolism in the liver. Impaired 25-hydroxylation of vitamin D in liver related to the degree of hepatic dysfunction has been reported in patients with alcoholic cirrhosis( Reference Hepner, Roginsky and Moo 40 ). In rats, bile duct ligation led to a 64 % decrease in hepatic 25-hydroxylation of vitamin D( Reference Bolt, Sitrin and Favus 41 ). In addition, the active form of vitamin D induces vitamin D receptors which in turn act as receptors for secondary bile acids, such as lithocholic acid and 3-ketocholanic acid, and result in their catabolism via induction of cytochrome 3A enzymes( Reference Makishima, Lu and Xie 42 , Reference Xie, Radominska-Pandya and Shi 43 ).
We did not find any significant effect of combined Ca+vitamin D supplementation on birth size. Our results are in agreement with previous studies showing that taking 1500 mg Ca from week 20 of gestation among women with low Ca intake did not affect fetal and infant growth during the first year of life( Reference Abdel-Aleem, Merialdi and Elsnosy 44 ). In addition, others did not observe any significant effect of vitamin D supplementation during pregnancy on birth size( Reference Roth, Perumal and Al Mahmud 21 , Reference Hossain, Kanani and Ramzan 45 ). In contrast, some studies have indicated that either one oral dose of 1500 μg cholecalciferol or two doses of 3000 μg cholecalciferol in the second and third trimesters resulted in increased birth weight, length and head circumference( Reference Kalra, Das and Agarwal 35 ). Some studies that have been performed to determine the effect of maternal Ca status on birth size have generally reported larger birth weights with supplementation( Reference Villar and Repke 46 , Reference Lopez-Jaramillo, Delgado and Jacome 47 ). Discrepancies between our study and others might be explained by the different doses of Ca and vitamin D, different stages of gestation as well as the different durations of supplementation.
Limitations of the study
Our study has some limitations. First is the duration of this trial. We were unable to continue the Ca+vitamin D supplementation up to delivery time. Second, we did not assess the effects of Ca+vitamin D supplementation on other pregnancy outcomes including neonatal respiratory distress syndrome and Ca and vitamin D concentrations in amniotic fluid. Furthermore, in the current study, we did not assess cord blood levels of Ca and vitamin D. Further studies are suggested to determine the effect of Ca+vitamin D supplementation on cord blood levels of Ca and vitamin D. In the current study, we did not quantify serum vitamin D levels at the study baseline to see the percentage of women who were vitamin D deficient. Serum vitamin D levels were measured at the end of the trial to assess compliance to vitamin D supplements. When we found that some patients were vitamin D deficient (at the end of the study), we suggested they take vitamin D supplements. We calculated the sample size based on the primary outcome variable (newborn weight). Therefore, the study had enough power to detect differences for this parameter. However, we did not consider secondary outcome variables such as infant growth parameters or other pregnancy outcomes in the sample size calculation. As the study is not powered to detect differences in these outcome measures, one cannot make any conclusions about them. Therefore, further large-scale studies are needed to examine the effect of Ca+vitamin D supplementation on these pregnancy outcomes. Participants’ dietary intakes were examined through the use of 3 d dietary records to find differences in dietary intakes between the two groups. We did not observe any significant difference in dietary intakes between the two groups. However, it must be kept in mind that all dietary assessment methods, including dietary records, are subject to bias particularly when the dietary information is collected from household information. This limitation should also be taken into account in interpretation of our findings.
Conclusion
In conclusion, Ca+vitamin D supplementation for 6 weeks among pregnant women with GDM led to decreased caesarean section rate and maternal hospitalization, and decreased macrosomia, hyperbilirubinaemia and hospitalization in newborns compared with placebo; however, we did not find any significant effect on pre-eclampsia, maternal polyhydramnios, needing to progress to insulin therapy after the intervention, newborns’ birth size and Apgar scores.
Acknowledgements
Acknowledgements: The authors would like to thank the staff of Kossar Clinic (Arak, Iran) for their assistance in this project. Financial support: The present study was supported by the Vice-Chancellor for Research, Arak University of Medical Sciences, Arak, Iran (grant number 92–158). The funder had no role in the design, analysis or writing of this article. Conflict of interest: None. Authorship: M.K. and M.A-D. contributed in data collection and manuscript drafting. Z.A. and A.E. contributed in conception, design, statistical analysis and drafting of the manuscript. All authors read and approved the final version of the paper. Z.A. is the guarantor of this work; as such, he had full access to all data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. This study is registered at the Iranian registry of clinical trials website (registration number IRCT201407115623N23; www.irct.ir). Ethics of human subject participation: The present study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects were approved by the ethics committee of Arak University of Medical Sciences. Written informed consent was obtained from all participants.







