Breast cancer (BC) is the leading cause of cancer death in women worldwide( Reference Jemal, Siegel and Ward 1 ). In Western countries, age-standardized incidence rates range between 56·8 and 109·4 per 100 000 women, while lower rates are observed in Asia, Central America and sub-Saharan Africa( Reference Ferlay, Shin and Bray 2 ). Among Mexican women, the age-standardized incidence rate is 26·4 per 100 000 women( Reference Tavani, Gallus and La Vecchia 3 ).
Multiple risk factors for BC such as family history, obesity, lactation, adult attained height, and menstrual and reproductive history are well established but are generally difficult to modify( Reference Tavani, Gallus and La Vecchia 3 – Reference Li, Littman and White 7 ). A substantial amount of research has explored the influence of modifiable dietary risk factors on BC risk( Reference Franceschi, Favero and Decarli 8 – Reference Wakai, Tamakoshi and Date 13 ). Several foods as well as macro- and micronutrients (e.g. vegetables, dietary fibre and vitamins) have been investigated in relation to BC risk( Reference Smith-Warner, Spiegelman and Yaun 11 , Reference Gandini, Merzenich and Robertson 14 , Reference Michels, Mohllajee and Roset-Bahmanyar 15 ), although no consistent and statistically significant associations have been established. One convincing exception is for alcohol consumption( Reference Lof and Weiderpass 16 ).
Most epidemiological studies on diet and cancer have largely been on intakes of individual food items or nutrients( Reference Donaldson 17 , Reference Key, Schatzkin and Willett 18 ). This approach, however, does not fully take into account the complexity of human diets, in terms of the large number of foods consumed by individuals, as well as the inter-correlation between those foods( Reference Willett 19 ). There has been an increasing interest towards dietary patterns, rather than individual foods, as a way to investigate the aetiology of BC( Reference Baglietto, Krishnan and Severi 20 – Reference Mannisto, Dixon and Balder 22 ). A valuable alternative was constituted by a priori scores, defined on dietary guidelines and recommendations. The Healthy Eating Index (HEI), the Diet Quality Index (DQI) and the Recommended Food Score (RFS) are recent examples, but have little or no association with BC risk and/or mortality( Reference Fung, Hu and McCullough 21 , Reference Cade, Taylor and Burley 23 , Reference Kim, Willett and Fung 24 ). More robust evidence with BC risk and mortality was produced by using scores integrating dietary components with other lifestyle factors such as body fatness, physical activity, alcohol consumption and/or smoking habits( Reference Harnack, Nicodemus and Jacobs 25 – Reference Sanchez-Zamorano, Flores-Luna and Angeles-Llerenas 27 ).
In 2007, the World Cancer Research Fund (WCRF) in collaboration with the American Institute for Cancer Research (AICR) summarized the existing scientific evidence on the role of foods, nutrition and physical activity in the aetiology of cancer( Reference Coughlin 4 ). Accordingly, a list of recommendations (eight general and two special) on diet, physical activity and weight management were developed in order to reduce the incidence of cancer in the general population.
In the present study, we evaluated the association between the WCRF/AICR recommendations and the risk of BC in a case–control study of Mexican women within the Cancer de Màma (CAMA) study, overall and by menopausal status.
Materials and methods
Study population
CAMA recruitment procedures have been described in detail previously( Reference Beasley, Coronado and Livaudais 28 ). In brief, 1000 cases and 1074 controls, pre- and postmenopausal women aged 35–69 years, were recruited between January 2004 and December 2007 from three regions in Mexico and their surrounding metropolitan areas (Mexico City, Monterrey and Veracruz). Participants were resident from one of these regions during at least 5 years prior to recruitment in the study. Cases were identified by trained field staff at twelve hospitals from major health-care institutions in Mexico: the Mexican Institute of Social Security (Instituto Mexicano del Seguro Social (IMSS), six hospitals), the Social Security and Services Institute for State Employees (Instituto de Seguridad y Servicios Sociales de los Trabajadores del Estado (ISSSTE), two hospitals) and the Ministry of Health (Secretarıade Salud (SS), four hospitals). Inclusion criteria included: (i) patients with a new histologically confirmed diagnosis of BC, regardless of the stage of disease; (ii) patients with no previous treatment such as radiotherapy, chemotherapy or anti-oestrogens such as tamoxifen during the previous 6 months; (iii) patients who were not taking Aromasin® (exemestane), Femara® (letrozole), Arimidex® (anastrozole) or Megace® (megestrol) at the time of the study; and (iv) patients who were not pregnant. Cases known to be HIV-positive (n 1) were excluded from the study. After excluding in situ cases (n 20), 980 cases were eligible.
Control subjects were randomly selected by multiple-step random sampling and were frequency-matched to cases according to 5-year age groups, health-care institution and region. The response rate for controls was 87·4 % for Mexico City, 90·1 % for Monterrey and 97·6 % for Veracruz. The study personnel visited the selected households and determined willingness to participate in the study and conducted a face-to-face interview. Finally, an appointment was scheduled for each woman to attend the hospital for anthropometric measurements, mammography and a blood sample. A total of 1074 eligible controls were identified. Natural menopause was defined as twelve consecutive months of amenorrhoea without an obvious cause( Reference Bassol Mayagoitia 29 , Reference Salazar-Martinez, Lazcano-Ponce and Gonzalez Lira-Lira 30 ).
Ethics statement
Cases and controls provided written informed consent to participate in the study. The study protocol and data collection instruments were reviewed and approved by the Institutional Review Board at the National Institute of Public Health.
Data collection and dietary questionnaire
A trained interviewer administered a questionnaire to each selected participant to collect information on her health, physical activity and diet. The health questionnaire collected data on sociodemographic characteristics; reproductive factors (e.g. age at menarche and menopause, number of full-term pregnancies, pregnancy outcomes, breast-feeding, menopausal status); use of oral contraceptives and hormone replacement therapy; family and individual history of chronic diseases (e.g. hypertension, diabetes mellitus, BC); personal history of sexually transmitted diseases; history of body size, smoking, alcohol consumption; and history of X-ray and mammographic studies.
Information on dietary habits was obtained through questions on food consumption during the 12 months preceding the symptoms (for BC cases) or the recruitment (for controls), using a semi-quantitative FFQ adapted from the Nurses’ Health Study( Reference Willett 19 ) for the Mexican population and validated in Mexico City( Reference Hernandez-Avila, Romieu and Parra 31 , Reference Romieu, Parra and Hernandez 32 ). The FFQ included 104 items and ten multiple-choice frequency categories of consumption: ‘6 or more per day’, ‘4–5 per day’, ‘2–3 per day’, ‘1 per day’, ‘5–6 per week’, ‘2–4 per week’, ‘1 per week’, ‘1–3 per month’, ‘less than 1 per month’ and ‘never’. For each food item, the nutrient content per average unit (specified serving size: slice, glass or natural unit) was compiled( 33 ) and women were asked how often they had consumed an amount of each food on average over the previous year. Nutrient intakes were computed by multiplying the frequency response by the nutrient content of specified portion sizes using Microsoft® Office Access 2007. The database for calculating the nutrient intake took advantage of information from the US Department of Agriculture food composition tables( 33 ) and it was complemented, when necessary, with a nutrient database developed by the National Institute of Nutrition in Mexico( Reference Morales de Leon, Babinsky and Bourges 34 ).
To assess physical activity within the last 12 months, a semi-structured interview-based questionnaire was used to assess individuals’ time spent in physical activity (light-, moderate- and vigorous-intensity, as well as sleep) during a regular week. The questionnaire was based on the 7 d recall questionnaire proposed by Sallis et al.( Reference Sallis, Haskell and Wood 35 ).
World Cancer Research Fund/American Institute for Cancer Research score composition
An index score reflecting adherence to the WCRF/AICR recommendations for cancer prevention was constructed; hereafter referred to as the ‘WCRF/AICR score’. Out of ten recommendations (components), the following seven were retained to determine the score in women( Reference Ledikwe, Blanck and Khan 36 ): body fatness, physical activity, intake of foods and drinks that promote weight gain, intake of plant foods, intake of animal foods, consumption of alcoholic drinks and breast-feeding in women. Information on the construction of the score is detailed below in Table 2.
The score was designed on recent work evaluating the association between WCRF/AICR guidelines and cancer risk in the European Prospective Investigation into Cancer (EPIC) cohort( Reference Romaguera, Vergnaud and Peeters 26 ). The score was constructed using quantitative criteria supplied in the WCRF/AICR recommendations. Briefly, for each component, 1 point was assigned when the recommendation was met, 0·5 points when it was partially met and 0 points otherwise. In some cases, arbitrary a priori cut-off values were defined for intermediate categories, not based on the distribution of a given variable in our study. For the recommendations including several sub-recommendations (foods and drinks that promote weight gain and plant foods), the final score was the average of each sub-recommendation score. Three recommendations were not implemented in the present work: (i) the recommendation on preservation, processing and preparation of foods because insufficient data were available; (ii) the recommendation on dietary supplements which could not be operationalized in terms of cancer prevention without further assumptions about type or dose of supplementation; and (iii) the special recommendation related to cancer survivors which was outside the scope of the present study. As the WCRF/AICR recommendations were not ranked according to priority, all major recommendations were summed to contribute equally to the total WCRF/AICR score. Therefore, the total WCRF/AICR score ranged from 0 to 7 in the present study, with higher scores indicating greater adherence to the WCRF/AICR recommendations.
Statistical analyses
The t test was used to assess differences between cases and controls for continuous variables, i.e. height, weight, waist circumference, waist-to-hip ratio, BMI, age at menarche, age at first pregnancy, number of births, energy intake, breast-feeding, alcohol consumption and physical activity. A χ 2 test was used to test for differences between cases and controls for categorical variables, including socio-economic status, family history of BC, history of fibrocystic disease, use of oral contraceptives, use of hormone therapy, education, smoking and marital status. To estimate the association between the WCRF/AICR score and the risk of BC, conditional logistic regression models were used to compute odds ratios and associated 95 % confidence intervals.
Matching accounted for age category, health-care system and region (model 1). Confounding factors were then included in the model (model 2), i.e. family history of BC (yes/no), age at menarche, age at first pregnancy, parity (number of children born alive), socio-economic status (lower, middle and upper), hormone replacement therapy (yes/no) and total energy consumption (kcal/d). Smoking status and use of oral contraceptive were not included in the different models because their inclusion in the statistical model did not change the results. Analyses were carried out for all women, and separately among pre- and postmenopausal women.
The score was categorized into quartiles based on the distribution of controls. The lowest quartile (from 0 to 3·25 points) was considered as the reference.
Also, the association of each component of the WCRF/AICR recommendations was evaluated in models mutually adjusted for all other components of the score. Tests for trends were computed using a continuous variable with values from 0 to 7 and P values were determined (P trend). Throughout the work, P<0·05 was considered statistically significant. All analyses were conducted using the statistical software package SAS version 9·2.
Results
Postmenopausal women represented 59 % and 56 % of cases and controls, respectively, as displayed in Table 1. The response rate in control women was high in the three regions (87 %, 90 % and 97 % in Mexico City, Monterrey and Veracruz, respectively). Cases and controls were similar with respect to the frequency of ever smoking, ever use of oral contraceptives and age at menarche. Compared with controls, cases displayed lower BMI values (29·3 kg/m2 v. 30·5 kg/m2, P<0·01), were more likely to have a family history of BC (6 % v. 4 %, P=0·01), had on average fewer children and were more likely to have children later in life.
Table 1 Clinical characteristics of the study participants: women aged 35–69 years, incident BC cases and controls matched on age, region and health-care system, CAMA study, Mexico, January 2004–December 2007
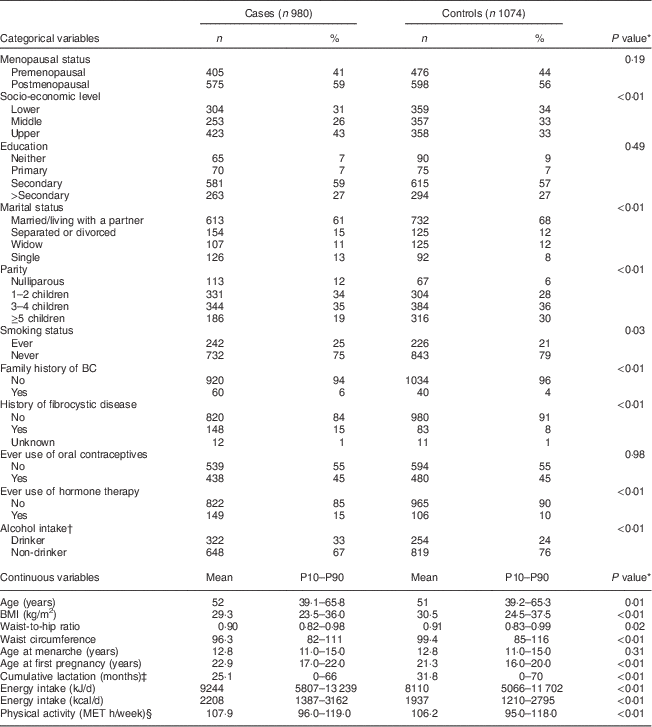
BC, breast cancer; CAMA, Cancer de Màma; MET, metabolic equivalent of task; P10–P90, 10th–90th percentile.
* From χ 2 test for categorical variables and t test for continuous variables.
† Median (interquartile range) among drinkers (330 cases and 254 controls).
‡ Among parous women.
§ Estimated from 7 d activity diary that queried all activities (working and leisure).
The different components of the WCRF/AICR score are described in Table 2. Compared with controls, cases displayed a lower frequency of high physical activity (43 % v. 61 %) and of breast-feeding for longer than 6 months (64 % v. 74 %), but higher frequency of ‘fruits and vegetables’ intake larger than 600 g/d (63 % v. 48 %) and of ‘dietary fibre’ intake larger than 25 g/d (62 % v. 45 %).
Table 2 WCRF/AICR recommendations for cancer prevention and operationalization of the WCRF/AICR score in the CAMA study
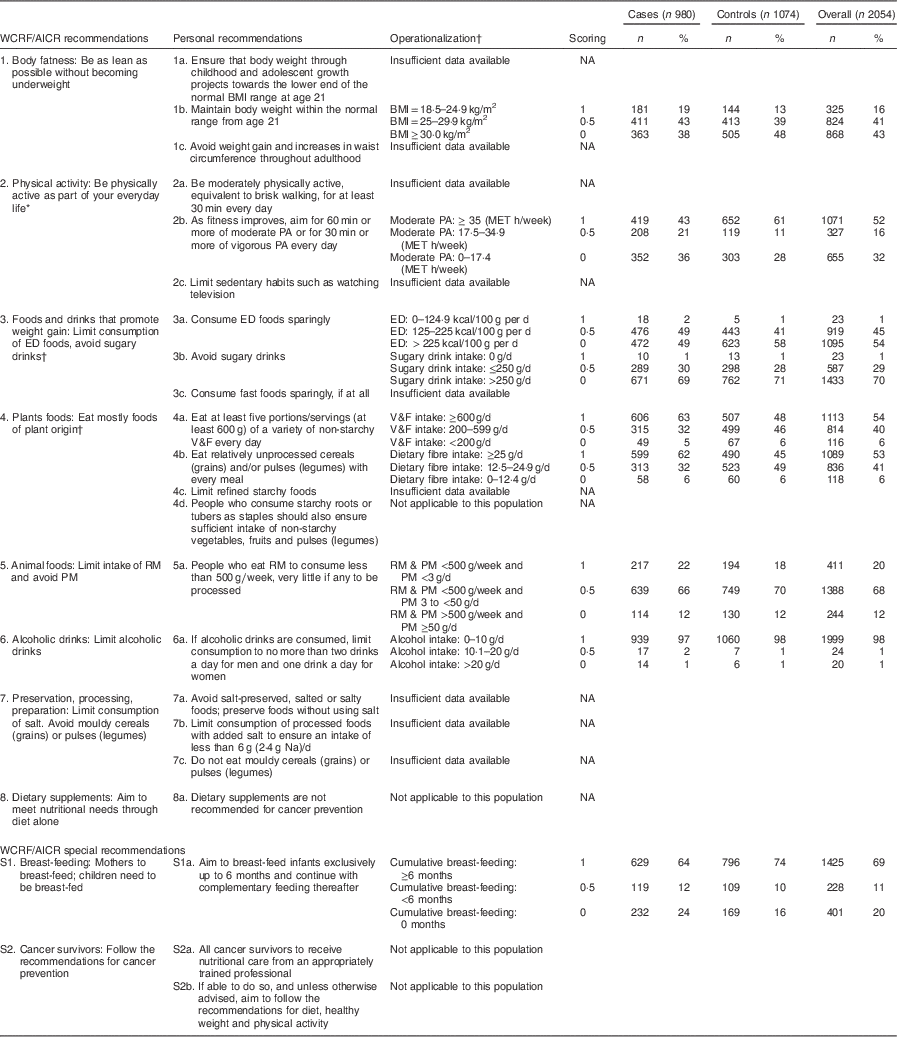
WCRF/AICR, World Cancer Research Fund/American Institute for Cancer Research; CAMA, Cancer de Màma; ED, energy-dense; RM, red meat; PM, processed meat; PA, physical activity; V&F, vegetables and fruits; MET, metabolic equivalent of task; NA, not available.
* Using the moderate physical activity (MET h/week) that combines recreational and occupational activities.
† The score for recommendations 3 and 4 was the result of averaging the scores of each sub-recommendation (3a and 3b; 4a and 4b).
‡ ED food was calculated as energy (kcal; 1 kcal=4·1868 kJ) from foods (solids foods and semi-solid or liquid foods such as soups) divided by the weight (g) of these foods. Drinks (including water, tea, coffee, juice, soft drinks, alcoholic drinks and milk) were not included in the calculation( Reference Sanchez-Zamorano, Flores-Luna and Angeles-Llerenas 27 ). Sugary drinks included both soft drinks and fruit and vegetable juices.
After controlling for confounding factors, the WCRF/AICR score was not associated with risk of BC overall or by menopausal status, with OR comparing the score in the top v. bottom quartile (ORQ4–Q1) equal to 1·17 (95 % CI 0·75, 1·82, P trend=0·26) and 0·97 (95 % CI 0·64, 1·46, P trend=0·39) in pre- and postmenopausal women, respectively (Table 3).
Table 3 Multivariate-adjusted odds ratios and 95 % confidence intervals between WCRF/AICR score and BC risk, overall and by menopausal status, among women aged 35–69 years, incident BC cases and controls matched on age, region and health-care system, CAMA study, Mexico, January 2004–December 2007
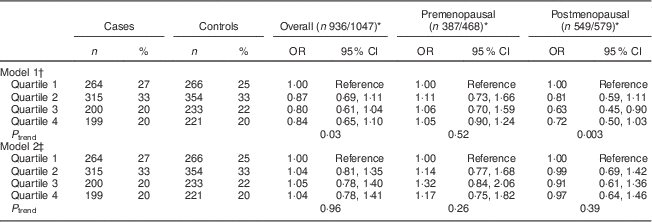
WCRF/AICR, World Cancer Research Fund/American Institute for Cancer Research; BC, breast cancer; CAMA, Cancer de Màma.
* Frequencies of case and control participants, only includes participants from informative case sets.
† Assessed by analysing BC cases and their individual matched controls by conditional logistic regression, conditioning for matching factors (age, region and health-care institution).
‡ Further adjusted for age at first pregnancy, number of full-term pregnancies, energy intake, socio-economic status, age at menarche, hormone therapy and family history of BC (no/yes).
Sensitivity analyses were carried out by excluding, in turn, each component of the WCRF/AICR recommendations (Table 4) from the overall score. Notably, the exclusion of the BMI component resulted in a marked reduction of BC risk overall, with ORQ4–Q1=0·68 (95 % CI 0·49, 0·92, P trend=0·03), and among postmenopausal women, with ORQ4–Q1=0·60 (95 % CI 0·39, 0·94, P trend=0·03). Two individual components were significantly associated with BC risk, as shown in Table 5. An inverse association was observed between BMI and risk of BC, with OR comparing obese v. normal-weight women equal to 0·57 (95 % CI 0·42, 0·76, P trend<0·01) and 0·48 (95 % CI 0·31, 0·73, P trend<0·01) overall and among premenopausal women, respectively. Women with high physical activity levels (≥35 MET h/week) compared with low physical activity (≤17·5 MET h/week; MET=metabolic equivalent of task) displayed OR equal to 0·61 (95 % CI 0·49, 0·76, P trend<0·01) and 0·42 (95 % CI 0·31, 0·59, P trend<0·01) overall and among postmenopausal women, respectively.
Table 4 Odds ratios and 95 % confidence intervals for BC risk according to WCRF/AICR score and after alternate subtraction of each of its components, overall and by menopausal status, among women aged 35–69 years, incident BC cases and controls matched on age, region and health-care system, CAMA study, Mexico, January 2004–December 2007
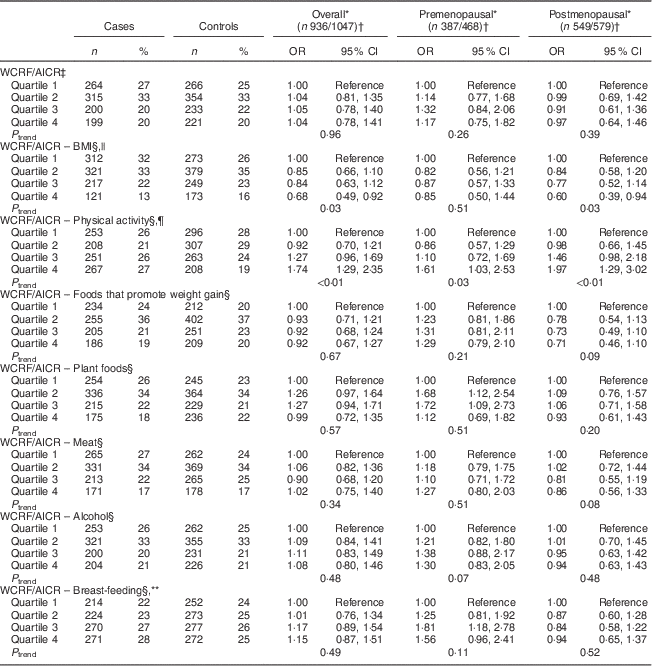
WCRF/AICR, World Cancer Research Fund/American Institute for Cancer Research; BC, breast cancer; CAMA, Cancer de Màma.
* Assessed by analysing BC cases and their individual matched controls by conditional logistic regression, conditioning for matching factors (age, region and health-care institution) and adjusted for age at first pregnancy, number of full-term pregnancies, energy intake, socio-economic status, age at menarche, hormone therapy and family history of BC (no/yes)·
† Frequencies of case and control participants, only includes participants from informative case sets.
‡ Total WCRF/AICR score range: 0–7.
§ The WCRF/AICR score minus one component ranged from 0 to 6.
|| Statistical model was further adjusted for BMI.
¶ Statistical model was further adjusted for physical activity.
** Statistical model was further adjusted for breast-feeding.
Table 5 Mutually adjusted odds ratios and 95 % confidence intervals for BC risk associated with the components of the WCRF/AICR score, overall and by menopausal status, among women aged 35–69 years, incident BC cases and controls matched on age, region and health-care system, CAMA study, Mexico, January 2004–December 2007
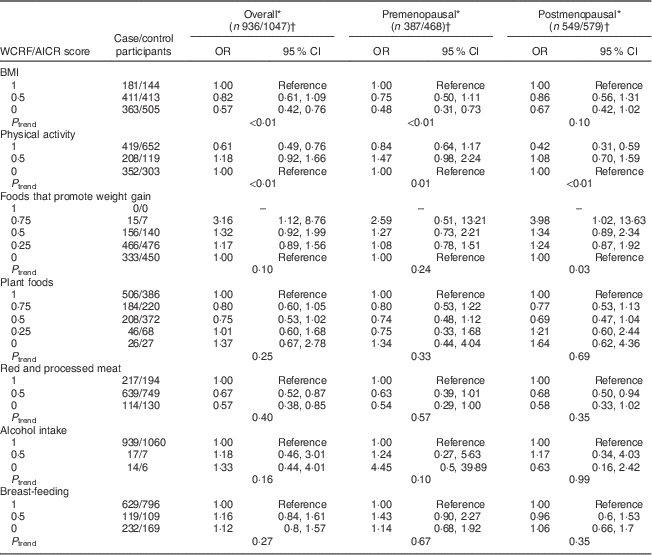
WCRF/AICR, World Cancer Research Fund/American Institute for Cancer Research; BC, breast cancer; CAMA, Cancer de Màma.
* Assessed by analysing BC cases and their individual matched controls by conditional logistic regression, conditioning for matching factors (age, region and health-care institution) and adjusted for age at first pregnancy, number of full-term pregnancies, energy intake, socio-economic status, age at menarche, hormone therapy, family history of BC (no/yes) and all the other WCRF/AICR components simultaneously.
† Frequencies of case and control participants, only includes participants from informative case sets.
Discussion
In a case–control study conducted in Mexico we observed that an index of adherence to the WCRF/AICR cancer prevention recommendations was not associated with the risk of BC. However, after excluding BMI, the WCRF/AICR score index was inversely associated with BC risk overall and among postmenopausal women, while a marginal effect was observed among premenopausal women.
Two prospective studies assessing the impact of the WCRF/AICR recommendations on BC have been published to date( Reference Romaguera, Vergnaud and Peeters 26 , Reference Hastert, Beresford and Patterson 37 ). Within the EPIC cohort, women within the highest WCRF/AICR score were 16 % less likely to develop BC compared with those in the first category of the score (with a similar operationalization to our score) with hazard ratio equal to 0·84 (95 % CI 0·78, 0·90)( Reference Romaguera, Vergnaud and Peeters 26 ). The VITamins And Lifestyle (VITAL) study cohort found that postmenopausal women meeting at least five of the WCRF/AICR recommendations had 60 % lower BC risk (hazard ratio=0·40; 95 % CI 0·25, 0·65) compared with women not meeting any recommendation( Reference Hastert, Beresford and Patterson 37 ). A possible explanation is that in our study BMI was differentially associated with BC risk compared with the EPIC and VITAL cohorts. No major change in BC risk was observed in both EPIC and VITAL studies after excluding BMI from their respective index scores, indicating that BMI played only a partial role in the observed associations. Moreover, when physical activity was removed from the index, the WCRF/AICR score was associated with an increase in BC risk. We believe that the observed association might be due to the major role that BMI plays in the WCRF/AICR score related to BC risk.
In our study, when BMI was excluded from the WCRF/AICR score, a statistically significant inverse association between the WCRF/AICR score and BC risk was observed overall and among postmenopausal women. BMI was inversely associated with BC risk overall and among premenopausal women. A weak non-statistically significant decrease in BC risk was also observed among postmenopausal women. These findings are in contrast with positive relationships between BMI and BC risk consistently observed among postmenopausal women( Reference Cheraghi, Poorolajal and Hashem 38 – Reference van den Brandt, Spiegelman and Yaun 41 ) and with inverse relationships among premenopausal women in Caucasian populations( Reference Renehan, Tyson and Egger 39 , Reference Ma, Bernstein and Ross 42 – Reference Weiderpass, Braaten and Magnusson 44 ). Few studies have investigated the association of BMI and BC risk in women from Hispanic origin and results have been conflicting( Reference John, Sangaramoorthy and Phipps 45 – Reference Sexton, Franzini and Day 47 ). While, in a case–control study, delays in ascertainment of cancer onset may possibly lead to an underestimation of habitual weight among cases, this is unlikely to explain our results given that BC does not usually lead to loss of weight and that only incident cases were included in the study. The lack of positive association between BMI and BC among postmenopausal women, which has been observed in many studies conducted among Caucasian populations, might be explained by the different fat distribution of Hispanic women( Reference Amadou, Torres Mejia and Fagherazzi 48 ).
Studies in the USA show that American women of African or Hispanic origin are more likely to be obese than Caucasian women( Reference Wang and Beydoun 49 ). In our Mexican study population, 85 % of women were overweight or obese, whereas in the EPIC and VITAL cohorts, the frequencies were 61 % and 52 %, respectively( Reference Romaguera, Vergnaud and Peeters 26 , Reference Hastert, Beresford and Patterson 37 ). This observation may suggest that the thresholds of BMI customarily used to identify normal-weight, overweight and obese individuals may not adapt to populations other than in Europe and North America( Reference Deurenberg, Deurenberg-Yap and Guricci 50 , Reference Liu, Byrne and Kagawa 51 ). A WHO expert consultation addressed this issue in Asian populations and considered whether population-specific cut-off points for BMI were necessary( 52 ). To the best of our knowledge, there is no similar debate around Latin American populations. Therefore, adaptation of the WCRF/AICR recommendations outside Caucasian populations may need to consider other markers of adiposity or weight gain.
Several studies have shown that regular physical activity is beneficial to control weight and may also decrease the risk of some types of cancer, including BC( 53 – Reference Slattery, Sweeney and Edwards 56 ). Recently, moderate-intensity physical activity for 3 h/week was associated with a lower risk of BC in both pre- and postmenopausal women, also suggesting differential associations with respect to menopausal status( Reference Angeles-Llerenas, Ortega-Olvera and Perez-Rodriguez 57 ). Other recent epidemiological studies have shown that BC risk reduction in relation to physical activity was greater in post- than in premenopausal women( Reference Friedenreich and Orenstein 55 , Reference Angeles-Llerenas, Ortega-Olvera and Perez-Rodriguez 57 – Reference Vainio, Kaaks and Bianchini 60 ). In our study, high physical activity was heterogeneously associated with BC risk compared with low physical activity level, with a 16 % and 58 % BC risk reduction among pre- and postmenopausal women, respectively. These results call for further investigation on the role of lack of physical activity as a risk factor in Latin American populations, possibly using prospective cohort studies.
Several limitations of the present study should be considered in interpreting our findings. Recall bias is a source of misclassification in case–control studies assessing diet through self-reported questionnaire measurements, possibly differentially expressed among cases and controls. However, interviewing women close to the time of diagnosis may have reduced the impact of potential changes in dietary and other lifestyle habits, likely to occur in cancer patients. The fact that in Mexico there is limited awareness about lifestyle risk factors related with BC may have attenuated the extent of differential classification. FFQ in general are subject to measurement error and this may have limited our ability to accurately measure relevant dietary components. The questionnaire measurements were validated and shown to perform reasonably well( Reference Hernandez-Avila, Romieu and Parra 31 ). Not all WCRF/AICR recommendations were included in our score, either because of a lack of available data or because they were not applicable to the study population. This refers mostly to processing and preservation of foods, salt intake and vitamin supplementation. In addition, while epidemiological studies showed that abdominal adiposity such as waist circumference may be a better predictor of some cancer types, such as colorectal or pancreatic( Reference Berrington de Gonzalez, Spencer and Bueno-de-Mesquita 61 , Reference Pischon, Lahmann and Boeing 62 ), waist circumference was not part of the WCRF/AICR recommendations. However, additional analyses including waist circumference in the WCRF/AICR score instead of BMI produced very similar results.
In conclusion, adherence to the WCRF/AICR recommendations was not related to the risk of BC in the CAMA study. However, after exclusion of BMI from the original index, a statistically significant inverse association between the WCRF/AICR score and BC risk was observed. Further large prospective studies are required to clarify the role and relevance of adherence to the WCRF/AICR recommendations, and the role of adiposity, on BC risk in the Mexican population.
Acknowledgements
Acknowledgements: The authors would like to acknowledge the funders for the financial support provided for this work and deeply thank all physicians responsible for the project in the different participating hospitals: Dr Germán Castelazo (IMSS, Hospital de la Raza, Ciudad de México, DF), Dr Sinhué Barroso Bravo (IMSS, Hospital siglo XXI, Ciudad de México, DF), Dr Fernando Mainero Ratchelous (IMSS, Hospital de Gineco-Obstetricia No 4 ‘Luis Castelaco Ayala’,’ Ciudad de México, DF), Dr Hernando Miranda Hernández (SS, Hospital General de México, Ciudad de México, DF), Dr Joaquín Zarco Méndez (ISSSTE, Hospital 20 de Noviembre, Ciudad de México, DF), Dr Edelmiro Pérez Rodríguez (Hospital Universitario, Monterrey, Nuevo León), Dr Jesús Pablo Esparza Cano (IMSS, Hospital No. 23 de Ginecologìa, Monterrey, Nuevo León), Dr Heriberto Fabela (IMSS, Hospital No. 23 de Ginecologìa, Monterrey, Nuevo León), Dr José Pulido Rodríguez (SS, Hospital Metropolitano Dr ‘Bernardo Sepulveda’, Monterrey, Nuevo León), Dr Manuel de Jesús García Solis (SS, Hospital Metropolitano Dr ‘Bernardo Sepulveda’, Monterrey, Nuevo León), Dr Fausto Hernández Morales (ISSSTE, Hospital General, Veracruz, Veracruz), Dr Pedro Coronel Brizio (SS, Centro Estatal de Cancerología ‘Dr Miguel Dorantes Mesa’, Xalapa, Veracruz), Dr Vicente A. Saldaña Quiroz (IMSS, Hospital Gineco-Pediatría No 71, Veracruz, Veracruz) and M.C. Teresa Shamah Levy (INSP, Cuernavaca, Morelos). Financial support: This work was supported by Consejo Nacional de Ciencia y Tecnologia (CONACyT; grant number 2002-C01-7462) and the National Institutes of Health (grant number 1U54CA13238). The funding organizations had no role in design and conduct of the study; collection, management, analysis and interpretation of the data; or preparation, review and approval of the manuscript. Conflict of interest: None. Author contributions: I.R. initiated, acquired the main funding and designed this investigation. A.F. conducted the statistical analysis under supervision of P.F. A.F. drafted the first version of the manuscript with important contributions from P.F. and I.R. All authors were involved with collection of data, data interpretation, critical revisions of the paper, and approval of the final version. I.R. had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Ethics of human subject participation: The study protocol and data collection instruments were reviewed and approved by the Institutional Review Board at the National Institute of Public Health. Cases and controls provided written informed consent to participate in the study.








