In accordance with the WHO, Health Canada’s Nutrition for Healthy Term Infants (NHTI) recommends that infants be exclusively breast-fed during the first 6 months of life( 1 , 2 ). These recommendations are based on a large body of scientific evidence which indicates that exclusive breast-feeding during this critical period promotes optimal nutrition, immunological protection and growth of the developing infant( 1 , 3 ). It is also advised that complementary foods, i.e. solid foods and liquids other than breast milk or formula, be avoided during the first 6 months of an infant’s life( 1 , 2 ). The NHTI provides clear recommendations for both the timing of complementary feeding generally and the order in which food groups should be introduced. For example, NHTI recommendations outline optimal first foods and foods to be delayed( 3 ). Introducing complementary foods before 6 months may increase the risk of adverse health outcomes including diabetes, unhealthy weight status and eczema( Reference Moss and Yeaton 4 – Reference Gaffney, Kitsantas and Cheema 7 ). Many studies indicate that breast-fed infants may have a lowered likelihood of obesity later in life( Reference Moss and Yeaton 4 , Reference Huh, Rifas-Shiman and Taveras 8 , Reference Yan, Liu and Zhu 9 ), but some inconsistent findings indicate the need for further investigation( Reference Yan, Liu and Zhu 9 , Reference Vehapoglu, Yazici and Demir 10 ). Despite potential health risks, several Canadian and international studies have reported that infants are frequently introduced to complementary foods before 6 months( Reference Briefel, Reidy and Karwe 11 – Reference Tatone-Tokuda, Dubois and Girard 14 ).
It is important to understand which of the NHTI recommendations require increased support in order for health professionals to best inform caregivers and most effectively design programmes. The public health literature addresses only a few of the published recommendations; in particular, exclusive breast-feeding and the timing of complementary food introduction. Many studies demonstrate low rates of exclusive breast-feeding in Canada and other countries, and that both exclusive and partial breast-feeding are associated with later introduction to complementary foods( 3 , Reference Briefel, Reidy and Karwe 11 , Reference Caton, Ahern and Hetherington 15 – Reference Wright, Parkinson and Drewett 24 ) while formula feeding is associated with earlier introduction to complementary foods( Reference Caton, Ahern and Hetherington 15 , Reference Giovannini, Riva and Banderali 18 , Reference Schiess, Grote and Scaglioni 25 – Reference Scott, Binns and Graham 27 ). Fewer studies are available regarding, for example, vitamin D supplementation, the importance of introducing iron-rich foods first and delaying the introduction of honey and certain milks. There is also limited understanding of the extent to which breast-feeding at 6 months, with or without formula supplementation, may be linked to these recommendations. Exploring these issues, as well as gaining an understanding of the order in which particular foods are introduced by breast-feeding and non-breast-feeding mothers, would allow public health initiatives to be tailored to mothers who would most benefit from support.
The current study aimed to address these questions by investigating adherence to the NHTI recommendations, in place at the time of the study, in a sample of mothers from the Kingston, Frontenac, and Lennox & Addington (KFL&A) region of Ontario, Canada( 28 ). Mothers who breast-fed to any degree at 6 months and those who exclusively formula-fed were compared with respect to feeding practices and adherence to NHTI recommendations. This comparison was selected over exclusive to non-exclusive breast-feeders to produce results that have more generalizable implications, as low rates of exclusive breast-feeding rates at 6 months are observed in the local maternal population. Similar classification schemes have been used in other research, recognizing that there are immunological benefits to even partial breast-feeding( Reference Yan, Liu and Zhu 9 , Reference Caton, Ahern and Hetherington 15 ). To ensure a comprehensive approach in summarizing the relationship between breast-feeding and complementary food introduction, and to control for known confounders, confirmatory analyses were conducted to examine if partial breast-feeding delays the introduction of complementary foods. The current study also examined changes in breast-feeding rates and the sources of infant feeding information that parents accessed as a means to examine potential timing and sources of interventions to promote NHTI recommendations. This thereby enables us to discuss implications directly relevant to public health. Thus, the primary objectives were to:
-
1. determine the extent to which breast-feeding and non-breast-feeding mothers were following the NHTI recommendations in place at the time of the Infant Feeding Survey;
-
2. describe first complementary foods and investigate differences by breast-feeding status;
-
3. confirm whether any breast-feeding is associated with earlier introduction to complementary foods relative to non-breast-feeding, after controlling for potentially confounding factors; and
-
4. determine needs for improvements in timing and resources of interventions by examining breast-feeding rates over time and information sources used by mothers.
Materials and methods
Study design
Data used in the present study came from a subset of mothers who completed the Infant Feeding Survey, which was conducted in 2008 in the KFL&A region of Ontario, Canada. Maternal characteristics, attitudes and behaviours surrounding infant feeding practices were examined. Women were eligible for the study if they gave birth to a live infant of at least 36 weeks’ gestation and a birth weight of at least 1500 g at Kingston General Hospital between 1 January 2008 and 31 July 2008. Mothers whose infants were admitted to the neonatal intensive care unit were approached to participate in the survey only after their baby was well enough to be discharged from hospital. Only the first born of multiples was included. Of the 1055 women who gave birth to a live infant of eligible gestation and birth weight, 463 (43·9 %) met the Infant Feeding Survey inclusion criteria and agreed to participate, with 325 (70·2 %) of these mothers providing sufficient information regarding the introduction of complementary foods to be included in the current analysis. All mothers provided informed consent prior to participation. Mothers were asked to complete the initial survey at the end of their hospital stay and were subsequently interviewed by telephone at 2, 4, 6 and 12 months after birth. Follow-up information was linked to the in-hospital survey results to provide individual records for each mother. The study was approved by the Queen’s University and Associated Teaching Hospitals Research Ethics Board. All measures came from the KFL&A Public Health Infant Feeding Survey( 29 ).
Introduction to complementary foods
Complementary foods were addressed by a twenty-nine-item food questionnaire developed by the Reproductive Health Team at KFL&A Public Health, which is comprised of public health nurses, managers and a public health dietitian. Mothers were asked at all time points in the study whether or not they had given their infant any of the specified food or drink items more than once. When responses were affirmative, mothers were asked the age of the infant when the item was introduced. Timing of introduction was coded in weeks; for coding purposes each month was considered to be four weeks.
Breast-feeding status
Breast-feeding status was categorized as ‘any breast-feeding’ or ‘non-breast-feeding’. Mothers were considered to be in the any breast-feeding group if they were feeding breast milk to their infant at 6 months, regardless of whether they were exclusively breast-feeding or supplementing with formula and/or complementary foods. The non-breast-feeding group consisted of women exclusively formula-feeding their infant at 6 months, with or without feeding complementary foods. Breast-feeding status was measured at each follow-up interview and breast-feeding trends over the study period were used to address Objective 4.
Covariates and other measures
Covariates used during model building included the mothers’ age (15–24, 25–34, ≥35 years), education (high school or less, some education after high school, college or university diploma or degree), household income (≤$CAN 39 999, $CAN 40 000–79 999, ≥$CAN 80 000), smoking during pregnancy (yes, no), attendance at a breast-feeding or prenatal class (yes, no), parity (1, ≥2), prior breast-feeding status (yes or no to breast-feeding a previous child), infant birth weight (1500–2499 g, 2500–3999 g, ≥4000 g) and infant admission to an intensive care unit (yes, no). Given the large number of missing values, ‘prior breast-feeding’ was excluded from the analysis.
Foods were categorized into groups for analysis. ‘Infant cereals’ included rice, barley, oat or wheat infant cereals or Pablums™. ‘Other grains’ included cooked cereals such as oatmeal, teething biscuits, bread, crackers, breadsticks or unsweetened breakfast cereals, rice and pasta. ‘Vegetables and fruit’ included vegetables and fruit that were fresh, frozen or in jars or cans, and diluted or undiluted fruit juice. ‘Whole milks’ referred to whole milk only. ‘Other milks’ included 2 % fat, 1 % fat, skimmed and chocolate milks, and evaporated, soya and rice milks. ‘Milk products’ included milk products such as cheese and yoghurt. ‘Meats and alternatives’ included meats and alternatives such as fish, beans and tofu, and mixed dishes containing meats (stews). Eggs are not included here as the survey did not capture the timing of introduction of this food item. ‘Foods not recommended’ were foods not recommended by the version of Canada’s Food Guide (CFG) in place in 2008( 30 ). These foods included sugary drinks such as soft drinks, Kool-Aid® and artificial fruit drinks, processed meats such as hot dogs, and salty or sweet foods like potato chips, candy or chocolate. There is no age at which the foods in this final category are recommended; these foods have little or no nutritional value and may be high in sugar or salt. Infants have high energy needs and small stomachs, and their hunger should not be satiated by foods that do not promote optimal growth and development( Reference Klein and Moeschberger 31 ).
Mothers reported the sources they used most frequently to gather information on introducing complementary foods to their infant, as well as the sources they considered most useful. The top five sources accessed and the five rated as most useful for information on introducing complementary foods were used to address Objective 4.
Statistical analysis
Analyses to address Objectives 1 and 2 included frequencies, cross-tabulations, means and proportions. Then χ 2 tests and one-tailed t tests were conducted to test for significant differences in proportions or means between any breast-feeding and non-breast-feeding groups. For Objective 3, Cox proportional hazards models were used to examine the association between breast-feeding status and time to introduction of complementary foods, while controlling for confounding characteristics. A mother’s breast-feeding status can change from any breast-feeding to non-breast-feeding at any time during the follow-up period and thus breast-feeding status was treated as a time-dependent variable. One advantage of using a Cox proportional hazards model is that it allows time-dependent variables as covariates in the model. Even though the proportional hazards assumption is no longer valid with the introduction of time-dependent variables in Cox’s model, its estimation method still provides valid estimates( 32 ). Thus, this model can be used to assess the effect of the time-dependent breast-feeding status on time to introduction of complementary foods. Using a critical value of P=0·25, the sociodemographic, infant and maternal characteristics to be included in the final model were determined. Based on this criterion, infant admission to an intensive care unit, household income and maternal education level were not included in the final model. The final Cox proportional hazards model examines the association between breast-feeding status (as a time-varying covariate) and time to introduction of complementary foods after controlling for mother’s age, parity, smoking history, attendance at prenatal classes and infant birth weight. Hazard ratios and 95 % confidence intervals are reported and a P value of <0·05 was considered statistically significant. To investigate Objective 4, life-table analyses and frequencies were conducted to examine breast-feeding trends and reported sources of infant feeding information, respectively. All statistical analyses were conducted using the statistical software package Stata version 11 (2009).
Results
Study sample
Participant retention for the Infant Feeding Survey was 82 % (n 379) at 2 months, 75 % (n 348) at 4 months, 71 % (n 330) at 6 months and 65 % (n 302) at 12 months. For the purposes of the present paper, mothers who gave adequate information on the introduction of complementary foods to their infant on one of the 4-, 6- or 12-month surveys were included in the analysis (n 325). To be included, information regarding the introduction of both iron-rich foods and other foods recommended by CFG was required.
Characteristics of the study mothers are described in Table 1. Due to some missing responses, sample sizes are presented separately for each variable in the table. Two-thirds of participating mothers were between the ages of 25 and 34 years (66·6 %), the majority had at least some post-secondary education (89·1 %) and 46·9 % reported an annual household income level in excess of $CAN 80 000. Approximately half of the sample had attended a breast-feeding or prenatal class during pregnancy (48·3 %) and were first-time mothers (49·2 %). The rate of smoking during pregnancy in the current sample was 9·7 %. A total of 7·8 % of infants were reported by their mothers to be admitted to the intensive care unit, and the majority of infants in the sample (82·3 %) weighed between 2500 and 3999 g at birth. Just over half of mothers, 51·2 %, were providing at least some breast milk to their infant at 6 months, but only 6·8 % of mothers were exclusively breast-feeding at this time (Table 2). Mothers who were in the any breast-feeding group at 6 months were older, more educated, had a higher total household income, had lower smoking rates, and were more likely to have attended prenatal or breast-feeding classes than mothers who were not breast-feeding at 6 months.
Table 1 Characteristics of the study sample according to breast-feeding status; Kingston, Frontenac, and Lennox & Addington (KFL&A) Infant Feeding Survey, Ontario, Canada, 2008
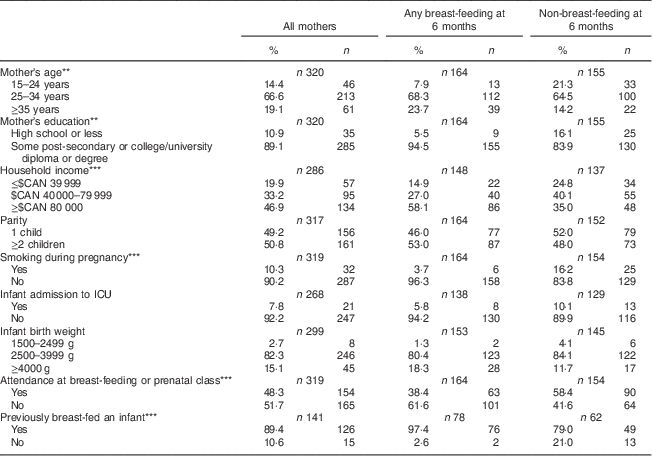
ICU, intensive care unit.
Significant difference between groups: **P<0·01, ***P<0·001.
Table 2 Proportion of mothers who followed the NHTI recommendations( 28 ) according to breast-feeding status; Kingston, Frontenac, and Lennox & Addington (KFL&A) Infant Feeding Survey, Ontario, Canada, 2008
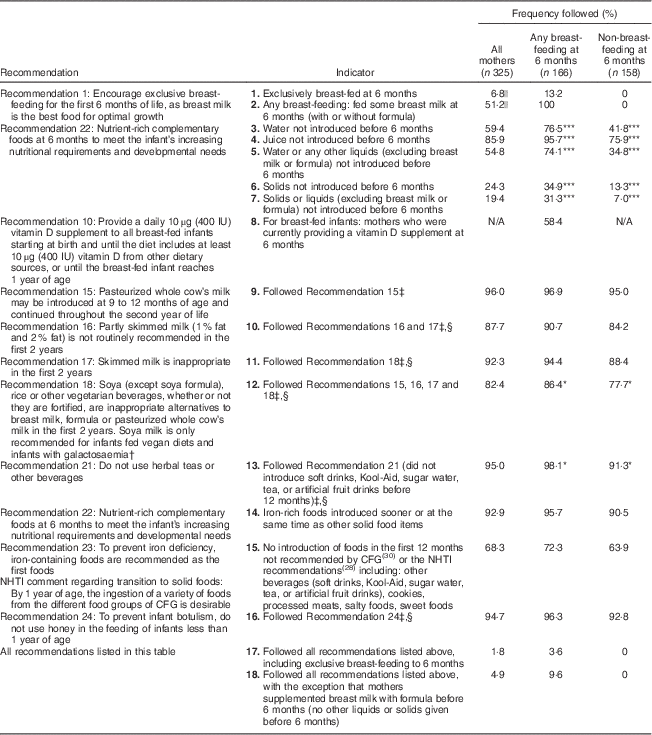
NHTI, Nutrition for Healthy Term Infants; CFG, Canada’s Food Guide; N/A, not applicable. Significant difference between groups: *P<0·05, ***P<0·001.
† Note that the most recent recommendations recognize that a soya-based commercial infant formula may be given for cultural, religious or health reasons( Reference Klein and Moeschberger 31 ).
‡ The denominator is 301 as twenty-four mothers did not complete the 12 month survey and thus foods given between 6 and 12 months could not be determined.
§ Although other milks are not recommended for the first 2 years of life, the Infant Feeding survey covered only the first 12 months.
|| The denominator is 324 as one mother’s breast-feeding status could not be defined at 6 months.
Objective 1: Determine the extent to which breast-feeding and non-breast-feeding mothers were following the Nutrition for Healthy Term Infants recommendations at the time of the Infant Feeding Survey
Table 2 presents the proportion of mothers who followed the NHTI recommendations that could be addressed using the Infant Feeding Survey data. Proportions are provided for the whole sample, as well as by the breast-feeding status of the mother. Since data for the current study were collected in 2008, NHTI recommendations available to mothers in 2008 were included in the table( 28 ). The proportion of mothers who adhered to the recommendations ranged from 1·8 % to 96·0 %, depending on the particular recommendation(s) under investigation. Only six mothers (1·8 %) followed all the recommendations listed in Table 2. Ten mothers (4·9 %) followed all the recommendations, except that they supplemented with formula prior to 6 months. Twenty-two mothers (6·8 %) exclusively breast-fed to 6 months.
With regard to the overall introduction of solids and liquids, 19·4 % of mothers followed Recommendation 22 and did not introduce complementary foods before 6 months. One-quarter (24·3 %) of the sample did not introduce solids before 6 months, and just over half (54·8 %) did not provide their infant with other liquids before 6 months. Most women followed the recommended order of complementary foods, with iron-rich foods preceding or offered in the same week as other recommended foods, regardless of the age at which these foods were introduced (92·9 %).
Mothers who were not breast-feeding at 6 months were significantly more likely than breast-feeding mothers to introduce complementary foods prior to the recommended age of 6 months. Specifically, they were more likely to introduce water and juice before 6 months, to give their infants beverages not recommended in the NHTI, to provide inappropriate types of milk within the first year, to provide infant cereals, vegetables and fruit before 6 months, and to give foods not recommended by CFG (e.g. foods that are high in sugar and salt). No other statistically significant differences in following the recommendations emerged between breast-feeding and non-breast-feeding mothers.
Other than exclusive breast-feeding, recommendations with the lowest adherence included supplementing with vitamin D for breast-fed infants at 6 months (58·4 % of breast-feeding mothers complied), avoiding foods not recommended in the CFG (e.g. soft drinks, cookies, processed meats, salty or sweet snacks; 68·3 % of mothers complied) and providing types of milk appropriate to the infant’s age (82·4 % of mothers complied).
Objective 2: Describe first complementary foods and investigate differences by breast-feeding status
Figure 1 shows the distribution of infant age at which liquids only, solids only, and either liquids or solids were first introduced. Figure 2 provides a graphical representation of the introduction of complementary foods by food group and the breast-feeding status of the mother. In Figs 1 and 2 the ‘first’ introduction refers to the time at which infants were introduced more than once to foods in each food group. Mothers who were not breast-feeding at 6 months introduced complementary foods significantly earlier (median introduction=16 weeks) than mothers who were breast-feeding to any extent at 6 months (median introduction=22 weeks, P<0·001; Table 3). Results in Fig. 2 indicate that mothers who were in the any breast-feeding group at 6 months introduced other grains, vegetables and fruit, water and foods not recommended in CFG significantly later than women who were not breast-feeding at 6 months. It is noteworthy that several women introduced solids or liquids before their infant was 2 months of age, more than 4 months earlier than recommended.

Fig. 1 Distribution of infant age at which liquids only (![]() ), solids only (
), solids only (![]() ), and either liquids or solids (
), and either liquids or solids (![]() ) were first introduced; Kingston, Frontenac, and Lennox & Addington (KFL&A) Infant Feeding Survey, Ontario, Canada, 2008
) were first introduced; Kingston, Frontenac, and Lennox & Addington (KFL&A) Infant Feeding Survey, Ontario, Canada, 2008
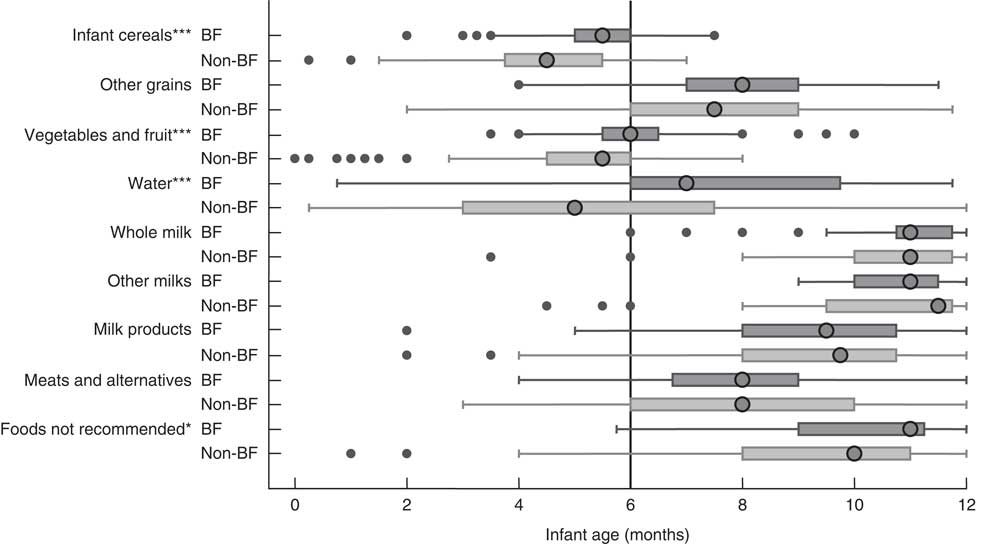
Fig. 2 First introduction to complementary foods by breast-feeding (BF) status (![]() , BF;
, BF; ![]() , non-BF) at 6 months; Kingston, Frontenac, and Lennox & Addington (KFL&A) Infant Feeding Survey, Ontario, Canada, 2008. Data are presented as box-and-whisker plots in which the large circle indicates the median age at first introduction; the left and right edge of the box represent the 25th and 75th percentile, respectively (i.e. interquartile range); the left and right whisker represent the minimum and maximum age at first introduction, respectively; and small dots indicate the outliers. Non-BF includes mothers who were not breast-feeding when infant was 6 months old and BF includes mothers who were breast-feeding to any extent (‘any breast-feeding’) when infant was 6 months old. Significant difference between groups: *P<0·05, ***P<0·001
, non-BF) at 6 months; Kingston, Frontenac, and Lennox & Addington (KFL&A) Infant Feeding Survey, Ontario, Canada, 2008. Data are presented as box-and-whisker plots in which the large circle indicates the median age at first introduction; the left and right edge of the box represent the 25th and 75th percentile, respectively (i.e. interquartile range); the left and right whisker represent the minimum and maximum age at first introduction, respectively; and small dots indicate the outliers. Non-BF includes mothers who were not breast-feeding when infant was 6 months old and BF includes mothers who were breast-feeding to any extent (‘any breast-feeding’) when infant was 6 months old. Significant difference between groups: *P<0·05, ***P<0·001
Table 3 Median time of first introduction of solids and liquids by breast-feeding status at 6 months; Kingston, Frontenac, and Lennox & Addington (KFL&A) Infant Feeding Survey, Ontario, Canada, 2008
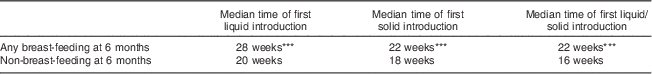
Significant difference between groups: ***P<0·001.
Objective 3: Examine whether any breast-feeding is associated with earlier introduction to complementary foods relative to non-breast-feeding, after controlling for potentially confounding factors
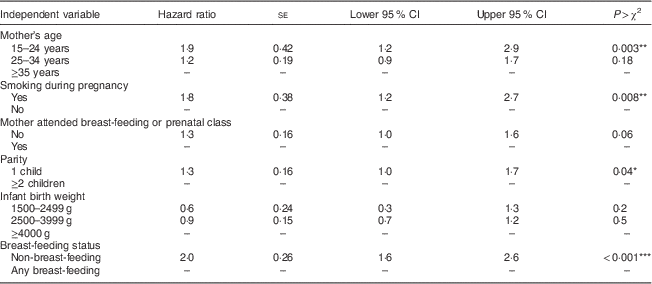
Table 4 presents the results of a Cox proportional hazards model examining the association between time to introduction of complementary foods and breast-feeding status, while controlling for infant birth weight and maternal age, parity, smoking and prenatal/breast-feeding class attendance. Results indicate that at any time point prior to 6 months for mothers who had not already introduced complementary foods to their infants, non-breast-feeding mothers were twice as likely as breast-feeding mothers to introduce complementary foods (hazard ratio=2·0, 95 % CI 1·6, 2·6, P<0·001). In addition, younger mothers (hazard ratio=1·9, 95 % CI 1·2, 2·9, P=0·003), first-time mothers (hazard ratio=1·3, 95 % CI 1·0, 1·7, P=0·04) and mothers who smoked (hazard ratio=1·8, 95 % CI 1·2, 2·7, P=0·008) introduced complementary foods earlier, regardless of breast-feeding status.
Table 4 Cox proportional hazards model examining associations of breast-feeding status with time to introduction to complementary foods in infants prior to age 6 months, after controlling for other variables (n 325); Kingston, Frontenac, and Lennox & Addington (KFL&A) Infant Feeding Survey, Ontario, Canada, 2008
– indicative of comparison group.
*P<0·05, **P<0·01, ***P<0·001.
Objective 4: Determine needs for improvements in timing and resources of interventions by examining breast-feeding rates over time and information sources used by mothers
Earlier results indicated that the timing of the introduction of complementary foods is linked to breast-feeding status. Breast-feeding trends were, therefore, examined to provide insight into the optimal time to provide additional support to families with new infants. Trends during the first 6 months after birth can be seen in Fig. 3. Results indicate that the percentage of mothers exclusively breast-feeding dropped sharply in the first 2 months. Rates of exclusive breast-feeding dropped from 80·2 % at discharge to 30·6 % at 2 months, and rates of any breast-feeding dropped from 89·2 % to 63·9 %. The percentage of women in the any breast-feeding group remained fairly steady from 2 to 6 months (63·9 % to 51·5 %). Efforts to promote both exclusive and any breast-feeding should, therefore, be geared toward mothers with infants younger than 2 months.
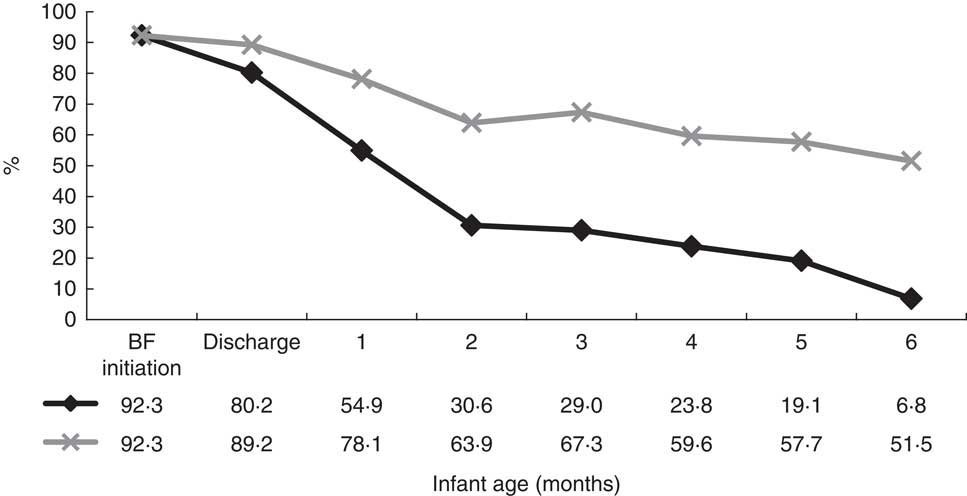
Fig. 3 Breast-feeding trends during the first 6 months after birth (![]() , exclusive breast-feeding from birth;
, exclusive breast-feeding from birth; ![]() , any breast-feeding); Kingston, Frontenac, and Lennox & Addington (KFL&A) Infant Feeding Survey, Ontario, Canada, 2008
, any breast-feeding); Kingston, Frontenac, and Lennox & Addington (KFL&A) Infant Feeding Survey, Ontario, Canada, 2008
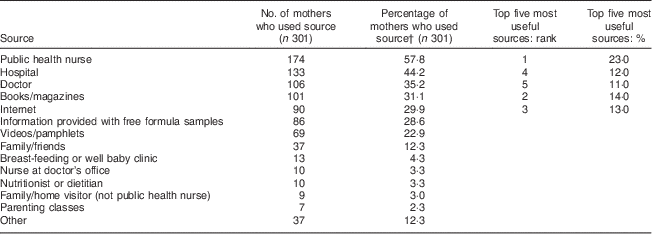
The sources most often used by mothers to obtain information on introducing complementary foods were investigated to determine potential avenues for public health intervention. Public health nurses were reported most frequently (57·8 %), followed by hospitals (44·2 %), doctors (35·2 %), books/magazines (31·1 %) and the Internet (29·9 %). Mothers in the any breast-feeding group were more likely than mothers in the non-breast-feeding group to use public health nurses and books to access information regarding introduction to complementary foods. When asked which sources were most useful, the top five were ranked as follows: (i) public health nurses (23·0 %); (ii) books/magazines (14·0 %); (iii) the Internet (13·0 %); (iv) hospitals (12·0 %); and (v) doctors (11·0 %; Table 5). No differences in the usefulness of resources emerged between breast-feeding and non-breast-feeding mothers. Therefore, these sources appear to be most promising when deciding where to direct interventions to promote NHTI recommendations.
Table 5 Sources mothers used and ranked as most useful to obtain information on introducing complementary foods to their infant; Kingston, Frontenac, and Lennox & Addington (KFL&A) Infant Feeding Survey, Ontario, Canada, 2008
† Mothers were allowed to provide more than one option, thus the percentages total greater than 100 %. The percentage shown uses the denominator of all mothers who answered this question.
Discussion
In the present study, fewer than 2 % of mothers followed all NHTI recommendations and only 19·4 % of mothers waited until 6 months to introduce complementary foods. The latter finding is supported by previous work( Reference Briefel, Reidy and Karwe 11 , Reference Tatone-Tokuda, Dubois and Girard 14 , Reference Scott, Binns and Graham 27 ). The present study adds to this body of literature, however, by investigating other, less commonly addressed NHTI recommendations. For example, 45·2 % of mothers introduced liquids other than breast milk or formula (most commonly water or fruit juices) before 6 months. These other liquids may compromise adequate intake of nutrients and increase health-related risks, including dental caries and nursing bottle syndrome( 28 ). Results also indicate that almost a third of mothers introduced complementary food items not listed in the CFG to their infant before 1 year of age. The present study did not assess the quantity of these food items fed to infants, but it is recommended that foods with high salt and sugar content be avoided, as they may interfere with the nutritious energy-dense foods needed for rapid growth. Limiting these foods items also allows the infant to experience natural food flavours and helps to promote lifelong eating habits( Reference Klein and Moeschberger 31 ). Lastly, over 40 % of breast-fed infants were not supplemented with vitamin D, which is lower than reported in previous studies( Reference Gallo, Jean-Philippe and Rodd 33 , Reference Crocker, Green and Barr 34 ), and necessary for infant health as breast milk is not a dependable source of this nutrient( 28 ).
Despite low adherence to several recommendations, it is promising that some foods are introduced in the appropriate order, with iron-fortified foods (i.e. infant cereals) fed first or in the same week as other foods. Introducing iron-rich foods first is encouraged to promote infant growth and development, and to reduce the risk of long-term health effects caused by iron deficiency in infancy( 3 ). More effort is needed to promote the earlier introduction of meat and meat alternatives, since these were not common first foods. Meat and meat alternatives are advised alongside iron-fortified cereals due to their high iron content and the benefits obtained from haem iron contained in meat, poultry and fish( 3 ).
No previous research, to our knowledge, has examined differences in the introduction of various foods by breast-feeding status. Consistent with other studies, vegetables and fruit (including juice), water and infant cereals were introduced earliest to all infants( Reference Friel, Hanning and Isaak 12 , Reference Grummer-Strawn, Scanlon and Fein 35 ). However, it is noteworthy that the present study found that mothers who did not breast-feed to 6 months introduced these food items sooner than mothers breast-feeding at 6 months. The early introduction of water, especially prior to other foods and liquids, has important implications, since it offers no nutritional value and, similar to other foods, may satiate the infant and reduce intake of breast milk. In addition, mothers who did not breast-feed at 6 months were more likely to provide their infants with non-recommended beverages and inappropriate types of milk within the first year. They also provided foods that are high in sugar and salt significantly earlier than mothers who breast-fed to any extent at 6 months. These foods should be avoided due to their low nutritional value and to help the infant establish lifelong healthy eating habits( Reference Grummer-Strawn, Scanlon and Fein 35 ). No significant differences were found between breast-feeding and non-breast-feeding mothers with regard to the other food categories. Thus, not all food groups are introduced earlier in mothers who do not breast-feed at 6 months, and special attention must be paid to delaying the introduction and frequency of these particular liquids and solids in infants of non-breast-feeding mothers.
Overall, mothers who did not breast-feed to 6 months introduced complementary foods to their infants significantly earlier than mothers who breast-fed to any extent at 6 months, even when accounting for sociodemographic, infant and maternal characteristics. Previous research has also reported breast-feeding as an indicator of the timelier introduction of complementary foods( Reference Briefel, Reidy and Karwe 11 , Reference Caton, Ahern and Hetherington 15 – Reference Wright, Parkinson and Drewett 24 ). The control of confounders was critical to the interpretation of results to ensure that differences observed by breast-feeding status could not be explained by known confounders in this particular sample. Since the present study was observational, not a controlled experiment, the possibility that the relationship revealed may be subject to residual confounding by unobserved sociodemographic factors cannot be completely ruled out. There is not, however, concrete evidence of existence of such sociodemographic factors that may confound the relationship based on a review of the literature on this topic. Without knowing the reasons for initiating complementary foods in the study, it is difficult to explain this association. A recent study investigated whether breast-feeding and formula-feeding mothers differed in their reasons for introducing complementary foods and found that mothers who formula feed may be starting complementary foods earlier on the advice of health-care professionals( Reference Clayton, Li and Perrine 36 ). This suggests that women who formula feed need to be made aware that formula is adequate nutrition until their infant is 6 months of age.
In examining breast-feeding trends and the introduction of complementary foods, it is evident that greater support for mothers is needed prior to 2 months in order to promote exclusive breast-feeding, and from 2 to 4 months in order to promote the delayed introduction to complementary foods. The greatest decline in rates of exclusive and any breast-feeding were seen prior to 2 months in the current sample whereas complementary foods were introduced among non-breast-feeding and breast-feeding mothers at approximately 4 months and 5·5 months, respectively. Previous research has reported similar timing in terms of declines in breast-feeding( Reference Brown, Dodds and Attenborough 37 ) and differences in early introduction to complementary foods by breast-feeding status( Reference Scott, Binns and Graham 27 , Reference Clayton, Li and Perrine 36 ). It may be useful to target sources already used by mothers to access information regarding introducing complementary foods. Mothers reported using public health nurses, hospitals and doctors most often to gain this information, and also cited these sources as very useful. Mothers who breast-fed to any extent at 6 months were more likely than non-breast-feeding mothers to report use of public health nurses and books to gain information. Of potential concern is that previous research indicates that mothers may be receiving unclear or inconsistent advice from health-care professionals regarding infant feeding recommendations( Reference Arden 38 ). It is imperative that health-care professionals provide consistent, evidence-based and up-to-date information, and that they be educated on ways to encourage mothers to overcome barriers to adherence( Reference Arden 38 ). Effort should be made to ensure that support from public health nurses continues to be available to mothers after they stop breast-feeding.
It should be noted that in order for a mother to follow the NHTI recommendations, she must be aware of and agree with them( Reference Caton, Ahern and Hetherington 15 , Reference Scott, Binns and Graham 27 ). There are a number of programmes and services that public health can offer to increase mothers’ knowledge, agreement and self-efficacy in adhering to the recommendations, and several health units across Ontario do so. For example, KFL&A Public Health offers breast-feeding clinics, ‘introduction to solids’ classes and programmes to support families from the prenatal period until the transition to school. Greater effort is needed to ensure that other NHTI recommendations, such as vitamin D supplementation, are also promoted through public health. As far as timing of programmes and services, early infancy appears to be a critical period to promote adherence to recommendations. Effort should be made to ensure higher enrolment in existing programmes by reducing barriers to access and targeting vulnerable populations. As indicated by the present results, for example, interventions should target mothers who do not breast-feed to any extent, are younger, smoke during pregnancy, are first-time mothers and have not attended a prenatal class. Finally, it is important to foster a primary care and public health partnership to ensure consistent messaging across health settings and to assist in addressing the educational and resource needs of primary care. It is likely that combined support and resources from public health, primary care and other health services will be needed to increase knowledge, skill level, self-efficacy and adherence to the NHTI recommendations.
Limitations
Several limitations for the present study should be noted. First, Infant Feeding Survey participants were older, more educated and of higher socio-economic status than the general population of women giving birth in the region. Previous research indicates that mothers with these characteristics are more likely to breast-feed and to follow infant feeding recommendations( Reference Briefel, Reidy and Karwe 11 , Reference Coleman, Gutmanis and Larsen 16 , Reference Schiess, Grote and Scaglioni 25 , Reference Scott, Binns and Graham 27 , Reference Alder, Williams and Anderson 39 , Reference Pfluger, Winkler and Hummel 40 ). This suggests that the proportion of mothers who do follow the infant feeding recommendations is less than that found in the current study, and therefore the need for guidance and support in following the recommendations is expected to be even greater than is evident here.
Second, the sample size was relatively small and almost 30 % of the initial study sample was lost to follow-up at 6 months. Of the 463 mothers who met the inclusion criteria and agreed to participate at the beginning of the Infant Feeding Survey, only 325 mothers provided adequate data for the current analyses. The small sample size prohibited us from classifying breast-feeding type into exclusive breast-feeding, mixed feeding and formula only. Instead, ‘any breast-feeding’ and ‘non-breast-feeding’ categories were used.
Third, introduction of complementary foods and breast-feeding status were measured using self-report, thereby introducing the potential for reporting bias. The questionnaire items that assessed introduction of complementary foods were developed by the Reproductive Health Team, including a public health dietitian, at KFL&A Public Health, but its validity was not evaluated.
Fourth, there are some limitations associated with using NHTI’s recommended 6-month cut-off for introducing complementary foods. Although 6 months is a guideline, it is recognized that some infants may safely be ready for complementary foods slightly earlier or later than this( 28 ). It is unclear what the clinical implications are of introducing foods slightly before the recommended 6-month period (e.g. at 5·5 months) or introducing non-recommended foods in very small quantities before the recommended age. More work is needed to investigate the implications of not following recommendations exactly.
Lastly, the Infant Feeding Survey did not ask why women initiated complementary foods; thus, we are unable to examine the reasons for mothers’ decisions to introduce complementary foods early. Future research could be conducted in this area to help determine effective interventions.
Conclusion
There is evidence of a significant need for public health efforts to encourage optimal infant feeding. By examining the recommendations individually, and comparing feeding practices by breast-feeding status, we were able to highlight certain recommendations and target populations that should receive extra attention through public health initiatives. Encouragement of breast-feeding and introducing foods at the recommended times are important, but several other NHTI recommendations also require attention. Both breast-feeding and non-breast-feeding mothers are in need of support, and it is suggested that interventions target mothers with infants less than 2 months of age and through common points of access.
Acknowledgements
Acknowledgements: The authors would like to acknowledge Tracy McDonough, a public health dietitian at KFL&A Public Health, for providing insight into implications from study results and feedback on manuscript drafts. Financial support: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors. Conflict of interest: None. Authorship: S.F., K.S.O. and E.B. formulated the research questions. S.F. and Y.P. analysed the data. E.B., S.F. and K.S.O. wrote the first draft of the article. All authors edited and contributed to the final draft. Ethics of human subject participation: This study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects/patients were approved by the Queen’s University and Associated Teaching Hospitals Research Ethics Board.











