It is well known that Fe is one of the major trace minerals needed for good health. Inadequate or excess Fe levels can cause several health issues since it is involved in various metabolic processes including oxygen transport, muscle development, oxidative metabolism and bone homeostasis(Reference Girelli, Ugolini and Busti1,Reference Balogh, Paragh and Jeney2) . Despite its relevance, Fe deficiency is the most common micronutrient deficiency in the world, affecting a third of the world’s total population(Reference Lopez, Cacoub and Macdougall3). A recent epidemiological survey in the Middle East revealed that ~10–20 % of the population in the region has Fe deficiency(Reference Hwalla, Al-Dhaheri and Radwan4). In Saudi Arabia, similar to other countries, Fe deficiency is mostly observed among women of reproductive age and growing children, but other notable risk factors include infrequent consumption of red meat(Reference Alzaheb and Al-Amer5), maternal Fe deficiency(Reference Abu-Ouf and Jan6), presence of β-thalassaemia which is relatively common in eastern and western regions in Saudi Arabia(Reference Alaithan, Abdulazeez and Borgio7), and overall deficient intakes of essential micronutrients especially among Saudi children(Reference Nasreddine, Kassis and Ayoub8).
Vitamin D is another important micronutrient and its deficiency is considered an epidemic worldwide(Reference Van Schoor and Lips9). The main reason for its widespread deficiency is the absence of sufficient exposure to UV from the sun, a major initiator of vitamin D production in skin. As such, universal supplementation of vitamin D, at least for infants, and lifelong vitamin D supplementation for high-risk populations are recommended to prevent nutritional rickets and osteomalacia(Reference Uday and Hogler10). The vitamin D level required to reach optimum effects for health is 25-hydroxyvitamin D (25(OH)D) >75 nmol/l (30 ng/ml)(Reference Al-Daghri, Al-Saleh and Aljohani11). In the absence of adequate sun exposure, about 20–25 µg (800–1000 IU) of oral vitamin D daily is required to achieve sufficient levels in adults and children(Reference Holick and Chen12).
Recent evidence suggests an association between vitamin D deficiency and low Fe level(Reference Blanco-Rojo, Perez-Granados and Toxqui13,Reference Monlezun, Camargo and Mullen14) . Vitamin D is thought to have an important role in erythropoiesis and the regulation of hepcidin, the major hormone responsible for modulating Fe concentration(Reference Smith and Tangpricha15). However, there is limited information on the association between vitamin D and Fe in the general adolescent population(Reference Albar, Banjar and Bokhari16), especially within the Arabian region where both these micronutrient deficiencies are prevalent(Reference Hwalla, Al-Dhaheri and Radwan4). Furthermore, while vitamin D level has been observed to be significantly associated with cardiometabolic parameters, with beneficial effects on weight, blood pressure, lipid and glucose profiles in Arab adolescents(Reference Al-Daghri, Al-Saleh and Aljohani17,Reference Al-Daghri, Ansari and Sabico18) , the same associations with Fe level have not been studied. Hence, the present study aimed to determine whether Fe indices are influenced by vitamin D status and other metabolic markers among Arab adolescents.
Methods
Participants
A total of 170 apparently healthy Saudi adolescents aged 10–17 years (seventy males and 100 females) were randomly selected from the Vitamin D School Project Database of the Prince Mutaib Chair for Biomarkers of Osteoporosis in King Saud University, Riyadh, Saudi Arabia. In brief, more than 1000 apparently healthy students were recruited from the different government schools in Riyadh, Saudi Arabia to ascertain the effects of several vitamin D correction strategies including sunlight exposure, oral supplementation and vitamin D-fortified diets(Reference Blanco-Rojo, Perez-Granados and Toxqui13,Reference Monlezun, Camargo and Mullen14) . All participants, despite coming from different schools, were selected from the same geographical location and have the same environmental conditions, as described previously(Reference Masoud, Alokail and Yakout19). Participants completed a questionnaire on past medical history, general health status and demographic information. For the purpose of the present study, participants who were apparently healthy with no acute medical conditions, who were not on vitamin D, Fe or multivitamin supplements, and no had history of anaemia, liver and/or kidney diseases based on medical history, were included. Exclusion criteria included participants with known blood disorders such as thalassaemia and those with hepatic and/or renal impairments.
Anthropometrics
Anthropometric data were extracted from the database. Anthropometric measurements were done by trained school nurses. Anthropometry included mean systolic and diastolic blood pressure (mmHg), height (rounded to the nearest 0·5 cm) and weight (rounded to the nearest 0·1 kg). BMI was calculated as weight in kilograms divided by the square of height in metres (kg/m2).
Sample collection
Blood samples (5 ml) were extracted on a scheduled date. All participants were reminded to come in a fasting state (≥8 h) for blood extraction prior to beginning of classes. Blood samples were centrifuged for serum isolation. The collected sera were then transferred to pre-labelled tubes, stored in ice and delivered to the Prince Mutaib Chair for Biomarkers of Osteoporosis at King Saud University for immediate storage at −20°C until analysis.
Biochemical measurements
Serum glucose and lipid profile (TAG, total cholesterol, HDL-cholesterol, LDL-cholesterol) were extracted from the database. These parameters had previously been measured routinely (Konelab, Espoo, Finland). Serum 25(OH)D was measured in a DEQAS (Vitamin D External Quality Assessment Scheme)-certified laboratory (Prince Mutaib Chair for Biomarkers of Osteoporosis in King Saud University) using a COBAS e-411 automated analyser (Roche Diagnostics, Indianapolis, IN, USA). For serum 25(OH)D analysis, the inter- and intra-assay CV were 8·0 and 5·6 %, respectively, with a lower detection limit of 7·5 nmol/l(Reference Al-Daghri, Al-Saleh and Aljohani17,Reference Al-Daghri, Ansari and Sabico18) . Serum Fe and total iron-binding capacity (TIBC) were measured using a colorimetric ferrozine-based assay by UV spectrophotometer. The percentage of transferrin saturation was measured using the formula: TSAT (%) = [serum Fe (µg/l)/TIBC (µg/l)] × 100(20).
Statistical analysis
The G*power calculator was used to determine sample and effect size by sex (0·52) post priori. A sample size of n 170 (eighty-five per group) had 95 % level of significance at α = 0·05. The statistical software package IBM SPSS Statistics version 21·0 was used to analyse the data. Continuous data were presented as mean and standard deviation for normal variables and non-Gaussian variables were presented as median and 25th–75th percentiles. Categorical data were presented as frequencies and percentages. The Kolmogorov–Smirnov test was used to check for normality in all continuous variables. Non-Gaussian variables were log-transformed prior to parametric analysis. The independent-sample t test was used to compare mean differences. Bivariate correlations between variables were done using Pearson’s correlation coefficient (r). Multiple stepwise linear regression analysis was done to determine the significant predictors using Fe, TIBC and TSAT (%) as dependent variables and age, BMI, glucose and lipid profile as independent variables. Fe indices were not included together in the model of independent variables due to multicollinearity. P < 0·05 was considered significant.
Results
In the present study, all participants were vitamin D insufficient (25(OH)D < 50 nmol/l), while 68·8 % were in the severely vitamin D deficient range (25(OH)D < 25 nmol/l). About 37 % were obese (age- and sex-specific cut-offs for children) and the rest were normal weight (data not shown). Comparisons between boys (n 70) and girls (n 100) are presented in Table 1. Boys had significantly higher BMI, systolic and diastolic blood pressure, glucose and 25(OH)D than girls (P = 0·003, <0·01, 0·03 and <0·001, respectively). Girls, on the other hand, had significantly higher levels of total cholesterol and HDL-cholesterol as well as TIBC (P < 0·001, <0·01 and 0·05, respectively). Age, TAG, serum Fe and TSAT (%) were not significantly different between boys and girls (Table 1). Table 2 shows the significant bivariate, unadjusted associations of all parameters measured with vitamin D status and Fe indices in all participants and after stratification according to sex. In all participants, the data showed a significant association of age with serum Fe (r = 0·22; P < 0·01) and TIBC (r = 0·21; P < 0·01). Also, 25(OH)D was significantly associated with diastolic blood pressure (r = 0·18; P < 0·05) and TAG (r = 0·25; P < 0·01) but inversely with TIBC (r = −0·20; P < 0·01). As expected, serum Fe was highly associated with TIBC and TSAT (%).
Table 1 Clinical characteristics of all participants and comparison by sex (boys v. girls): Arab adolescents aged 10–17 years randomly selected from the Vitamin D School Project Database, King Saud University, Riyadh, Saudi Arabia (2014–2016)
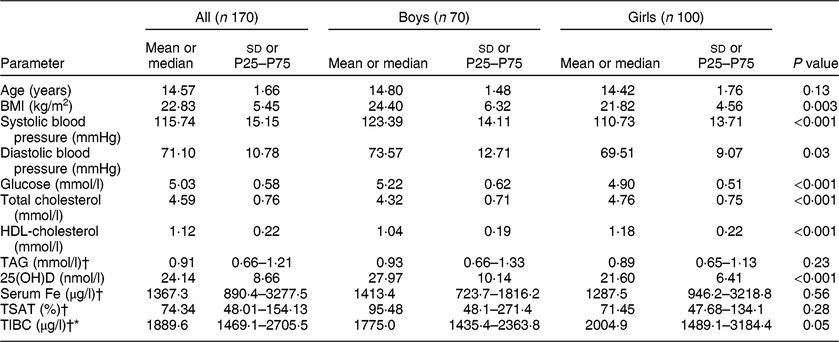
P25, 25th percentile; P75, 75th percentile; 25(OH)D, 25-hydroxyvitamin D; TSAT (%), percentage of transferrin saturation; TIBC, total iron-binding capacity.
P < 0·05 indicates statistical significance.
Data are presented as mean and SD for normal continuous variables.
† Denotes continuous variables with non-Gaussian distribution, presented as median and P25–P75.
Table 2 Significant associations between iron indices, vitamin D status and cardiometabolic parameters for all participants and by sex: Arab adolescents aged 10–17 years (n 170; 100 girls and seventy boys) randomly selected from the Vitamin D School Project Database, King Saud University, Riyadh, Saudi Arabia (2014–2016)
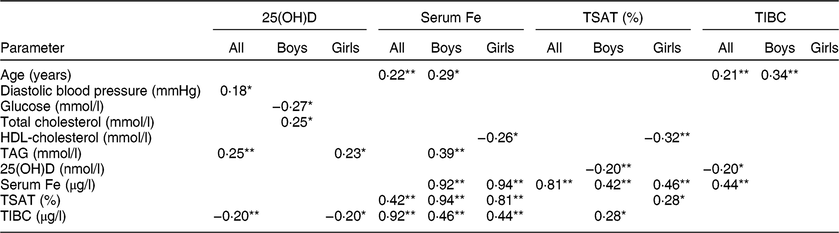
25(OH)D, 25-hydroxyvitamin D; TSAT (%), percentage of transferrin saturation; TIBC, total iron-binding capacity.
Data are presented as Pearson correlation coefficients (r). Only significant associations are presented.
* Denotes significance at 0·05 level.
** Denotes significance at 0·01 level.
In boys, vitamin D status was significantly correlated with total cholesterol (r = 0·25; P < 0·05) but inversely with blood glucose (r = −0·27; P < 0·05). Age was significantly associated with serum Fe (r = 0·29; P < 0·05) and TIBC (r = 0·34; P < 0·01). Serum Fe was also significantly associated with TAG (r = 0·39; P < 0·01). Among girls, vitamin D status was positively associated with TAG (r = 0·23; P < 0·05) but inversely with TIBC (r = −0·20; P < 0·05). HDL-cholesterol was significantly and inversely associated with both serum Fe (r = −0·26; P < 0·05) and TSAT (%) (r = −0·32; P < 0·01). Finally, vitamin D status was inversely associated with TIBC in girls (r = −0·20; P < 0·05; Table 2).
Figure 1 shows the inverse relationship between vitamin D and TIBC in all participants (R = −0·20; P < 0·001). Figure 2 shows the significant direct correlation between age and TIBC in all participants (R = 0·22; P = 0·004).
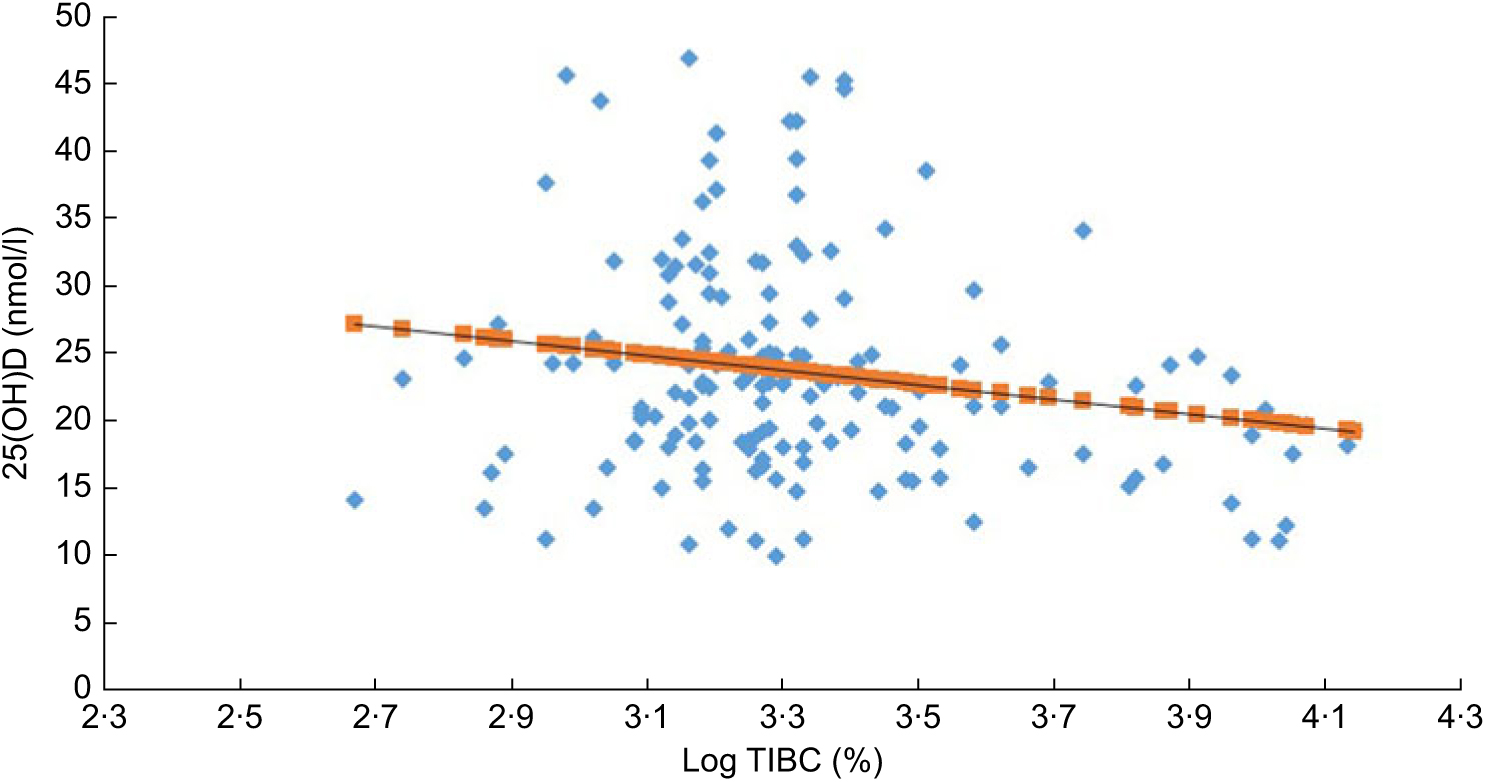
Fig. 1 (colour online) The inverse association (R = –0·205, P < 0·001) between 25-hydroxyvitamin D (25(OH)D) and total iron-binding capacity (log TIBC (%)) in all participants: Arab adolescents aged 10–17 years (n 170; 100 girls and seventy boys) randomly selected from the Vitamin D School Project Database, King Saud University, Riyadh, Saudi Arabia (2014–2016)
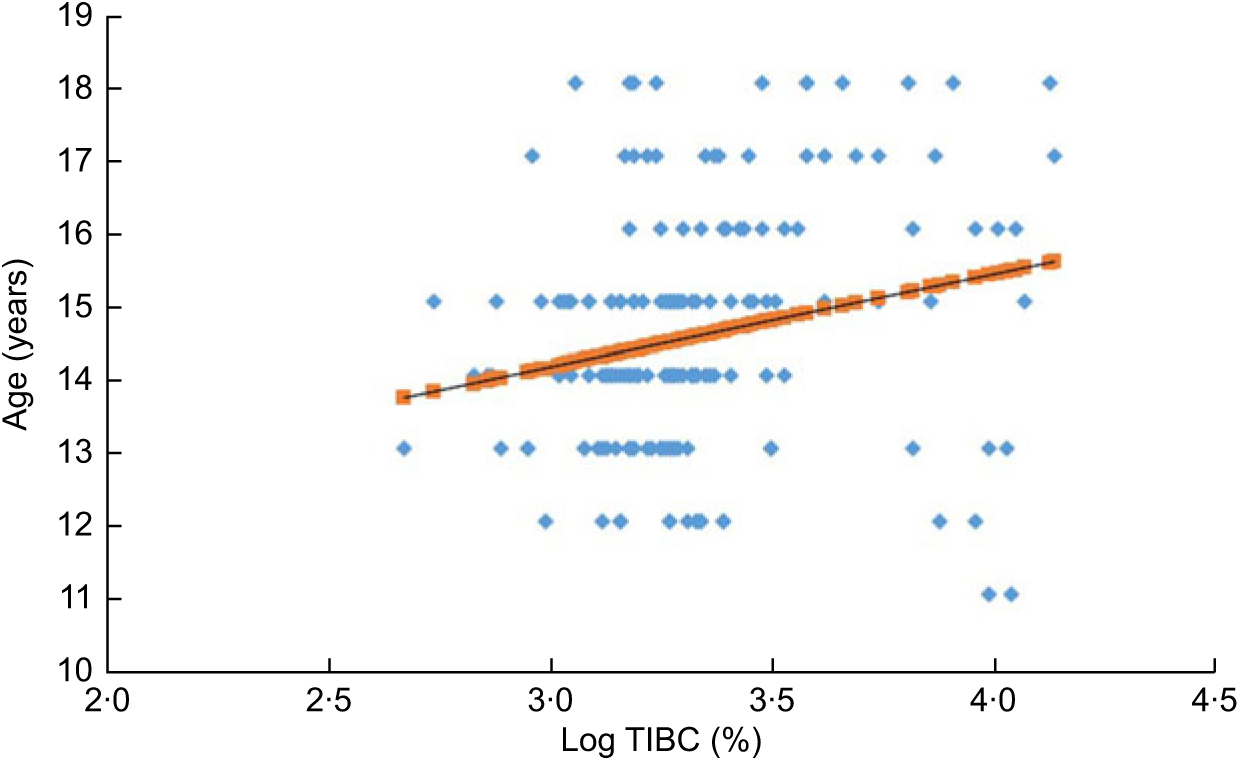
Fig. 2 (colour online) The positive association (R = 0·220, P = 0·004) between age and total iron-binding capacity (log TIBC (%)) in all participants: Arab adolescents aged 10–17 years (n 170; 100 girls and seventy boys) randomly selected from the Vitamin D School Project Database, King Saud University, Riyadh, Saudi Arabia (2014–2016)
Multiple stepwise regression analysis using the different Fe indices as dependent variables and age, BMI, glucose, lipid profiles and vitamin D status as independent variables revealed that age was the most significant predictor (β = 0·24) of serum Fe in all participants, accounting for 5 % (R 2 = 0·053; P = 0·004) of the variance perceived in circulating Fe levels. Serum 25(OH)D and age, on the other hand, were the most significant predictors observed for TIBC, accounting for 10·1 % (R 2 = 0·101; P < 0·001) of the variance perceived. When stratified according to sex, age was the most significant predictor of TIBC for boys (R 2 = 0·14; P = 0·002) and 25(OH)D for girls (R 2 = 0·07; P = 0·01). No significant predictors were observed for TSAT (%) (data not shown).
Discussion
The present study aimed to address the associations between circulating vitamin D, Fe indices and cardiometabolic parameters in a cohort of Saudi adolescents. While no significant association was found between Fe and vitamin D status in all participants, TIBC was inversely associated with vitamin D, with both vitamin D and age being significant predictors of TIBC in the adolescent population. Furthermore, while serum Fe was observed to be significantly associated with circulating TAG and HDL-cholesterol, stepwise regression showed that only age was a significant predictor explaining 5 % of the variance in Fe levels of Arab adolescents.
Previous population-based reports done in other ethnicities observed a mutual association between vitamin D and Fe levels in adolescents(Reference Sim, Lac and Liu21–Reference Sharma, Jain and Dabla23), but not all(Reference Orysiak, Mazur-Rozycka and Fitzgerald24), including the present findings. The present results are in alignment with a recent a large-scale study conducted in more than 5000 German adolescents which found an inhibitory role between vitamin D level and several haematological indices, including Hb(Reference Doudin, Becker and Rothenberger25). Among the limited interventional studies on healthy participants, cholecalciferol (vitamin D3) supplementation showed a downward but non-significant change in the levels of serum Fe, serum ferritin, Hb and TSAT among 251 adult ethnic minorities in Norway(Reference Madar, Stene and Meyer26). Furthermore, the longest interventional study on the effects of vitamin D supplementation on Fe indices was recently conducted in the present cohort of Arab adolescents with suboptimal vitamin D and revealed a modest but significant decrease in levels of Fe and TIBC, parallel to the increase in vitamin D status(Reference Masoud, Alokail and Yakout19).
The exact mechanism of the association between vitamin D and Fe is still unclear. It was proposed that vitamin D directly stimulates erythropoiesis in the bone marrow(Reference Smith and Tangpricha15). Another possible mechanism is that Fe, via the haem-containing cytochrome p450, is an essential cofactor for many enzymes such as 1α-hydroxylase in the kidney necessary for hydroxylation of 25(OH)D to 1,25-dihydroxyvitamin D(Reference Bikle27). The mutual association of these micronutrients appears to be more apparent among patients with compromised vascular or renal function(Reference Zittermann, Jungvogel and Prokop28,Reference Ernst, Zittermann and Pilz29) . In the absence of such disorders among healthy individuals, vitamin D seems to inhibit Fe and complements the functional role of vitamin D as a chemopreventive agent in down-regulating erythropoiesis and angiogenesis, which, in turn, inhibits proliferation of certain cells, cancer cells in particular(Reference Ma, Johnson and Trump30).
A highlight of the present study worthy of further investigation is the sex-specific differences in associations of Fe indices with circulating vitamin D and the cardiometabolic parameters measured. It has been proven at the proteomic level that vitamin D elicits a sexually dimorphic effect in the expression of several key proteins involved in major pathways such as blood coagulation, lipoproteins and other metabolic cycles(Reference Al-Daghri, Al-Attas and Johnston31). This observation has also been documented among studies in athletes where the association between Fe and vitamin D was not apparent in males(Reference Orysiak, Mazur-Rozycka and Fitzgerald24,Reference Hennigar, Gaffney-Stromberg and Lutz32) , but significant in females(Reference Malczewska-Lenczowska, Sitkowski and Surala33).
Lastly, the present study showed a significant association between Fe and lipid indices, specifically TAG in boys and inversely with HDL-cholesterol in girls. One of the multiple roles of Fe in metabolism is involvement in hepatic lipogenesis. As mentioned previously, Fe is an essential part of several key enzymes, one of those include transporters involved in lipid metabolism which may exert a direct effect on hepatic lipid load, intrahepatic metabolism and hepatic lipid secretion(Reference Ahmed, Latham and Oates34). Possible explanations for the sexual dimorphism exhibited in lipid and Fe associations in the present study may involve endogenous hormones, specifically oestrogen, which has been demonstrated to directly affect Fe metabolism(Reference Yang, Xu and Wang35) and the well-known differences in body fat distribution and muscle mass among males and females. The exact regulatory mechanisms between these differences may need further investigation.
The authors acknowledge some limitations. The present findings may apply only to the adolescent Arab population given the nutritional, economic and geographical variations in other ethnicities, factors that significantly influence micronutrient status. Several confounders that may independently influence the association of these two micronutrients, such as genetic and nutritional factors, were not taken into account. Important markers such as ferritin and hepcidin were not measured and their inclusion would have provided a more conclusive explanation on the mechanisms involved in the associations observed. Lastly, the cross-sectional nature of the study limits its findings to suggestive, at best. Nevertheless, the present study is one of the few to determine the sex-specific associations between two common micronutrient deficiencies in apparently healthy Arab adolescents, thereby adding new evidence to previous research. Findings can serve as a basis for larger epidemiological and longitudinal studies to validate the link between 25(OH)D and Fe in this population.
Conclusion
In summary, vitamin D status appears to modestly inhibit Fe indices, specifically TIBC, in healthy Arab adolescents. This association is sex-specific and is observed only in girls. Further studies to include other important markers such as ferritin and hepcidin, as well as other vitamin D metabolites, may shed light on the mechanisms involved in these associations. The study adds to the growing evidence that the mechanistic link between vitamin D and Fe is not straightforward and may be influenced by several factors including endogenous sex hormones and overall metabolic status.
Acknowledgements
Acknowledgements: The authors thank the volunteers and the research team from the different primary care centres for the recruitment of participants. The authors are also thankful to Mr Malak Nawaz Khan Khattak for the statistical analysis done in the study. Financial support: The study was funded by the Chair for Biomarkers of Chronic Diseases (CBCD), Deanship of Research Chairs in King Saud University, Riyadh, Saudi Arabia. CBCD had no role in the design, analysis or writing of this article. Conflict of interest: The authors declare no conflict of interest. Authorship: M.S.M. and S.M.Y. contributed in the design, participant recruitment and data collection. M.S.M., S.M.Y., O.S.A.-A. and M.S.A. carried out sample analysis, interpretation, and preparation of the draft manuscript. S.M.Y. carried out analysis and initial drafting of the manuscript. M.S.A., O.S.A.-A. and N.M.A.-D. edited the final version of the manuscript. All authors read and approved the final manuscript. Ethics of human subject participation: The present study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving research study participants were approved by the Ethics Committee of the College of Science Research Center, King Saud University, Riyadh, Saudi Arabia (reference number 15/0502/IRB; project number E-15-1667). Prior to inclusion in the study, written informed consent was obtained from parents, as well as assent from all the participants.






