Cervical cancer (CC) is a major concern for public health, representing the fourth most common cancer in women after breast, colorectal and lung cancers(Reference Arbyn, Weiderpass and Bruni1). Although the persistency of high-risk human papillomavirus (HPV) infection represents a necessary event for tumour transformation(Reference Bray, Ferlay and Soerjomataram2), it alone is not sufficient for the progression of cervical intraepithelial neoplasia (CIN) to CC(Reference Koshiyama3–Reference Agodi, Barchitta and La Rosa7). For this reason, further physiological and pathological mechanisms might be involved in the aetiology of CC, such as chronic inflammation and oxidative stress. Interestingly, the interaction between chronic inflammation and oxidative stress might trigger HPV-associated carcinogenesis and sustain tumour progression(Reference Di Domenico, Foppoli and Coccia8–Reference Salganik10). In fact, a chronic inflammatory state leads to the production of reactive oxygen species and the release of pro-inflammatory factors (e.g. IL-1 and IL-6, tumour necrosis factor-α and interferon γ)(Reference Koshiyama3). Moreover, chronic inflammation in HPV-infected cells seems also associated with a decreased level of antioxidants(Reference Chih, Lee and Colville11). This significantly reduces the antioxidant activity against reactive oxygen species at cellular level, and hence increases oxidative DNA damage(Reference Di Domenico, Foppoli and Coccia8,Reference De Marco, Bucaj and Foppoli9) .
The interaction between chronic inflammation and oxidative stress can also provide a partial explanation of biological aspects underpinning the risky effect of several factors involved in CC development (e.g. cigarette smoking, dietary deficiencies, sedentary lifestyle and use of contraceptives)(Reference Louie, Castellsague and de Sanjose4,Reference Di Domenico, Foppoli and Coccia8,Reference De Marco, Bucaj and Foppoli9,Reference Barchitta, Maugeri and Quattrocchi12–Reference Asthana, Busa and Labani14) . Among these, dietary factors have been estimated to contribute to 20–60 % of cancers(Reference Kushi, Doyle and McCullough15). Accordingly, it is now well established that consuming healthy foods – such as fruit, vegetables, whole grains, and fish – protects against several non-communicable diseases, such as cardiometabolic diseases and certain types of cancer. This evidence has been proven by different studies evaluating the intake of single foods, specific dietary indexes and the adherence to dietary patterns derived a priori or a posteriori(Reference Barchitta, Maugeri and Quattrocchi12,Reference Willett16–Reference Steck and Murphy20) .
In this scenario, several studies have been conducted to investigate the effect of foods, nutrients and dietary patterns against HPV infection and CC risk(Reference Koshiyama3,Reference Agodi, Barchitta and La Rosa7,Reference Di Domenico, Foppoli and Coccia8,Reference Barchitta, Maugeri and Quattrocchi12,Reference Guo, Zhu and Lin21–Reference Romney, Ho and Palan33) . Among them, there were some epidemiological studies, systematic reviews and meta-analyses focusing on dietary factors with antioxidant anti-inflammatory properties(Reference Di Domenico, Foppoli and Coccia8,Reference Guo, Zhu and Lin21–Reference Sreeja, Lee and Kwon26,Reference Siegel, Salemi and Villa30,Reference Kim, Kim and Lee31,Reference Romney, Ho and Palan33) . Despite increasing evidence in this field of research, the combined effect of foods and nutrients with antioxidant and anti-inflammatory activity remains to be clarified. To our knowledge, only the study by Sreeja and colleagues suggested that adherence to a pro-inflammatory diets increased the risk of CC, warranting further studies to confirm their findings(Reference Sreeja, Lee and Kwon26). With respect to dietary antioxidant intake, instead, there is a lack of supporting evidence. In a previous study on women with normal cervical epithelium, we demonstrated a negative association between dietary antioxidant intake and the risk of HPV infection(Reference Barchitta, Maugeri and La Mastra25). Here, we aim to evaluate the associations of the Composite Dietary Antioxidant Index (CDAI) and the Dietary Inflammatory Index (DII) – two of the most used indexes to assess antioxidant and inflammatory potential of diet – with the overall prevalence of CIN2, CIN3 and carcinoma in situ among Italian women.
Methods
Study design
The present cross-sectional study was conducted on all women who consecutively underwent CC screening at the Cervical Cancer Screening Unit of the Azienda Sanitaria Provinciale of Catania (Italy) from 2012 to 2015. The following inclusion criteria had to be satisfied: (1) women with abnormal Papanicolaou test; (2) who were subjected to high-risk HPV screening and (3) histological test through colposcopy. At recruitment, the screening for high-risk HPV was performed using the Digene HC2 HPV DNA Test (Qiagen, Milan, Italy) and allowed to classify women in HPV-negative and HPV-positive. The Digene HC2 HPV DNA Test was applied to cervical specimens and used the signal-amplified nucleic acid hybridisation for the qualitative detection of the following 13 types of high-risk HPV: 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59 and 68. At the same time, women were also classified according to histological diagnosis into those with normal cervical epithelium or CIN1 (from now on referred to as controls) and those with a diagnosis of CIN2, CIN3 or carcinoma in situ (from now on referred to as cases). A full description of study protocols and characteristics of the study population has been previously reported(Reference Barchitta, Maugeri and Quattrocchi12,Reference Barchitta, Maugeri and La Mastra25,Reference Barchitta, Quattrocchi and Maugeri34) .
Data collection
At recruitment, women were interviewed by trained epidemiologists to collect information on sociodemographic characteristics, behaviours and anthropometric measures. Face-to-face interviews were conducted with the aid of a structured questionnaire. With respect to sociodemographic characteristics, women were classified as having a low (primary education) or medium-high (secondary and tertiary education) educational level, and as being employed or unemployed (i.e. including students and housewives). Regarding behaviours, women were classified as current or non-current smokers (i.e. including both former and never smokers), as well as in users or non-users of oral contraceptives and supplements (i.e. including multivitamin and/or multimineral supplements). Women were also asked to report their height and weight that were used to calculate BMI according to the WHO criteria(35).
Dietary assessment
At recruitment, we also collected dietary data using a semi-quantitative FFQ, as fully described elsewhere(Reference Barchitta, Maugeri and Quattrocchi12,Reference Agodi, Barchitta and Quattrocchi36–Reference Maugeri, Barchitta and Fiore39) . In brief, the FFQ consisted of 95 items (i.e. both foods and beverages) for which frequency of consumption and portions size were collected. The daily dietary intake was obtained by multiplying the daily frequency of consumption by the portion size for each item. Here, the FFQ was used to calculate daily intake of foods and nutrients with antioxidant and anti- or pro-inflammatory properties during the month before the recruitment. Specifically, nutrient composition of each food was determined using the US Department of Agriculture (USDA) Food Composition Database.
Prior to further analyses, women with extreme total energy intake (i.e. those in the 5th or 95th percentile) were excluded. Dietary intakes of remaining women were further adjusted for total energy intake using the residual method(Reference Willett40). Based on energy-adjusted nutrient intakes, we computed two dietary indexes which reflected the cumulative dietary antioxidant intake and the inflammatory potential of diet. Specifically, we adapted the CDAI proposed by Wright and colleagues(Reference Wright, Mayne and Stolzenberg-Solomon41) and the DII developed by Shivappa and colleagues(Reference Shivappa, Steck and Hurley42). The CDAI was calculated as the sum of Z-scores of the dietary intakes of Zn, Se, Mg, vitamin A, vitamin C, vitamin E, β-carotene and flavonoids. With respect to the DII, we first calculated the Z-score for each dietary factor. Next, Z-scores were converted into percentile scores, with a symmetrical distribution (i.e. mean 0 and range from −1 to 1). The DII was then calculated as the sum of the products of percentile scores of dietary factors and their respective inflammatory effect scores. Inflammatory effect scores were previously derived from a systematic review of studies evaluating the inflammatory potential of forty-five dietary factors based on six inflammatory markers (i.e. C-reactive protein, IL-1β, IL-4, IL-6, IL-10 and tumour necrosis factor-α)(Reference Shivappa, Steck and Hurley42). In the current study, we used the following thirty-three of the forty-five dietary factors considered in the original DII: alcohol, vitamin B12, vitamin B6, β-carotene, coffee, carbohydrates, cholesterol, energies, total fats, fibre, folic acid, Fe, Mg, MUFA, niacin, proteins, PUFA, riboflavin, saturated fats, Se, thiamin, trans fats, vitamin A, vitamin C, vitamin D, vitamin E, Zn, black/green tea, flavan-3-ol, flavones, flavonols, flavonones and anthocyanidins. Finally, women were classified according to the tertile distribution of each dietary index, so that the cumulative dietary antioxidant intake and the pro-inflammatory potential increased from the 1st to the 3rd tertile.
Statistical analysis
All statistical analyses were performed on Stata (version 16.0). Prior to analysis, the Kolmogorov–Smirnov test was applied to test the normality of quantitative variables. Descriptive statistics were used to characterise the study population, in terms of percentages for qualitative variables and median with interquartile range for quantitative variables. Comparisons were performed using Mann–Whitney U test or the Kruskal–Wallis test for quantitative variables, and the chi-square test for qualitative variables. The Bonferroni correction was applied to adjust for multiple comparisons. Logistic regression models were applied to evaluate the association of dietary indexes with histological diagnosis of CIN2 or more severe lesions. For the DII index, results were reported as OR and 95 % CI of women in the 2nd or 3rd tertile compared with those in the 1st tertile. For the CDAI index, the reference group was constituted by women in the 3rd tertile. Model 1 was adjusted for age and HPV status, while Model 2 was further adjusted for educational level, BMI, smoking status, parity, use of oral contraceptives and supplements. We also tested for interaction of DII or CDAI indexes with the above-mentioned covariates. All statistical tests were two-sided with a significance level of 0·05.
Results
Study population
Overall, the current analysis included 539 women (mean age = 40·2 years; sd = 10·0 years), who satisfied the inclusion criteria. Figure 1 shows the composition of the study population according to high-risk HPV status and histological diagnosis. Specifically, 44·0 % of women (n 237) were HPV-negative and none of them was diagnosed with CIN2, CIN3 or carcinoma in situ. For this reason, they were all included in the control group. By contrast, 56·0 % of women (n 302) were HPV-positive and further classified as cases (n 127) and controls (n 175) according to histological diagnosis. Thus, the study population was finally split into 127 cases and 412 controls, independent of their HPV status. The characteristics of women according to HPV status and histological diagnosis have already been described elsewhere(Reference Barchitta, Maugeri and Quattrocchi12,Reference Barchitta, Maugeri and La Mastra25,Reference Barchitta, Quattrocchi and Maugeri34) . However – to better understand the current analysis – it is worth underlying that cases were younger (P < 0·001) and heavier (P = 0·012) than controls. Moreover, they were also more likely to be smokers (P = 0·001), nulliparous (P = 0·011) and users of oral contraceptives (P = 0·039).
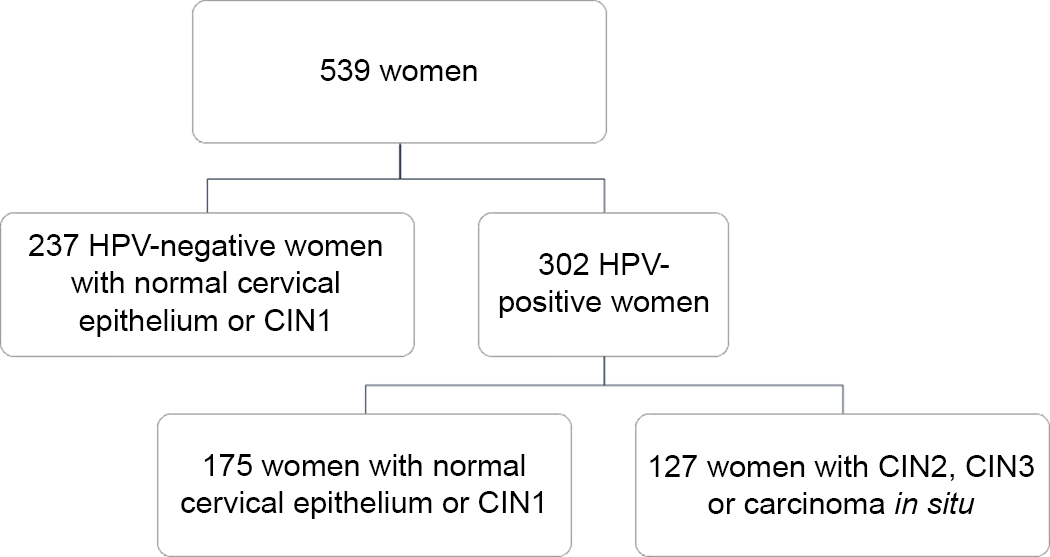
Fig. 1 Description of the study population according to high-risk HPV status and histological diagnosis. CIN, cervical intraepithelial neoplasia; HPV, human papillomavirus
Intake of foods and nutrients with antioxidant and anti-inflammatory potential
We first observed that cases reported lower intakes of energies and other foods or nutrients with antioxidant and anti-inflammatory potential (i.e. coffee, black and green tea, carbohydrates, fibre, PUFA, vitamins A, B6, C, and E, β-carotene, niacin, Mg, and flavonoids; Fig. 2 and Table 1). However, none of the above-mentioned foods and nutrients was significantly associated with histological diagnosis after adjusting for multiple comparison.

Fig. 2 Radar plot illustrating dietary intakes between cases and controls. This plot shows the Z-scores of dietary factors and their comparison between cases (CIN2 + women; blue line) and controls (women with diagnosis of normal cervical epithelium or CIN1; green line). CIN, cervical intraepithelial neoplasia
Table 1 Comparison of daily dietary intakes between controls and cases

Daily dietary intakes are reported as median (interquartile range) and compared using the Mann–Whitney U test between women with CIN2 or more severe lesions (CIN2+) and those with diagnosis of CIN1 or normal cervical epithelium (controls).
Composite Dietary Antioxidant Index
Next, we investigated the synergistic effect of dietary antioxidants using the CDAI, on the basis of which women were classified into tertiles. We found that women with higher CDAI were older (P < 0·001) and heavier (P < 0·001), less educated (P = 0·001), more likely to have children (P < 0·001), and less prone to use oral contraceptives than those with lower dietary antioxidant intake (1st tertile). We also noted a lower proportion of HPV-positive women among those with higher CDAI (P < 0·001) (Table 2).
Table 2 Comparison of population’s characteristics across tertiles of Dietary Inflammatory Index (DII) and Composite Dietary antioxidant Index (CDAI)

Data are reported as median (interquartile range) or percentage (%) and compared using the Kruskal–Wallis or the chi-square tests.
Dietary Inflammatory Index
Similarly, we computed the DII and categorised women according to its tertile distribution. In this case, women with higher DII were younger (P < 0·001) and thinner (P < 0·001), less likely to have children (P < 0·001), more likely to be smokers (P < 0·001), more educated (P < 0·001), more prone to use oral contraceptives (P < 0·001), and less prone to use supplements (P = 0·020) than those with lower DII. With respect to HPV status, we found more HPV-positive women among those with higher DII (P < 0·001) (Table 2).
The associations of dietary antioxidant and inflammatory indexes with cervical cancer
In line with findings presented above, we next sough to evaluate the associations of CDAI and DII with the diagnosis of CIN2, CIN3 or carcinoma in situ. As depicted in Fig. 3(a), the proportion of cases decreased from the 1st tertile to the 3rd tertile of CDAI. However, this difference was not statistically significant (P = 0·070). In fact, also considering the effect of covariates, we failed in demonstrating an association between CDAI and the diagnosis of CIN2 or more severe lesions (Table 3). In contrast, the proportion of cases significantly increased from the 1st tertile to the 3rd tertile of DII (P < 0·001) (Fig. 3(b)). Accordingly, after adjusting for age and HPV status, women with medium or high DII (2nd or 3rd tertile) had higher odds to be diagnosed with CIN2 or more severe lesions than those with low DII (1st tertile) (OR = 2·02; 95 % CI 1·05, 3·88; P = 0·035 and OR = 2·51; 95 % CI 1·33, 4·73; P = 0·004, respectively). Notably, these relationships remained significant further adjusting for age, HPV status, educational level, BMI, smoking status, parity, use of oral contraceptives and supplements (OR = 2·15; 95 % CI 1·11, 4·17; P = 0·024 and OR = 3·14; 95 % CI 1·50, 6·56; P = 0·002, respectively) (Table 3). No interactions of DII or CDAI with other covariates were evident (P-values >0·05).

Fig. 3 Proportions of cases and controls across tertiles of Dietary Inflammatory Index (a) and Composite Dietary Antioxidant Index (b). The bars represent the proportion women with CIN2 or more severe lesions (cases; blue bars) and those with diagnosis of normal cervical epithelium or CIN1 (controls; green bars). ***P-value<0·001 based on the chi-square test
Table 3 Association of Dietary Inflammatory Index and Composite Dietary antioxidant Index with CIN2 or more severe lesions

Results of the logistic regressions are reported as OR and 95 % CI.
Model 1 was adjusted for age and HPV status.
Model 2 was further adjusted for educational level, BMI, smoking status, parity, use of oral contraceptives and supplements. Reference groups (Ref) were the 1st tertile for the Dietary Inflammatory Index and the 3rd tertile for the Composite Dietary Antioxidant Index, respectively.
Discussion
To the best of our knowledge, the current cross-sectional study is the first evaluating the prevalence of CIN2 or more severe lesions associated with DII and CDAI in a Mediterranean population. These are respectively two of the most used indexes for dietary inflammatory potential and cumulative antioxidant intake in epidemiological research. For this purpose, we first evaluated dietary intakes of foods and nutrients with antioxidant, anti- or pro-inflammatory potential among women with abnormal Papanicolaou test results. In this population, we previously demonstrated that the adherence to a Mediterranean-like dietary pattern – characterised by high intake of legumes, vegetables and olive oil – was associated with lower risk of CIN2 or more severe lesions(Reference Barchitta, Maugeri and Quattrocchi12). Here, we added to this evidence, showing that women with CIN2 or more severe lesions had a lower intake of energies and of other dietary factors with antioxidant and anti-inflammatory potential (i.e. coffee, black and green tea, carbohydrates, fibre, PUFA, vitamins A, B6, C, and E, β-carotene, niacin, Mg, and flavonoids) than their counterpart with negative histological diagnosis or CIN1. In line with these findings, we hypothesised that the cumulative antioxidant intake and/or the inflammatory potential of diet might be associated with the odds of having CIN2+ lesions.
With respect to the CDAI, we reported that the proportion of HPV-positive women decreased with increasing antioxidant score, both here and in a previous study on women with normal cervical cytology(Reference Barchitta, Maugeri and La Mastra25). In fact, dietary antioxidant intake might counteract oxidative stress and DNA damage induced by HPV persistence(Reference De Marco, Bucaj and Foppoli9,Reference De Marco43) , producing a cellular environment that could help viral clearance(Reference Chih, Lee and Colville11,Reference García-Closas, Castellsagué and Bosch44) . Yet, despite it has been suggested that antioxidants also prevents CC progression(Reference García-Closas, Castellsagué and Bosch44), we did not find an association between CDAI and CIN2+ status after adjusting for HPV status and other covariates. This should encourage further research to understand if antioxidants exert their protective role in the entire carcinogenesis process or, on the contrary, if they intervene only in the first phase(Reference Barchitta, Maugeri and La Mastra25).
In the current study, we also evaluated the DII, a cumulative score based on the anti- and pro-inflammatory potential of foods and nutrients(Reference Shivappa, Steck and Hurley42). We first noted that the proportion of HPV-positive women increased with increasing dietary inflammatory potential. But even more interestingly, we demonstrated that women with higher DII – and hence women with a pro-inflammatory diet – were more likely to have CIN2 or more severe lesions than those with lower index. The association between DII and CIN2+ status remained significant after adjusting for HPV status and other covariates. This was consistent with the study by Sreeja and colleagues(Reference Sreeja, Lee and Kwon26), which represented the first attempt in this field of research. Our findings, together with those Sreeja and colleagues(Reference Sreeja, Lee and Kwon26), indicated that dietary inflammatory potential might modulate the risk of CC independent of HPV infection and other risk factors (e.g. age, cigarette smoking, parity and use of oral contraceptives). Looking at other types of cancer, it has already been shown that higher DII was associated with the risk of other cancers, albeit with some variations related to study design and type of cancer(Reference Zheng, Merchant and Wirth45–Reference Fowler and Akinyemiju47). For instance, Shivappa and colleagues concluded that a pro-inflammatory diet was associated with increased risk of bladder cancer(Reference Shivappa, Hébert and Rosato46). By contrast, Zheng and colleagues did not support a direct association of DII with pancreatic cancer; however, their results differed according to different follow-up times(Reference Zheng, Merchant and Wirth45). In 2017, the meta-analysis by Fowler and colleagues tried to solve some controversies. Interestingly, upon stratification by cancer type, they demonstrate a significant association with breast, colorectal and lung cancers(Reference Fowler and Akinyemiju47).
Although no other study has ever considered the relationship with CC, our results are in line with the notion that healthy foods, nutrients and dietary patterns might reduce the risk of CC. By contrast, the consumption of high-energy, high-fat and processed foods might increase the risk(Reference Barchitta, Maugeri and Quattrocchi12). From a biological point of view, a previous cross-sectional study demonstrated that a pro-inflammatory diet increased serum levels of inflammatory biomarkers (e.g. tumour necrosis factor-α, and interferon γ, IL-1, and IL-2) among healthy people(Reference Shivappa, Hebert and Marcos48). Indeed, impaired cell-mediated immune response via deregulation of immune system mediators might promote CC progression. However, other physiological and molecular mechanisms could be involved, motivating at least partially the protective effect of foods and nutrients against CC. For instance, it has been shown that foods and nutrients might interact with the DNA methylation process(Reference Agodi, Barchitta and Quattrocchi36–Reference Barchitta, Maugeri and Magnano San Lio38,Reference Maugeri, Mazzone and Giuliano49–Reference Maugeri, Barchitta and Mazzone51) , resulting in aberrant DNA methylation of retrotransposable elements associated with the risk of CC (e.g. long interspersed nuclear elements 1; LINE-1)(Reference Piyathilake, Badiga and Kabagambe27,Reference Barchitta, Quattrocchi and Maugeri34,Reference Piyathilake, Henao and Macaluso52) . However, this field of research is continuously expanding, with several factors and diseases associated with LINE-1 methylation(Reference Woo and Kim53–Reference Maugeri, Barchitta and Fallico57). Thus, further research is needed to understand molecular mechanisms underpinning the protective or detrimental effect of dietary factors against CC also considering genetic susceptibility and epigenetic markers(Reference Agodi, Barchitta and Cipresso6,Reference Agodi, Barchitta and Quattrocchi58) .
The current study had some limitations to be considered, such as its cross-sectional design that hindered to evaluate the causality of observed associations. Moreover, the inclusion criteria – and therefore the selection of women who were screened for HPV and tested through colposcopy – cannot exclude potential selection bias. However, this was a convenience sample that allowed us to test the association with high-grade CIN and to adjust our findings for HPV status. With respect to dietary assessment, we used a FFQ that might be affected by measurement errors and inaccuracies. To partially manage reporting bias, we excluded from the analysis all women with the lowest and highest values of total energy intake (i.e. those in the 5th and 95th percentiles). We also adjusted food and nutrient intakes for total energy intake prior to further analyses. Although the FFQ used in this study allowed us to obtain the intake of thirty-three of the forty-five dietary factors included in the DII, the residual effect of other foods and nutrients with anti- or pro-inflammatory potential was not evaluated. Moreover, the DII was characterised by intrinsic limitations common to all a priori dietary obtained through structured questionnaires (e.g. dependence on memory, incomplete data collection and different thresholds for classification), which also made difficult the comparison with previous studies on other types of cancer. Another weakness of our study was the absence of any sort of validation using biomarkers, which for its part would have allowed to overcome some limits of dietary indexes. It is worth mentioning that the FFQ used in our study collects dietary data related to 1-month prior the recruitment and that it does not take into account any changes related to food cooking. In general, FFQ are relatively simple, cost-effective and time-efficient tools to collect dietary data in large epidemiological studies. However, doubts on their accuracy were raised in the last decades, an issue that is still debated among nutritional epidemiologists(Reference Mozaffarian, Rosenberg and Uauy59). Finally, we cannot rule out the possible impact of unmeasured factors, which might be associated with dietary habits or CC risk. However, it is worth noting that our analyses were adjusted for age, HPV status, educational level, BMI, smoking status, parity, use of oral contraceptives and supplements. Thus, further prospective studies evaluating additional factors, as well as inflammatory markers in the serum of patients, should be recommended.
In conclusion, our study provided a comprehensive analysis of foods and nutrients with antioxidant and inflammatory potential among women at risk for CC. While the proportion of HPV-positive women was lower among those with high CDAI, the dietary antioxidant intake was not associated with the prevalence of CIN2 or more severe lesions. In contrast, we demonstrated that women who adhered to a pro-inflammatory diet – specifically those with higher DII – exhibited increased odds of CIN2 or more severe lesions. Although these findings should be confirmed by further research, at the present time they suggested how some foods and nutrients might modulate the risk of CC.
Acknowledgements
Acknowledgements: We are grateful to all the women who gave their consent to participate in the study. Financial support: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors. Conflict of interest: There are no conflicts of interest. Authorship: Conceptualisation: A.M., M.B. and A.A.; methodology: A.M., M.B., R.M.S.L. and A.S.; software: A.M. and R.M.S.L.; formal analysis: A.M. and R.M.S.L.; investigation: A.M., M.B., R.M.S.L. and A.S.; resources: A.A.; data curation: A.M. and R.M.S.L.; writing – original draft preparation: A.M. and R.M.S.L.; writing – review and editing: all the authors; supervision: A.A.; funding acquisition: A.A. All authors have read and agreed to the published version of the manuscript. Ethics of human subject participation: This study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving research study participants were approved by the Catania 2 ethics committee. Written informed consent was obtained from all subjects.









