Body weight and related health issues among Canadian children and adolescents are of growing concern for health professionals. In the past, hypertension was believed to be a concern directed to the adult population, yet researchers around the world are beginning to study the associations between BMI and blood pressure (BP) in children and adolescents to predict future cardiovascular risks. Although the most recent national data suggest that only 2·2 % of Canadian children/adolescents (aged 12–19 years) have high BP( Reference Paradis, Tremblay and Janssen 1 ), other studies have reported high BP among 3–13 % of Canadian youth( Reference Maximova, O'Loughlin and Paradis 2 – Reference Salvadori, Sontrop and Garg 5 ). However, all studies have used different methods to classify high BP in youth, since no recognized standards exist and/or have been agreed upon.
Salvadori et al. ( Reference Salvadori, Sontrop and Garg 5 ) reported being overweight or obese to be strongly associated with high BP in Canadian children after adjusting for a family history of hypertension and kidney disease. Further, Gopinath et al.( Reference Gopinath, Baur and Garnett 6 ) recently reported high BP among 21 % of overweight/obese pre-school children (3–6 years old, n 1294). High BP levels are thought to track from childhood to adulthood( Reference Chen and Wang 7 – Reference Lauer, Burns and Clarke 9 ), which may increase cardiovascular health risk in the future. Furthermore, high BP tracking seems to be even stronger among overweight and obese youth( Reference Burke, Beilin and Dunbar 10 – Reference Lauer, Mahoney and Clarke 12 ).
Several recent studies have investigated the impact of various lifestyle behaviours on BP( Reference Ekelund, Luan and Sherar 13 – Reference Sugiyama, Xie and Graham-Maar 15 ), albeit mainly physical activity behaviours. For example, using the International Children's Accelerometry Database (i.e. aged 4–18 years), Ekelund et al. ( Reference Ekelund, Luan and Sherar 13 ) reported that greater amounts of time spent in moderate-to-vigorous physical activity was associated with better cardiometabolic risk factors (including BP) regardless of the amount of sedentary time. Furthermore, Stabelini Neto et al. ( Reference Stabelini Neto, Sasaki and Mascarenhas 16 ) observed the metabolic syndrome (high waist circumference, high BP, low HDL-cholesterol, high TAG and high fasting plasma glucose) among inactive children/adolescents (14 years old, n 456). However, very little evidence exists on the associations between BP and nutrition among children/adolescents.
According to the Canadian Community Health Survey 2·2( Reference Garriguet 17 ), the median Na intake for 9–13-year-olds was 3515 mg/d and 2959 mg/d for males and females, respectively, and approximately 97 % (males) and 82 % (females) had Na intakes above the Tolerable Upper Intake Level (UL is <2200 mg/d). Other Canadian studies have also reported high Na intake levels( Reference Tanase, Koski and Laffey 18 , Reference Veugelers, Fitzgerald and Johnston 19 ), suggesting important possible health implications. Among adults, reducing Na intake is a common recommendation for hypertension, yet it is unclear whether Na is associated with BP in children/adolescents.
Furthermore, the prevalence of high body weight status is concerning among Canadian children and adolescents. Researchers and clinicians are striving to understand this potentially deleterious health concern. Very little work has investigated the potential negative associations of high Na consumption in children/adolescents. Therefore, the purpose of the present study was to examine the associations among body weight status, BP and daily Na intake among grade 7 students from south-western Ontario, Canada.
Participants and methods
All methods and procedures were approved by the University of Windsor Research Ethics Board, the Windsor Essex County Health Unit Research Ethics Board and each school board. Schools were chosen from two school boards (i.e. ninety-eight possible schools) and were selected to represent a cross-section of neighbourhoods based on a comparison of socio-economic and demographic variables (e.g. forward sortation code from the school's postal code) from the 2006 Census Tract Profile. The desired sample size was thirty schools but due to timing, twenty-six schools participated (at the school level, there were thirteen refusals to participate mainly due to timing of the study during the school year). All students in grade 7 were targeted, yet due to split classrooms, some grade 6 and grade 8 students were invited to participate. Out of a potential 1208 students from twenty-six schools, 1068 students participated (i.e. students in class on the day of the survey with parental consent, representing an 88 % student response rate). Data were collected from October 2010 to April 2011.
Procedure
The web-based Food Behaviour Questionnaire( Reference Hanning, Royall and Toews 20 ), including a 24 h diet recall, was used to assess nutrient intake. Participants completed the questionnaire independently in the school's computer lab over 30–40 min of class time. All surveys were completed on Tuesday–Friday, to ensure weekday recalls were obtained. A trained research assistant was present to respond to questions. The 24 h recall collected data separately for breakfast, lunch, dinner and other times. Nutrient analysis was completed using ESHA Food Processor software and the 2007 Canadian Nutrient File (CNF) database. The CNF, as published by Health Canada, is the standard reference food composition database reporting the amount of nutrients in foods commonly consumed in Canada and is used by Statistics Canada, the Canadian Food Inspection Agency as well as many hospitals, universities and some food manufacturers. It was important to use a Canadian-specific food database, as Na levels in foods are known to differ across countries( Reference Dunford, Webster and Woodward 21 ). Some participants (n 44, ∼4 %) were excluded from further analyses because of implausible energy intake data (e.g. <837 kJ/d (<200 kcal/d) or >25 104 kJ/d (>6000 kcal/d)) and/or food group intakes (e.g. determined to be false on visual inspection of any record with more than three times the upper servings recommendation)( Reference Hanning, Woodruff and Lambraki 22 – Reference Woodruff, Hanning and McGoldrick 25 ). Further, a measure of under-reporting was calculated using the ratio EI:BMRest of self-reported energy intake to BMR as estimated using the age- and sex-specific formulae outlined by the WHO( 26 ). Lower values of EI:BMRest (v. higher) represent more under-reporting (a reporting status cut-off of EI:BMRest = 1·74 has been used in previous works( Reference Vance, Woodruff and McCargar 23 , Reference Black 27 ) when defining as a categorical variable).
Na intake (mg/d) was based on the Na content of foods consumed and does not consider the potential addition of table salt. Na was used as both a continuous variable (e.g. mg/d within food record) and a categorical variable (less than and greater than the UL of 2200 mg/d and categorized by quartiles) to denote high Na intakes. The classification by quartiles was done in order to better describe intakes (as most participants were already consuming levels above the UL, this does not allow for discrimination among high(er) and low(er) intakes).
All physical measures (height, weight and BP) were taken by nurses and/or nursing students (senior undergraduate or master's students). Height and weight, without shoes, were measured using a stadiometer. Measured height and weight values were used to calculate BMI using the formula BMI = weight (kg)/[height (m)]2, from which body weight status was classified as underweight, normal weight, overweight or obese using the newly adopted WHO guidelines( Reference de Onis, Onyango and Borghi 28 ). BP was manually taken twice, using mercury sphygmomanometers, after participants had been sitting in a chair for at least 5 min and the two measures were taken at least 5 min apart. If the two measures differed by more than 10 % a third measure was taken. The final values of systolic BP (SBP) and diastolic BP (DBP) were calculated by averaging the two closest measurements. SBP and DBP measurements were used as both a continuous variable (e.g. mmHg) and a categorical variable to denote high BP (e.g. >120 mmHg for SBP and >80 mmHg for DBP).
Statistical analyses
Exploratory one-way ANOVA and χ 2 tests were completed for the continuous and categorical descriptive data, respectively (Table 1 by gender and Table 2 by body weight status). Separate one-way ANOVA were used to determine the effects of Na intake on SBP and DBP. An ordinal regression analysis was used to determine the overall impact of SBP, DBP and daily Na intake (by quartiles) on body weight status, controlling for sex, ethnicity and under-reporting (i.e. adjusted model).
Table 1 Descriptor variables by gender: grade 7 students (n 1008), south-western Ontario, Canada, October 2010 to April 2011

SBP, systolic blood pressure; DBP, diastolic blood pressure; EI, energy intake; BMRest, estimated BMR; n/a, not applicable.
Results are expressed as mean and standard deviation unless noted differently.
*Significance of the difference between genders.
Table 2 Descriptor variables by body weight status: grade 7 students (n 1008), south-western Ontario, Canada, October 2010 to April 2011
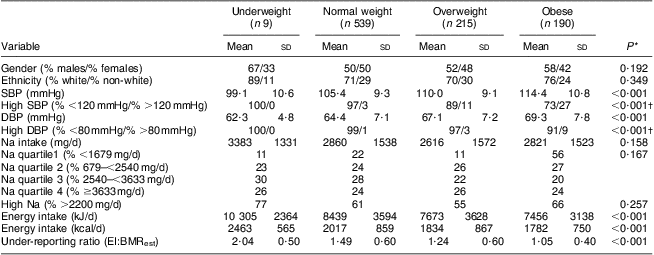
SBP, systolic blood pressure; DBP, diastolic blood pressure; EI, energy intake; BMRest, estimated BMR.
Results are expressed as mean and standard deviation unless noted differently.
*Significance of the difference among weight status groups.
†Analysis was completed without the underweight participants due to small sample size.
Results
Of the students who completed the web-based survey (n 1008), there were 522 males (52 %) and 486 females (48 %). Among them, one (0·1 %), thirty-six (4 %), 710 (70 %), 237 (24 %) and twenty-four (2 %) reported that they were 10, 11, 12, 13 and 14 years of age, respectively. While our original aim included grade 7 students (n 897, 89 %), twenty-four grade 6 students (2 %) and eighty-seven grade 8 students (9 %) completed the study. Students represented a wide variety of ethnic backgrounds including: white (72 %), black (6 %), Arab (5 %), South Asian (3 %), Chinese (2 %) and other (12 %). The major languages spoken at home were English (88 %), French (0·2 %), Arabic (3 %), Chinese (1 %) and other (8 %).
Table 1 describes height, weight, BMI, BMI status, SBP, prevalence of high SBP, DBP, prevalence of high DBP, ethnicity (white v. non-white), Na intake, prevalence of high Na intake, total energy intake and under-reporting status (EI:BMRest) by gender. Males consumed higher amounts of Na and more total energy than females (P < 0·001), yet no differences were observed between males and females when Na intake was normalized over total energy intake. Among all participants, 80 % and 60 % had Na intakes above the current Adequate Intake of 1500 mg/d and UL of 2200 mg/d, respectively.
Na intake, SBP and DBP are described by body weight status in Table 2. In bivariate analyses, both SBP (P < 0·001) and DBP (P < 0·001) were significantly different according to body weight status, yet body weight status was not associated with Na intake. Furthermore, Na intake was not associated with either SBP or DBP. High SBP was observed in 0 %, 3 %, 11 % and 27 % and high DBP was observed in 0 %, 1 %, 3 % and 9 % of underweight, normal weight, overweight and obese participants, respectively. However, in the adjusted model (Table 3), participants were more likely to be overweight or obese if they had higher SBP (v. lower: OR = 1·06, 95 % CI 1·05, 1·08, P < 0·001), higher DBP (v. lower: OR = 1·02, 95 % CI 1·00, 1·04, P = 0·043) and higher intakes of Na (3rd v. 1st quartile: OR = 1·72, 95 % CI 1·14, 2·59, P = 0·009; 4th v. 1st quartile: OR = 2·88, 95 % CI 1·76, 4·73, P < 0·001).
Table 3 Associations among body weight status categories and systolic and diastolic blood pressure, sodium intake quartiles and descriptor variables: grade 7 students (n 1008), south-western Ontario, Canada, October 2010 to April 2011
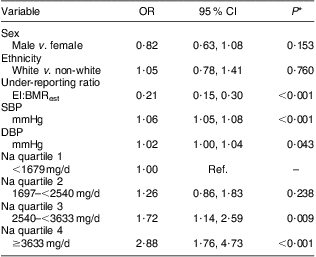
SBP, systolic blood pressure; DBP, diastolic blood pressure; EI, energy intake; BMRest, estimated BMR; Ref., referent category.
*Significance of the variable on body weight status category adjusted for all other variables in the model.
Discussion
Body weight status, BP (SBP and DBP) and Na intake of students in grade 7 from south-western Ontario were described through the Food Behaviour Questionnaire and physical measurements. Participants in the present study had higher rates of overweight and obesity compared with the national average( Reference Shields and Tremblay 29 ). Furthermore, higher SBP and DBP values were observed than what was expected, as the Canadian Health Measures Survey (CHMS) reported mean SBP and DBP of 98 mmHg and 63 mmHg, respectively, and a rate of elevated BP of 2·2 % among 12–19-year-olds( Reference Paradis, Tremblay and Janssen 1 ). Lastly, the current study observed higher (albeit more closely aligned than the CHMS data) SBP and DBP values than those in US children and adolescents from the National Health and Nutrition Examination Survey (NHANES III and NHANES IV), which reported 107·0/57·8 mmHg (males) and 103·1/58·0 mmHg (females)( Reference Going, Lohman and Cussler 30 ). Differences in the manner of measuring BP (e.g. a quiet room in the absence of staff as per the CHMS v. in a school setting) may have contributed to the differences in BP observations between studies.
Similar to others( Reference Gopinath, Baur and Garnett 6 , Reference Going, Lohman and Cussler 30 – Reference Moore, Eichner and Cohn 33 ), the current study observed higher BP measures among overweight/obese individuals. Excess weight is believed to influence BP through increased sympathetic nervous system activation( Reference Paradis, Tremblay and Janssen 1 ). While the strength of evidence is fairly strong for the association between BP and body weight status( Reference Couch and Daniels 34 ), Chiolero et al. ( Reference Chiolero, Bovet and Paradis 35 ) argue that the increase in BP measures has not paralleled the increase in body weight status over time. Further, other factors besides body weight status may influence BP such as low birth weight( Reference Lurbe, Carvajal and Torro 36 ), which was not studied in the current project. Regardless, among the overweight and obese participants, 36 % had at least one high measure of SBP or DBP and 7 % had both high SBP and high DBP, which may further exacerbate the cardiovascular health risks.
Interestingly, Na intake was not associated with BMI or BP in the bivariate analyses; however, it became positively associated in the adjusted model. The mechanistic influence of Na intake on BP is not fully understood, yet seems to be related to the inability of the kidneys to excrete large amounts of salt( Reference Meneton, Jeunemaitre and de Wardener 37 ), thus increasing overall blood volume. Significant( Reference Gillman, Oliveria and Moore 38 , Reference Jenner, English and Vandongen 39 ) and insignificant( Reference Simons, Obarzanek and Daniels 40 ) associations between Na intake and BP have been observed in the past, yet the inconsistencies are largely attributed to different methodologies. Further, several studies show mixed results for different age groups( Reference He, Marrero and Macgregor 41 , Reference Persson 42 ) or between genders( Reference Tucker, Smothers and Lewis 43 ). The results of the current study are based on a single 24 h diet recall and do not represent usual or long-term Na intake. Although high Na intakes are often reported among children/adolescents, high intra-individual daily variability could exist, thus displaying no relationships. Furthermore, there are inherent limitations in any nutrition survey. Self-reported survey data have the potential for recall error, inaccurate estimation of portion sizes, systematic bias in dietary reporting and providing socially desirable answers. The Food Behaviour Questionnaire was designed to minimize these limitations, and we have tried to account for some of the under-reporting in the analyses. Finally, it is recognized that the current findings are limited to the amounts of Na as designated by the CNF.
According to He and MacGregor( Reference He and MacGregor 44 ), 24 h urine collections are the most accurate method of assessing dietary salt intake. Studies using this methodology have also produced significant( Reference Cooper, Soltero and Liu 45 – Reference Watson, Langford and Abernethy 47 ) and insignificant( Reference Armstrong, Margetts and McCall 48 , Reference Ellison, Sosenko and Harper 49 ) associations between Na intake and BP. For example, among older children, Cooper et al. ( Reference Cooper, Soltero and Liu 45 ) reported a significant linear relationship between urinary Na and SBP in seventy-three 11–14-year-olds, even after controlling for confounding variables such as height, weight, pulse, age, sex and race. Due to the nature of the current study (and many other population/epidemiological studies), urinary Na measurements are not possible in the field, which may limit findings in large-scale studies.
There is a dearth of studies investigating the relationships among body weight status, BP and Na intake in children/adolescents, making it difficult to compare the results of the current study. Among longitudinal/intervention trials, Khang and Lynch( Reference Khang and Lynch 50 ) could not attribute the decreases in BP to nutritional intake (e.g. Na, K, total energy, protein and fat intake) even though they observed a reduction (∼8–10 mmHg for SBP) over the past 10 years among Korean children and adolescents. A recent meta-analysis reported that a reduction of salt intake by 42 % (3 g/d) causes a fall in SBP of 1·2 mmHg( Reference He and MacGregor 44 ); however, body weight status was not accounted for, thus limiting the findings to the current study. Interestingly, Rocchini et al. ( Reference Rocchini, Key and Bondie 51 ) reported lower mean arterial BP among obese boys (but not among normal weight boys) in a low-Na intervention. Several review papers( Reference Cappuccio, Capewell and Lincoln 52 – Reference Feber and Ahmed 54 ) convey that reducing salt intake from the diet will lower BP, regardless of gender, age or ethnicity. The Na intake in the present study was high (e.g. 60 % of males and 41 % of females were above the UL) without the addition of any table salt, suggesting that early education and/or intervention is necessary. Nutrition behaviours learned during childhood and adolescence are known to track into adulthood( Reference Larson, Neumark-Sztainer and Hannan 55 – Reference Story, Neumark-Sztainer and French 57 ), thereby emphasizing the importance of learning healthy eating habits early on in life.
Conclusion
High intakes of Na, coupled with high SBP and DBP, were associated with overweight and obesity status among the grade 7 sample from south-western Ontario, Canada. Health promotion strategies aimed at overweight and obese children should include BP screening and education, with a key focus on balancing physical activity with healthy nutritional daily practices.
Acknowledgements
Sources of funding: This research was supported by Southwestern Ontario in Motion, the Department of Kinesiology and the Faculty of Nursing at the University of Windsor. Conflicts of interest: The authors declare no conflicts of interest. Authors’ contributions: All authors contributed to the design, data collection and approved the manuscript.





