According to recent report released by UNICEF, almost half of all deaths in children under 5 years are attributable to poor nutritional status. Malnutrition is not a direct cause of death, but the vicious cycle of infection and undernutrition increases the chances of death from common infections by delaying the recovery and increasing the frequency and severity of infection(1).
India has one of the highest rates of child malnutrition and mortality among children under 5 years of age in the world. Malnutrition is estimated to contribute to almost 68·2 % of the total children under 5 years deaths and 17·3 % of the total disability-adjusted life years in India(2). Anthropometric data from the Comprehensive National Nutrition Survey (CNNS) – 2019 documented suboptimal level of nutrition in 0–5 years age group with one-third (33 per cent) children being underweight (10 % severe), 35 per cent being stunted (13 % severe), 17 per cent being wasted (5 % severe) and with ∼1 % having mid-upper arm circumference (MUAC) < 115 mm (in 6–59 months age group)(3).
Childhood undernutrition is a major global public health problem, and the major focus of research and interventional strategies has been the 6–59 months age group. As breast-feeding is a good nutritional source, it is believed that severe forms of undernutrition are rare in infants under 6 months of age, but rates of exclusive breast-feeding are suboptimal throughout the world(Reference Victora, Bahl and Barros4). Apart from the dietary intake, young infants can have a variety of health problems which can have impact on nutritional status(Reference Goh, How and Ng5). Infants under 6 months of age with severe malnutrition are more vulnerable due to the physiological and immunological immaturity leading to the high morbidity and mortality rates. A meta-analysis comparing the outcome of infants with severe acute malnutrition (SAM) aged under 6 months with that of 6–60 months receiving the same treatment documented that risk of death was significantly higher in <6 months of age group (RR 1·30, 95 % CI (1·09, 1·56); P < 0·01)(Reference Grijalva-Eternod, Kerac and McGrath6). Apart from mortality, long-term effects of early-life severe malnutrition can have significant effects on adult health and wellbeing with a higher likelihood of non-communicable disease-related risks(Reference Ruemmele7–Reference Lelijveld, Seal and Wells9).
A study published in 2011 analysed the Demographic and Health Survey (DHS) datasets of twenty-one countries and estimated that globally ∼8·5 million infants under 6 months of age had wasting and among these ∼3·8 million had severe wasting(Reference Kerac, Blencowe and Grijalva-Eternod10). Subsequently, analysis of National Family Health Survey-3 (NFHS-3) data from India showed that 31 % of infants less than 6 months of age had wasting, while 13 % had severe wasting(Reference Patwari, Kumar and Beard11). A recent analysis based on NFHS-4 documented a variable prevalence of severe wasting across different Indian states, ranging from 3·5 % to 21 % (median 14·8 %) among infants less than 6 months of age(Reference Choudhary, Srivastava and Chowdhury12). As this translates into a huge number of infants aged under 6 months having severe wasting and a higher risk of mortality as compared to older children, this age group needs to be prioritised for research and targeted interventions. Limited data, mainly from African settings, are available regarding the course and outcome of severe wasting in children aged less than 6 months. Most such information is from retrospective studies describing the immediate outcome of hospitalised children(Reference Desta13–Reference Dhanalakshmi and Devi15). Follow-up studies after discharge are available mainly for older children(Reference Dhanalakshmi and Devi15,Reference Bhatnagar, Kumar and Dua16) . We conducted this study to assess the course and outcome of severe wasting during a period of 3 months following discharge and to evaluate the risk factors for unsatisfactory outcome in children below 6 months of age.
Methods
This was a prospective observational hospital-based study conducted between January 2017 and October 2018 on inpatients at a hospital attached to a medical college and catering mainly to the urban low-income population of the Eastern part of Delhi and adjoining state of Uttar Pradesh. The study was approved by the Institutional Ethics Committee. Informed consent was obtained from parents or caregivers of all the participants.
All consecutive children with severe wasting between 1 and 6 months of age, presenting to outpatient department or paediatric casualty and requiring hospitalisation, were enrolled. Severe wasting was defined as weight-for-length Z-score (WLZ) less than −3 of the median reference WHO standard(17). Infants having any structural abnormalities such as cleft lip, cleft palate or any other major malformations, those with features suggestive of inborn error of metabolism such as seizures, recurrent vomiting, prolonged jaundice, those with the presence of chronic systemic illness (congenital heart disease, chronic renal disease, neurological conditions, chronic liver disease, primary or secondary immunodeficiency, and congenital hypothyroidism) or those born to a HIV-positive mother were excluded.
At the time of enrolment, a baseline assessment of all children was done and findings were recorded in a pre-designed case record form. Detailed clinical history, including dietary, immunisation and socio-economic history, was elicited from the parents/guardian, and demographic details including residential address, occupation, educational qualification and family income were recorded. Modified Kuppuswamy Scale 2017 update was used for the classification of socio-economic status. In this scale, a composite score is calculated based on the education and occupation of the head of the family along with total monthly income of the family, and socio-economic status of urban and peri-urban population is classified into upper, upper middle, lower middle, upper lower and lower(Reference Singh, Sharma and Nagesh18).
Baseline anthropometry (weight, length and head circumference) and detailed clinical examination for ruling out other causes of malnutrition were performed, and findings were recorded in a pre-designed case record form. Anthropometric measurements were taken using standard techniques(Reference Gupta and Gupta19,20) . Z-scores were calculated using age and sex appropriate WHO standards using the WHO Anthro software (21). Relevant investigations including blood sugar, electrolytes, Hb, cultures and X-rays were conducted at admission according to the clinical diagnosis. All enrolled children were managed according to the facility-based guidelines for the management of children with SAM prescribed by the National Rural Health Mission, Ministry of Health and Family Welfare (India) 2013(22). These infants received the similar general medical care as older children with SAM, including thermoregulation, maintaining normoglycemia, correction of dehydration and dyselectrolytemia, treatment and prevention of micronutrient deficiencies, antibiotics for presumed sepsis, and appropriate treatment of other medical complications. Initial feeding approach consisted of establishing or re-establishing effective exclusive breast-feeding by mother or caregiver. Support was given to re-lactate in the form of supplement suckling approach, wherever needed. In case of no prospect or failure of above approach, feeding with F-75 was started.
Outcomes were assessed as immediate outcome, that is, at discharge from hospital and overall outcome after 3 months of discharge (approximately 100 d from admission). Infants were discharged if all clinical conditions or medical complications, including oedema, were resolved; the infant had good appetite and was clinically well and alert; and weight gain on either exclusive breast-feeding or replacement feeding was satisfactory, that is, more than 5 g/kg/d for three successive days. Children received any pending immunisation and treatment of any comorbidity before discharge.
At discharge, mothers were counselled for dietary rehabilitation and childcare and advised to maintain a regular fortnightly follow-up with the hospital for 3 months. They were provided with the telephone numbers of the investigators and were told to contact them for any problem at any time during the follow-up. Clinical course and anthropometry were recoded every fortnightly following discharge for 3 months. Overall outcome after the completion of 3-month follow-up was categorised as recovered, cured, relapsed, lost to follow-up or died. Recovery was considered when an infant gained WLZ > −3 SD (recovered from severe wasting), cure was considered when WLZ > −2 SD (recovery from wasting) and this gain in weight for length was sustained over the follow-up period. Any infant who had achieved WLZ > −3 Z-score on treatment but again falls below it anytime during the follow-up was considered as relapse(17).
Statistical analysis
Data were analysed using IBM SPSS Statistics version 25.0. For analysis of risk factors of unsatisfactory outcome, enrolled infants were divided into two groups: favourable outcome group (recovered or cured) and unsatisfactory outcome group (those who died/left against medical advice/lost to follow-up/relapse). Chi-square or Fisher’s exact test, Cochran–Mante–Haenszel statistics and multivariable logistic regression were applied for calculating P-value, OR (95 % CI) and adjusted OR (95 % CI), respectively. Multivariable logistic regression was performed for the risk factors with P > 0·3 on univariate analysis.
Results
During the study period, sixty-four infants below 6 months of age with severe wasting were hospitalised, of which fifty were enrolled, and fourteen were excluded (six congenital heart disease, five suspected metabolic disorders and three congenital anomalies) (Fig. 1).
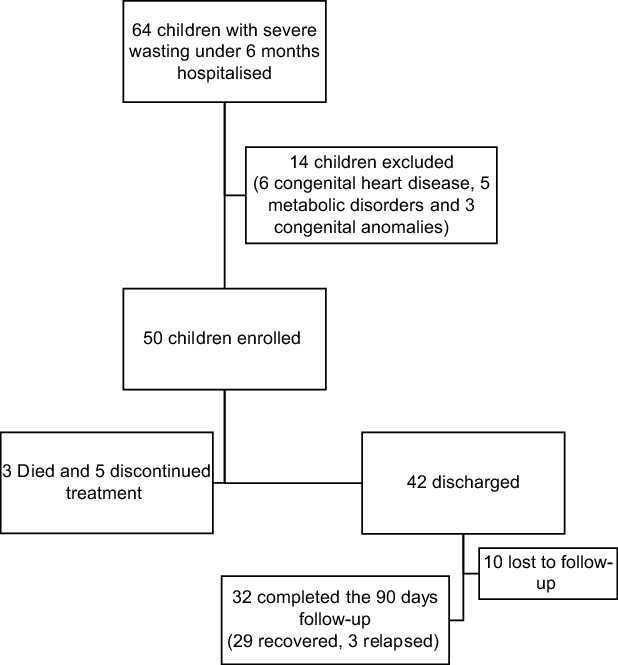
Fig. 1 Flow of participants in the study
The median (interquartile range (IQR)) age of enrolled infants was 108 (70, 148) d. These included twenty-nine (58 %) boys and twenty-one (42 %) girls. Only half of them had institutional delivery. Birth details were available for only twenty-three participants (median birth weight (IQR) 2500 (2250, 2900) g); 43 % of these were born as low birth weight. None of the enrolled infants had early initiation of breast-feeding. All of them were receiving replacement milk. Only eleven (22 %) were completely immunised for age. Diarrhoea was the presenting complaint in thirty-three (66 %) infants followed by fever, vomiting, cough, fast breathing, and decreased oral acceptance in 29, 28, 23,15 and 9 infants, respectively. None had edematous malnutrition, pallor was present in thirty-four (68 %) children and twenty-three (46 %) had dehydration (sixteen severe dehydration). Table 1 presents the socio-demographic profile of enrolled infants. Almost three-fourth of cases were from urban areas, two-third cases were from lower socio-economic strata (42 % fathers were illiterate or studied up to primary only and 50 % doing a desk job with an average earning < Rs. 6213 in three-fourth cases). More than 50 % mothers (n 28) were either illiterate or studied up to primary school only. Majority (∼80 %) of the families had a pucca (bricked or cemented) house with attached toilet and two-thirds were using municipal water supply.
Table 1 Socio-demographic profile of enrolled infants (n 50)
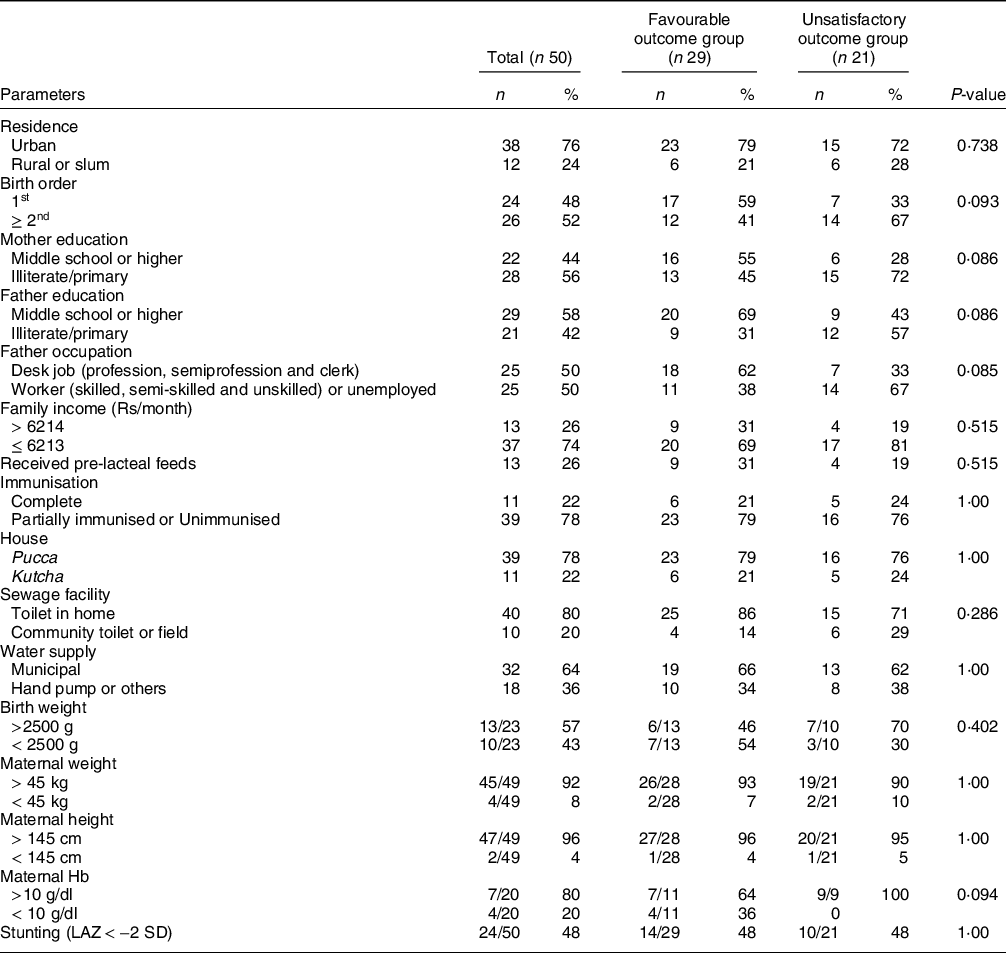
LAZ, length-for-age Z-score.
In the short-term, forty-two (84 %) infants were discharged, five (10 %) left against medical advice and three (6 %) died (Table 2). All deaths occurred within 1 week of hospitalisation, and the cause of death included – sepsis with acute gastroenteritis in two infants and meningitis in one infant. For those who were discharged (n 42), the median (IQR) duration of stay was 9·5 (6·5, 13·0) d. Overall, at the end of 3-month follow-up, twenty-nine (58 %) infants recovered, fifteen (30 %) cured, fifteen (30 %) left treatment in between/lost to follow-up, three (6 %) died and three (6 %) relapsed (Table 2).
Table 2 Outcomes of children under 6 months of age with severe wasting (n 50)
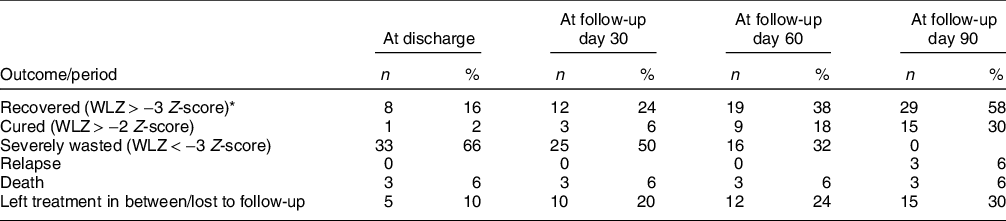
WLZ, weight-for-length Z-score.
* Includes the cured cases.
The anthropometric parameters at baseline, at discharge and during 3-month follow-up are shown in Table 3. Out of forty-two infants discharged, ten were lost to follow-up over 90 d. Of 32 remaining infants, 3, 9 and 15 achieved cure (WLZ > −2 SD) at the end of 1 month-, 2 month- and 3-month follow-up, respectively. Three infants relapsed and had to be re-hospitalised. In univariate analysis, none of the risk factors evaluated had a significant association with favourable or unsatisfactory outcome. On multivariable logistic regression, the adjusted OR for adverse outcomes was higher (>1) for higher birth order, low education status of the parents, absence of in-house toilet and father’s occupation, but these were not statistically significant (Table 4).
Table 3 Anthropometric parameters of infants less than 6 months with severe wasting from enrolment to 3 months after discharge
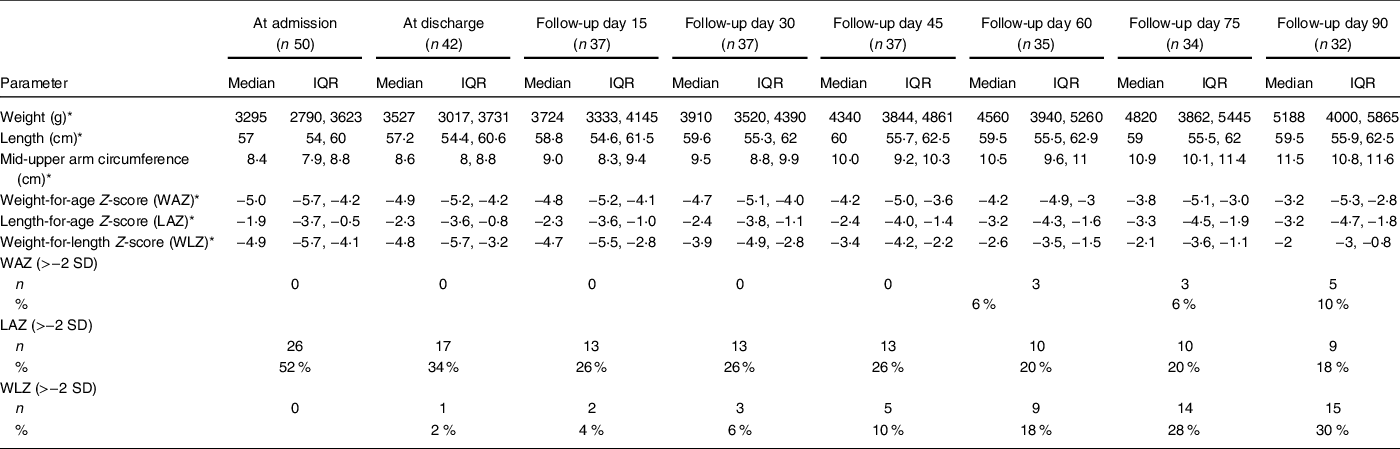
* All values are represented as median (interquartile range (IQR)).
Table 4 Association of risk factors and unsatisfactory outcome
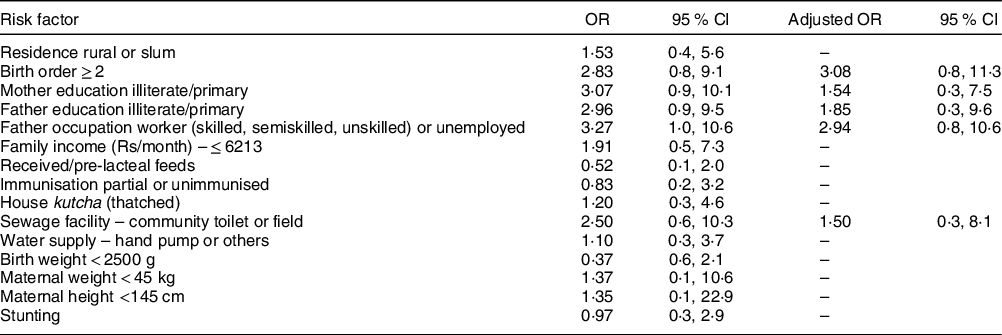
Figure 2 depicts the trends for weight-for-age Z-score, length-for-age Z-score and WLZ for thirty-two infants in whom complete 100 d of outcome (including hospital stay) was available (Online Fig. 3). And it depicts the pattern of (a) weight-for-age Z-score, (b) WLZ and (c) length-for-age Z-score of the individual children over the study period)
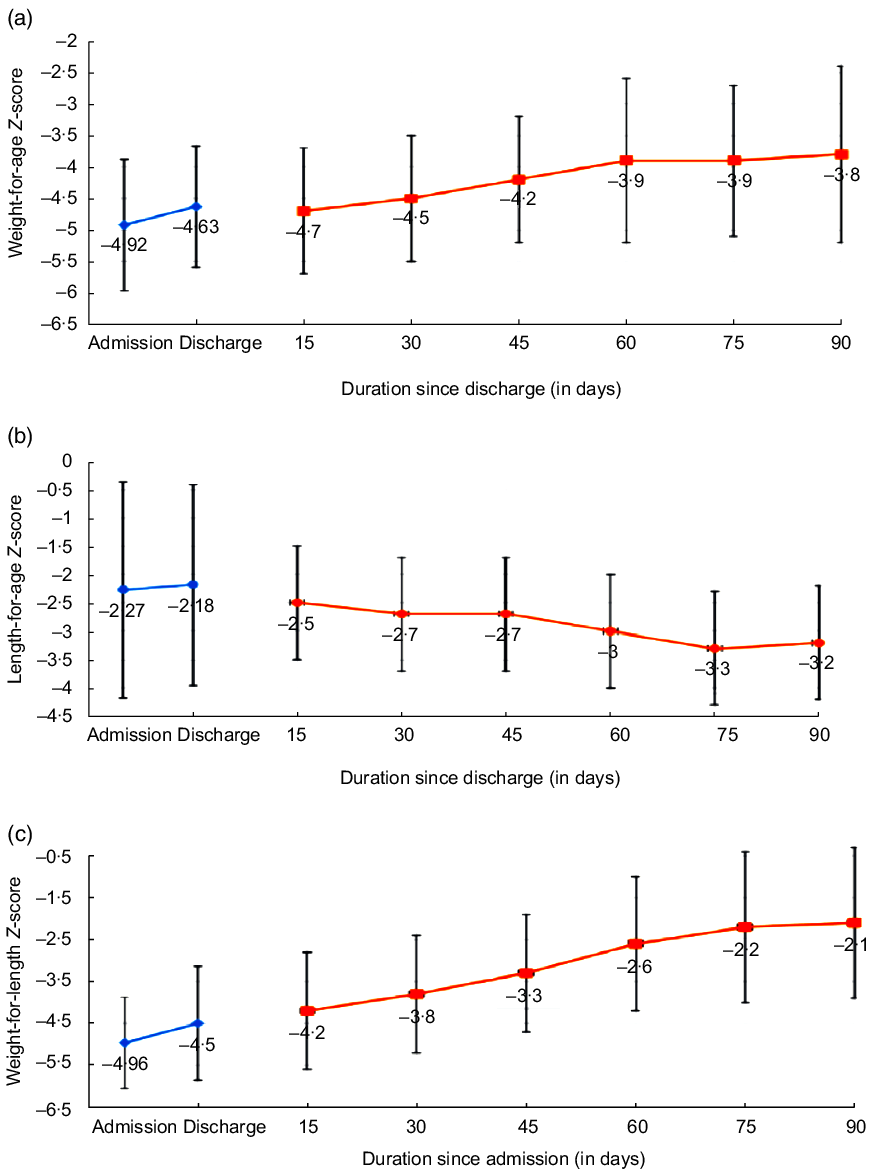
Fig. 2 Trends in (a) weight for age Z-score; (b) length-for-age Z-score and (c) weight-for-length Z-score during 3 months of follow-up (n 32)
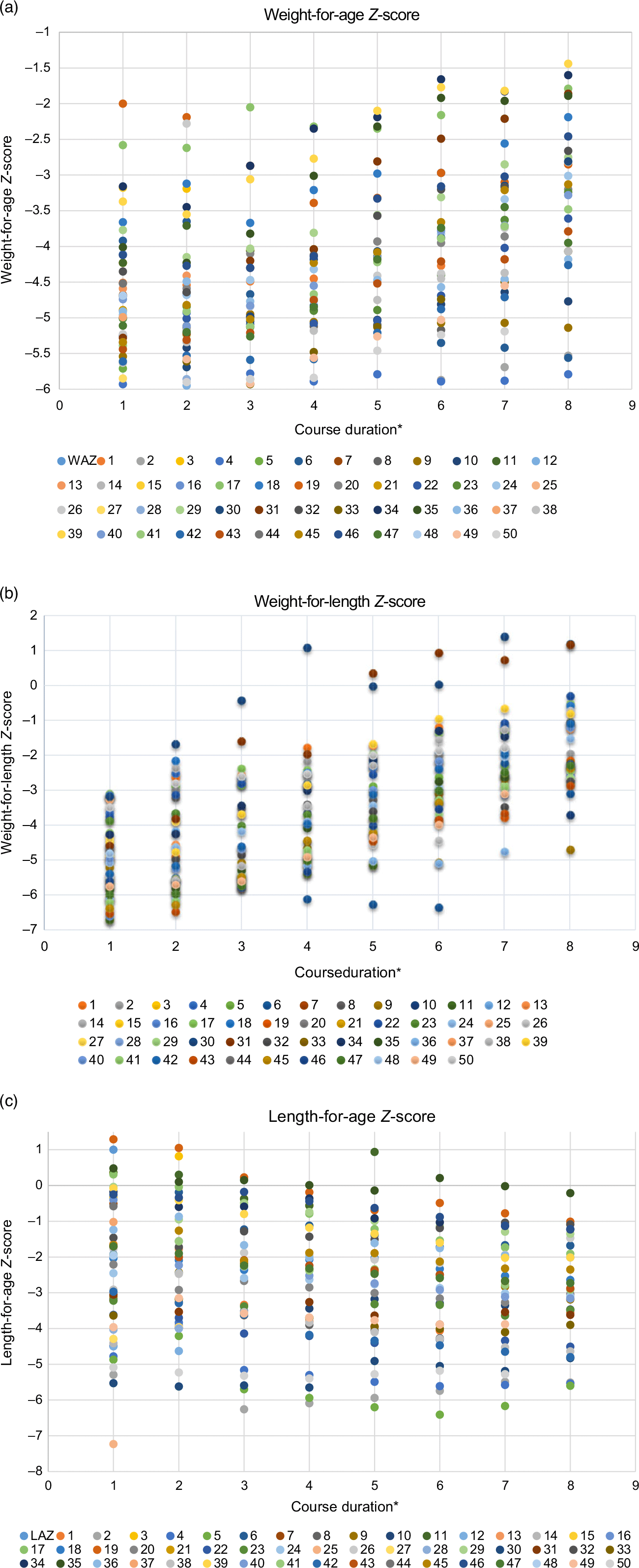
Fig. 3 Pattern of (a) weight-for-age Z-score; (b) weight-for-length Z-score and (c) length-for-age Z-score of the individual children over the study period
Discussion
In the present study that enrolled fifty infants with severe wasting under 6 months of age, the discharge rate from the hospital was 84 %, but cure could be achieved in only 30 % infants after 100 d of enrolment. Another 28 % recovered from severe wasting but remained in moderately wasted state. Overall mortality was 6 % and all deaths occurred in the first week. Overall relapse and default rates were 6 % and 30 %, respectively.
In a secondary analysis of data of 24 045 children (0–60 months) with SAM from 10 countries, 2939 (12 %) were aged below 6 months, and the outcomes in this age group at discharge were: 76 % recovered, 6 % defaulted and 5 % died(Reference Grijalva-Eternod, Kerac and McGrath6). A study from Bangladesh evaluating the outcome of SAM in infants enrolled at 4–8 weeks of age documented an overall recovery rate of 66 %, death rate of 4 % and default rate of 7 %, at the age of 6 months. Mother’s low education status and lower monthly family income were the important factors associated with poor outcome in these children(Reference Munirul Islam, Arafat and Connell23). We could not document any risk factor to be significantly associated with unsatisfactory outcome, probably because of inadequate sample size for such comparison.
The recovery rate in our study was lower and the default rate was higher compared to other similar studies(Reference Grijalva-Eternod, Kerac and McGrath6,Reference Dhanalakshmi and Devi15,Reference Vygen, Roberfroid and Captier24) . SPHERE Humanitarian Charter 2018 has suggested that in the management of SAM, the overall deaths should be <10 %, default rate should be < 15 % and recovery rate should be >75 %, where recovery is taken as discharge from the therapeutic care(25). In the present series, only one criteria of the Charter could be fulfilled. Two major reasons for this could be that majority of the parents belonged to the lower social class, working as daily wage earners and majority of the mothers were either illiterate or studied only up to middle school. There was no family or community support to these mothers following discharge, which could to be a major factor for high default and poor recovery.
Regarding the pattern of catch-up growth in our series, infants who completed 3 months of follow-up showed a significant improvement in all anthropometric parameters except in the length-for-age Z-score. This is similar to other studies having reported that during the rehabilitation phase in children with SAM (both in under 6 months and 6–59 months age group), there is minimal or no change in the length for age(Reference Munirul Islam, Arafat and Connell23,Reference Kerac, Bunn and Chagaluka26,Reference Ngari, Iversen and Thitiri27) . Studies from Bangladesh and Kenya had even reported a negative change in the height-for-age Z-score for children with SAM between 1 and 5 years age(Reference Ngari, Iversen and Thitiri27,Reference Khanum, Ashworth and Huttly28) . The postulated reasons for the minimal or no change in the height-for-age Z-score are that the catch-up in the height for age is restricted by the deficit in the weight for height, that is, the linear growth starts after reaching a threshold of for weight-for-age Z-score or WHZ, and persistence of adverse environmental factors like diarrhoea or respiratory illnesses even after recovering from SAM(Reference Ngari, Iversen and Thitiri27,Reference Walker and Golden29–Reference Golden34) . In the current study, our patients did not have any episode of diarrhoea or respiratory infection, but there was no increase in the linear growth during the follow-up period, suggesting the formal hypothesis.
The strength of our study is that it is a prospective study focusing on the long-term outcome of children with severe wasting under 6 months of age. The present study was limited by the short duration of follow-up and high loss to follow-up. As we have considered lost to follow-up as an unsatisfactory outcome, we might have underestimated the actual recovery rate. Request for early discharge and high attrition is a reality in urban low-income settings where families have no social support to compensate for the wage losses. Other limitations were lack of exact birth details in all infants, absence of the community linkage for home-based care of the discharged infants and small sample size. Also, our results may be applicable only to infants who have been identified to be undernourished based on the criteria of weight-for-length below −3 sd (severe wasting). Traditionally, children with severe wasting are classified as having SAM, but there is currently a move away from this terminology towards the use of ‘wasting’ or only ‘malnutrition’ due to high rates of co-existence of low length for age (stunting) in children identified as having SAM, and lack of clear differentiation between acute and chronic forms of malnutrition(35,Reference Kerac, McGrath and Connell36) . The validity of our results for less severe form of wasting (weight-for-length below −2 sd) and other forms of undernutrition identified on the basis of low weight-for-age or length-for-age needs to be documented in future studies.
We conclude that facility-based initial management, home-based rehabilitation and follow-up on outpatient basis does not appear to be an appropriate strategy for managing severely malnourished children below 6 months of age. These children may need longer hospitalisation with closer observation, supervised feeding and supportive care. A community linkage service is probably the most vital missing string, and further studies should evaluate the outcome of young infants with malnutrition throughout the continuum of care. Young children with wasting seem to need much more than facility-based initial care to improve compliance, food security and environment. Management of At-risk Mothers and Infants under 6 months of age (MAMI) Care Pathway approach can help the peripheral health workers to screen, assess and manage nutritionally at-risk infants under 6 months of age and their mothers and, thus, can help in achieving the better outcome in this age group(37).
Acknowledgements
Acknowledgements: Not applicable. Financial support: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors. Conflict of interest: There are no conflicts of interest. Authorship: D.S. and P.G. conceptualised the project, developed the protocol, supervised the enrolment and outcome assessment, and helped in writing the manuscript; J.P. had primary responsibility of patient screening, enrolment and data collection; R.K.M. performed the data analysis and writing the manuscript. All authors approved manuscript submitted. Ethics of human subject participation: This study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving research study participants were approved by the Institutional Ethics Committee-Human Research (IEC-HR) of University College of Medical Sciences, Delhi. Written informed consent was obtained from all guardians/caregivers of all the participants.
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S1368980021003268










