Introduction
Abnormalities in the function and structure of the hippocampus are often described in patients with major depression. For example, declarative memory deficits consistent with hippocampal impairment are a frequent accompaniment of depressive episodes (Zakzanis et al. Reference Zakzanis, Leach and Kaplan1998; MacQueen et al. Reference MacQueen, Campbell, McEwen, Macdonald, Amano and Joffe2003) and the hippocampus is known to show morphological changes in response to stress and excess cortisol secretion, both of which are implicated in the pathophysiology of mood disorders (MacQueen & Frodl, Reference MacQueen and Frodl2011; Moylan et al. Reference Moylan, Maes, Wray and Berk2013). Consistent with this, meta-analyses have consistently shown small but significant reductions in hippocampal volume in depressed patients (Campbell et al. Reference Campbell, Marriott, Nahmias and MacQueen2004; Kempton et al. Reference Kempton, Salvador, Munafo, Geddes, Simmons, Frangou and Williams2011). How far these volume reductions are linked to severe and recurrent illness is controversial (Moylan et al. Reference Moylan, Maes, Wray and Berk2013).
There is also some evidence that hippocampal volume may be reduced in people at increased familial risk of depression (Baaré et al. Reference Baaré, Vinberg, Knudsen, Paulson, Langkilde, Jernigan and Kessing2010; Chen et al. Reference Chen, Hamilton and Gotlib2010; Amico et al. Reference Amico, Meisenzahl, Koutsouleris, Resier, Moller and Frodl2011), though a history of childhood abuse may be an important moderating factor (de Gues et al. Reference de Geus, van't Ent, Wolfenberger, Heutink, Hoogendijk, Boomsa and Veltman2007; Rao et al. Reference Rao, Chen, Bidesi, Shad, Thomas and Hammen2010; Carballedo et al. Reference Carballedo, Lisiecka, Fagan, Saleh, Ferguson, Connolley, Meaney and Frodl2012). In a previous study we found that young women with a depressed parent but no personal history of depression showed impaired declarative memory in a verbal memory task (Mannie et al. Reference Mannie, Barnes, Bristow, Harmer and Cowen2009). In the same subjects we obtained evidence of increased activation of the neural substrate of working memory in a functional magnetic resonance imaging (fMRI) study (Mannie et al. Reference Mannie, Harmer, Cowen and Norbury2010).
The aim of the present study was to carry out a multimodal imaging study of hippocampal structure and function in a group of young people at increased familial risk of depression through virtue of having a depressed parent (Beardslee et al. Reference Beardslee, Versage and Gladstone1998). On the basis of the above studies we predicted that, relative to controls, participants at increased familial risk of depression would have lower hippocampal volumes and altered functional responses in hippocampal memory networks in an encoding task. It has also been suggested that loss of hippocampal volume in depression might be associated with increased glutamate activity (MacQueen & Frodl, Reference MacQueen and Frodl2011) and in a previous study we found that young people at increased familial risk of depression showed increased cortical levels of glutamate relative to controls (Taylor et al. Reference Taylor, Mannie, Norbury, Near and Cowen2011). In the present study, therefore, we also used magnetic resonance spectroscopy (MRS) to obtain a composite measure of glutamate and its precursor glutamine (Glx) in the right hippocampus.
Method
Participants
We recruited 62 young people (39 women, 23 men) with a mean age of 18.8 (s.d. = 1.0) years (range 16–20 years) who had never personally suffered from depression but who had a biological parent with a history of major depression (FH+). Potential participants were assessed with the Structured Clinical Interview for DSM-IV Axis I Disorders Schedule (SCID-I; First, et al. Reference First, Spitzer, Gibbon and Williams1997) to exclude a personal current or previous history of major depression. The presence of major depression in a parent was assessed by the family history method using the participant as an informant (Andreasen et al. Reference Andreasen, Rice, Endicott, Reich and Coryell1986). A history of bipolar disorder in a parent was an exclusion criterion. The criteria used included description of the symptoms of major depression together with the prescription of specific antidepressant treatment, either psychotherapy or medication. This was followed up by direct verification from the affected parent (by telephone or email), and where parental history could not be verified, participants were excluded. The verification was carried out by a psychiatric research nurse who asked the relevant parent specific questions taken from the SCID-I about diagnosis, number of depressive episodes, impact of episodes on functioning, form of treatment (out-patient or in-patient, antidepressants, psychotherapy or counselling) as well as the nature of the professional who had made the diagnosis (psychiatrist or general practitioner). We also recruited 59 controls (35 women and 25 men) with a mean age of 19.1 (s.d. = 0.8) years (range 16–20 years) who were determined by the same instruments to have no current or past history of major depression and no history of depression in a biological parent or other first-degree relative.
Current mood and anxiety symptoms were assessed with the self-rated Hospital Anxiety and Depression Scale (Zigmond & Snaith, Reference Zigmond and Snaith1983) while the Perceived Stress Scale (Cohen et al. Reference Cohen, Kamarck and Mermelstein1983) was used to provide a measure of subjective stress over the past month. Adverse life events and the impact of these events on emotional well-being were assessed with the Life Events Rating Scale which assesses adverse events at two time points; first, at a distant time point that includes childhood adversity and second, events experienced in the past year (Goodyer et al. Reference Goodyer, Herbert, Tamplin, Secher and Pearson1997). We assessed the quality of perceived parenting style for the first 16 years of life with the Parental Bonding Instrument (PBI), obtaining both maternal and paternal PBI scores (Parker, Reference Parker1979). All participants were right-handed as assessed with the Edinburgh Handedness Inventory (Oldfield, Reference Oldfield1971). All subjects gave full informed consent to the study, which was approved by the local ethics committee.
Structural MRI
Scanning was performed at the University of Oxford, Centre for Clinical Magnetic Resonance Research using a 3 Tesla Siemens Trio scanner with a 12-channel head-coil (Siemens, Germany). The neuroimaging protocol comprised structural and functional MRI sequences and MRS.
Three-dimensional high-resolution T1-weighted MR images were acquired using a MPRAGE (magnetization-prepared rapid acquisition gradient echo) sequence [repetition time (TR) = 2040 ms, echo time (TE) = 4.7 ms, flip angle = 8°, field of view = 192 mm, voxel dimension = 1 mm isotropic, acquisition time = 6 min]. Data analysis was carried out using FSL tools (FMRIB Software Library, www.fmrib.ox.ac.uk/fsl) (Smith et al. Reference Smith, Jenkinson, Woolrich, Beckman, Behrens, Johansen-Berg, Banister, De Luca, Drobnjak, Flitney, Niazy, Saunders, Vickers, Zhang, De Stefano, Brady and Matthews2004). Whole-brain analysis was carried out using a voxel-based morphometry (VBM)-style analysis (FSL-VBM) (Douaud et al. Reference Douaud, Smith, Jenkinson, Behrens, Johansen-Berg, Vickers, James, Voets, Watkins, Matthews and James2007), using default settings (as described at www.fmrib.ox.ac.uk/fsl/fslvbm/). In brief, brain extraction and tissue-type segmentation were performed and resulting grey matter (GM) partial volume images were aligned to standard space using first linear (FLIRT) and then nonlinear (FNIRT) registration tools. The resulting images were averaged, modulated and smoothed with an isotropic Gaussian kernel of 4 mm to create a study-specific template. Finally, a voxel-wise general linear model (GLM) was applied using a permutation non-parametric testing (5000 permutations), cluster determined by Z > 2.5 and a family-wise error (FWE)-corrected cluster significance threshold of p < 0.001 was applied to the superthreshold clusters.
Automatic segmentation of the hippocampus was performed with FSL FIRST (Patenaude et al. Reference Patenaude, Smith, Kennedy and Jenkinson2011) using the default settings (as described at http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/FIRST/UserGuide). Initially, segmentation was performed with the two-stage affine transformation to Montreal Neurological Institute (MNI) 152 standard space at 1 mm resolution followed by boundary correction to classify the boundary voxels as belonging to the hippocampus. To obtain intracranial volume (ICV), FSL-FAST was used to estimate GM, white matter (WM) and cerebrospinal fluid (CSF) while correcting for spatial intensity variations. ICV was calculated by adding the GM, WM and CSF volumes, and used to normalize for hippocampal volumes.
Functional MRI
Encoding memory was assessed using a single gradient echo EPI sequence covering the whole brain (TR = 3000 ms, TE = 28 ms, flip angle = 89°, field of view = 192 mm, voxel dimension = 3 mm isotropic, acquisition time = 9 min 6 s). The experimental task was carried out using Presentation software as described in detail elsewhere (Filippini et al. Reference Filippini, Bradley, MacIntosh, Hough, Goodwin, Frisoni, Smith, Matthews, Beckmann and Mackay2009). Briefly, a ‘novel versus familiar’ memory-encoding paradigm was used. A set of coloured images (representing animals and landscapes, similar in complexity, brightness and contrast, emotionally neutral and with no persons represented), presented in a ‘blocked design’ fashion, was shown inside the scanner. Six blocks of ‘familiar’ (eight images previously learnt outside the scanner, each time presented in a pseudorandom order) and six blocks of ‘novel’ images (eight new images presented in each block) were presented in an alternated order (image presentation = 3250 ms, inter-stimulus interval = 500 ms, block duration = 30 000 ms). Between each block of images were 15 000 ms of ‘rest’, during which subjects passively viewed a fixation cross (a total of 12 ‘rest’ blocks). Subjects were instructed to select from a two-button response according to whether the images contained animals. Responses were monitored by the scanner operators to ensure compliance and accuracy and were registered by the software in a text file. Participants were also instructed to try to remember the images for a subsequent memory task. Outside the scanner, approximately 50 min after encoding, 83 images were presented on a computer screen for 4000 ms each (inter-stimulus interval = 1000 ms). The 48 ‘novel’ images, the eight ‘familiar’ images and 27 ‘distractors’ (images never seen before, 13 animals and 14 landscapes) were displayed in pseudorandom order. Subjects had to select between two buttons according to whether the images had been seen inside the scanner or not.
Analysis of fMRI data was carried out using FEAT (FMRI Expert Analysis Tool version 5.98; http://www.fmrib.ox.ac.uk/fsl/feat5/) (Woolrich et al. Reference Woolrich, Behrens, Beckmann, Jenkinson and Smith2004). Pre-processing consisted of head motion correction, brain extraction, spatial smoothing using a Gaussian kernel of full width at half maximum (FWHM) 5 mm, and high-pass temporal filtering equivalent to 130 s. Time-series statistical analysis was carried out with local autocorrelation correction. A boxcar convolved with a γ haemodynamic response function and its temporal derivative was used to model the data. The main contrast of interest for the novelty detection paradigm was ‘novel versus familiar’. FMRI volumes were registered to the individual's structural scan and standard space images using a nonlinear registration tool (FNIRT). These transformations into standard space were applied to images of contrasts of interest and their variances. Higher-level (group-level) analysis was carried out using FMRIB's Local Analysis of Mixed Effect (FLAME) (Woolrich et al. Reference Woolrich, Behrens, Beckmann, Jenkinson and Smith2004). The GLM included the two groups. We tested for group averages and differences between groups for each of the contrasts of interest. Z (Gaussianized T/F) statistic images were thresholded using clusters determined by Z > 2.3 and a FWE threshold of p < 0.05 was applied to the superthreshold clusters. To reduce effects attributable to outliers, an automatic outlier deweighting tool was also applied (Woolrich, Reference Woolrich2008). To ensure that any group differences in fMRI data were not attributable to underlying structural differences, GM images of each subject were registered to standard space, smoothed to match the fMRI data, demeaned within each group and added to the model as voxel-wise covariates of no interest.
MRS
The structural MRI was used to guide placement of an MRS voxel measuring 30 × 15 × 10 mm3 in the right hippocampus (Fig. 1). The voxel was positioned to avoid overlap with the pons, peduncle or other midbrain structures that are associated with increased motion. All signal averages were stored individually, which enabled us to reject averages that were corrupted by motion, as well as to correct any frequency and phase drift errors (see Near et al. Reference Near, Edden, Evans, Pacquin, Harris and Jezzard2014). Single voxel-localized, short echo-time MRS data were acquired from the region of interest using the Point REsolved SpectroScopy (PRESS) sequence with the following sequence parameters: TR = 3000 ms, TE = 30 ms, flip angle = 90°, spectral width = 1200 Hz, 1024 points, 128 averages, acquisition time = 6.4 min (Bottomley, Reference Bottomley1987). Semi-automated processing of spectra was performed in MATLAB (MathWorks, USA) to remove motion-corrupted averages and frequency drift prior to data analysis in LCModel (http://s-provencher.com/pages/lcmodel.shtml).

Fig. 1. Sample Point REsolved SpectroScopy (PRESS) spectrum and voxel position in the right hippocampus. Glx, Glutamate/glutamine; ppm, parts per million.
Processed MRS data were analysed using LCModel (Provencher, Reference Provencher1993) and all metabolite concentrations were calculated with reference to total creatine (creatinine + phosphocreatine). Glutamate concentrations were assessed using the composite measure of glutamate + glutamine (Glx). Only Glx values with a Cramér–Rao lower bound uncertainty less than 30% were included in the statistical analyses.
Statistics
Statistical analyses were carried out using SPSS software (IBM, USA) and t tests were used for sociodemographic variables, brain structure volumes, memory performance and reaction times. Exact Fisher's test was used for categorical variables.
Results
Participants
The two groups did not significantly differ in age, gender, smoking, state and trait anxiety, perceived stress, anxiety or depression (Table 1). There was also no difference in recent or remote life events or in measures of parental bonding. Similarly, no group-related differences were observed for memory recognition performance or reaction times, both of which were recorded after fMRI task-related acquisition (all p values >0.5) (Supplementary Table S1).
Table 1. Sociodemographic and neuropsychological features of FH+ participants and controls

Data are given as mean (standard deviation) or as number of participants.
FH+, Familial risk of depression; HADS-D, Hospital Anxiety and Depression Scale – Depression; HADS-A, Hospital Anxiety and Depression Scale – Anxiety; PSS, Perceived Stress Scale; LERS-recent, Life Events Rating Scale – past year; LERS-lifetime, Life Events Rating Scale – lifetime; MPBI-care, Parental Bonding Instrument – Maternal Care; MPBI-overprotection, Parental Bonding Instrument – Maternal Overprotection; PPBI-care, Parental Bonding Instrument – Paternal Care; PPBI-overprotection, Parental Bonding Instrument – Paternal Overprotection.
Brain morphology and hippocampal volume
No group differences were observed in whole-brain volume or in segmented GM and WM (Table 2). Similarly, VBM revealed no differences between the two study groups in GM volume. Normalized hippocampal volumes (i.e. relative to ICV) were computed as the total volume of hippocampal GM (mm3)/ICV (mm3) × 1000. We were able to analyse structural data only from 54 FH+ and 52 control participants due to poor image registration in the remaining participants. There was no difference in hippocampal volume (absolute and normalized) between FH+ and controls as established with FSL FIRST (Table 3).
Table 2. Brain volume measures in FH+ participants and controls
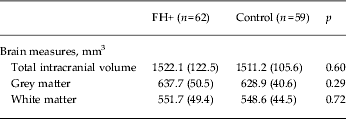
Data are given as mean (standard deviation).
FH+, Familial risk of depression.
Table 3. Hippocampal volumes (absolute and normalized) in FH+ participants and controls
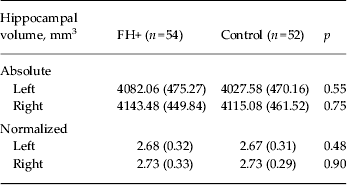
Data are given as mean (standard deviation).
FH+, Familial risk of depression.
fMRI
The encoding memory fMRI task successfully activated task-related areas associated with the encoding process by contrasting novel with familiar visual scenes. Group blood oxygen level-dependent (BOLD) fMRI signal intensity changes were observed in the hippocampus, temporal fusiform cortex, and parahippocampal gyrus bilaterally, and in association areas in the frontal, parietal and occipital lobes (See Fig. 2 a), as previously described (Fleisher et al. Reference Fleisher, Houston, Eyler, Frye, Jenkins, Thal and Bondi2005; Golby et al. Reference Golby, Silverberg, Race, Gabrieli, O'Shea, Knierim, Stebbins and Gabrieli2005; Filippini et al. Reference Filippini, Bradley, MacIntosh, Hough, Goodwin, Frisoni, Smith, Matthews, Beckmann and Mackay2009).

Fig. 2. Functional magnetic resonance imaging results for the ‘novel versus familiar’ contrast in the encoding task. (a) Mean activation for the novel versus familiar contrast for all 121 subjects: young people at familial risk of depression (FH+) and controls. Activation was found bilaterally in the primary and secondary visual cortices as well as regions involved in memory processes: the hippocampus, temporal fusiform cortex and parahippocampal gyrus. R, Right hemisphere; L, left hemisphere. Red-to-yellow colours define increases in brain activation. (b) Regions of significantly increased blood oxygen level-dependent (BOLD) signal intensity for FH+ participants relative to controls. Activation in a cluster of brain regions encompassing the insular and putamen regions bilaterally and the posterior portion of the anterior cingulate cortex was greater in the FH+ participants relative to controls (p < 0.05, corrected for multiple comparisons).
Fig. 2 b shows group differences in the novel versus familiar contrast. In general, an increased BOLD signal in FH+ participants relative to controls was found in a cluster of regions encompassing the insular cortices: the putamen and pallidum bilaterally (maximum Z score 4.15; cluster size 2082 voxels; peak coordinates in standard space 24, 18,–6; and maximum Z score – 3.78; cluster size 835 voxels; peak coordinates in standard space −28, −14, −6, respectively) and in the dorsal portion of the anterior cingulate cortex (maximum Z score 3.45; cluster size 325 voxels; peak coordinates in standard space 16, 20, 30). The addition of GM as a voxel-wise covariate of no interest did not affect BOLD-related group differences, suggesting that any subthreshold differences in brain structure did not influence the fMRI result.
MRS imaging
Valid scans were available from 57 FH+ participants and 57 controls. There were no group differences in hippocampal voxel contents (GM, WM and CSF, all p > 0.20) and spectral quality (FWHM, p > 0.30). The Glx:creatinine ratio in the hippocampus was significantly higher in FH+ subjects (Fig. 3). There was no change in the concentration of any other neurometabolites comparing FH+ with controls.

Fig. 3. Myoinositol (MI), glycerophosphocholine (GPc), N-acetylaspartate (Naa) and glutamate/glutamine (Glx) by group: young people at familial risk of depression (FH+) versus controls (Con). Values are means, with standard deviations represented by vertical bars. * Mean value was significantly different from that of the control group (p = 0.01).
Discussion
We found that young people at familial risk of depression exhibited overactivity of neural networks involved in memory encoding and higher levels of a composite measure (Glx) of glutamate and glutamine in the right hippocampus. However, there was no evidence of structural deficits in the hippocampus and more generally no change in brain morphology between the two participant groups.
As previously described, the fMRI task of memory encoding that we employed produced activation of the hippocampus and parahippocampal regions (Filippini et al. Reference Filippini, Bradley, MacIntosh, Hough, Goodwin, Frisoni, Smith, Matthews, Beckmann and Mackay2009). This task has been used successfully to identify overactivity of the hippocampus in carriers of APOE ε4 risk alleles, consistent with the increased risk of such individuals in later life for hippocampal-based learning deficits (Filippini et al. Reference Filippini, Bradley, MacIntosh, Hough, Goodwin, Frisoni, Smith, Matthews, Beckmann and Mackay2009). However, contrary to our hypothesis, we found no evidence of abnormal hippocampal activity in young people at risk of depression during encoding. Studies of hippocampal activity during encoding tasks in depressed patients have tended to report lower hippocampal recruitment (Bremner et al. Reference Bremner, Vythilingam, Vermetten, Vaccarino and Charney2004; Milne et al. Reference Milne, MacQueen and Hall2012; Kelly et al. Reference Kelly, Garrett, Cohen, Gomez, Lembke, Keller, Reiss and Schatzberg2013), though this is not always the case (Werner et al. Reference Werner, Meindl, Materne, Engel, Huber, Riedel, Reiser and Hennig-Fast2009) and more subtle changes such as a dysregulation of the normal relationship between hippocampal activation and encoding success have also been observed (Fairhall et al. Reference Fairhall, Sharma, Magnusson and Murphy2010). Fairhall et al. (Reference Fairhall, Sharma, Magnusson and Murphy2010) point out that numerous methodological factors might influence hippocampal activation in fMRI studies in depressed patients, for example, stage of disease, the use of psychotropic medication and whether the fMRI analysis is restricted to successful encoding. It is also likely that the emotional valence of the presented material can influence the extent of hippocampal activation, with depressed patients showing relatively less hippocampal response to the encoding of positive word pairs while the reverse is the case with negative words (Toki et al. Reference Toki, Okamoto, Onoda, Matsumoto, Yoshimura, Kunisato, Okada, Shishida, Kobayakawa, Fukomoto, Machino, Inagaki and Yamawaki2014).
We did find overactivity of other brain regions that have been linked to episodic memory such as the putamen, pallidum insula and anterior cingulate cortex (Rottschy et al. Reference Rottschy, Langer, Dogan, Reetz, Laird, Schultz, Fox and Eickhoff2012), suggesting that FH+ participants may need to recruit a wider neural network to maintain task performance during encoding. Interestingly, Yassa & Stark (Reference Yassa and Stark2008) observed, in healthy participants, a repetition-related decrease in the activation of putamen, insula and cingulate cortex over the time course of a novel episodic memory task. It is possible that FH+ participants do not show adaptation in this way, that is that novel information continues to remain ‘salient’. We also found altered neural responses in the anterior cingulate cortex in FH+ participants undertaking an emotional Stroop task and during the experience of reward and punishment (Mannie et al. Reference Mannie, Norbury, Murphy, Inkster, Harmer and Cowen2008; McCabe et al. Reference McCabe, Wiffindale, Harmer and Cowen2012). This suggests that abnormal activity in the anterior cingulate may characterize neural responses to a variety of cognitive and emotional tasks in FH+ subjects.
To the best of our knowledge, the kind of overactivity that we saw in FH+ participants in brain regions linked to episodic memory has not been reported in patients with established depression or bipolar disorder (see, for example, Fairhall et al. Reference Fairhall, Sharma, Magnusson and Murphy2010; Oertel-Knöchel et al. Reference Oertel-Knöchel, Reinke, Feddern, Knake, Knöchel, Prvulovic, Fuβer, Karakaya, Loellgen, Freitag, Pantel and Linden2013). However, people at familial risk of depression do overactivate subcortical, affect-linked, areas in a task requiring the shifting of attention from negative stimuli. This has been attributed to a compensatory ‘protective’ strategy in at-risk individuals (Lisiecka et al. Reference Lisiecka, Carbadello, Fagan, Ferguson, Meaney and Frodl2013), and it is possible that a similar effect could underlie the changes seen in our study.
Decrements of hippocampal volume are found fairly reliably in large samples of depressed patients (Campbell et al. Reference Campbell, Marriott, Nahmias and MacQueen2004; Kempton et al. Reference Kempton, Salvador, Munafo, Geddes, Simmons, Frangou and Williams2011), and there is evidence that this abnormality may extend to people at familial risk of depression. For example, using voxel-based morphometry, Chen et al. (Reference Chen, Hamilton and Gotlib2010) found smaller hippocampi bilaterally in 23 daughters (aged 9–15 years) of mothers with recurrent depression compared with age-matched controls. Baaré et al. (Reference Baaré, Vinberg, Knudsen, Paulson, Langkilde, Jernigan and Kessing2010) reported smaller hippocampal volumes in 59 high-risk twins where a co-twin suffered from major depression and Amico et al. (Reference Amico, Meisenzahl, Koutsouleris, Resier, Moller and Frodl2011) also found smaller right hippocampi in 30 participants with a first-degree relative with depression. Smaller hippocampal volumes were also found in three other familial risk studies; however, in the latter investigations, the effects of familial vulnerability on the hippocampus were partly dependent on an interaction with early life adversity (de Guess et al. Reference de Geus, van't Ent, Wolfenberger, Heutink, Hoogendijk, Boomsa and Veltman2007; Rao et al. Reference Rao, Chen, Bidesi, Shad, Thomas and Hammen2010; Carballedo et al. Reference Carballedo, Lisiecka, Fagan, Saleh, Ferguson, Connolley, Meaney and Frodl2012). In our study we found relatively low levels of childhood adversity that did not apparently distinguish our two participant groups, at least as measured by adverse life events and assessments of parental relationships. Our data therefore suggest that familial risk of depression may not by itself be associated with lowered hippocampal volume and that other factors such as childhood maltreatment may be involved in the low hippocampal phenotype.
We employed voxel-based morphometry as well as automatic segmentation of the hippocampus and we therefore think it unlikely that methodological factors can explain the differences between our findings in FH+ participants and the other studies cited here. Moreover, because we added GM maps as a covariate of no interest to the fMRI analyses, we have accounted for potential subthreshold differences that could have influenced our fMRI results. However, it is possible that more detailed structural analyses could have revealed differences in hippocampal subregions not captured by more conventional approaches (Cole et al. Reference Cole, Toga, Hojatkashani, Thompson, Costafreda, Clear, Williams, Bullmore, Scott, Mitterschiffthaler, Walsh, Donaldson, Mirza, Marquand, Nosarti, McGuffin and Fu2010).
Whether or not patients in a first episode of depression exhibit abnormalities in hippocampal structure has been disputed, but a recent meta-analysis of seven studies found a significant bilateral reduction in hippocampal volume in such participants (Cole et al. Reference Cole, Costafreda, McGuffin and Fu2011). If our finding in FH+ participants is correct, one interpretation is that in some ‘at-risk’ individuals hippocampal volume reduction becomes manifest at first presentation of clinical illness, perhaps as a result of an ongoing neurotoxic process (see below).
We did find increased levels of Glx in the hippocampus in FH+ participants. This is in partial agreement with a previous study in young FH+ subjects where, using a PRESS-J editing technique, we found increased glutamate but not Glx in the occipital cortex (Taylor et al. Reference Taylor, Mannie, Norbury, Near and Cowen2011). The PRESS sequence used in the present study does not allow separation of glutamate from glutamine and so the data are presented as Glx. Generally, in acutely depressed patients, Glx levels measured by proton MRS are lowered in anterior brain regions (Yuksel & Ongur, Reference Yuksel and Ongur2010), though the hippocampus has been relatively little investigated, with one study reporting lowered Glx levels in depression while another found no change (Block et al. Reference Block, Träber, von Widdern, Metten, Schild, Maier, Zobel and Jessen2008; Milne et al. Reference Milne, MacQueen, Yucel, Soreni and Hall2009). The hippocampus is a difficult area to image in MRS and it is possible that our voxel involved a contribution from the amygdala as well as the hippocampus. Because of time constraints, our MRS study was restricted to the right hippocampus so it is not clear whether similar changes in Glx might occur bilaterally. In view of our previous finding of raised glutamate in the occipital cortex in FH+ participants (Taylor et al. Reference Taylor, Mannie, Norbury, Near and Cowen2011) it will also be important in future studies to examine additional brain regions to determine whether any increases in glutamate and/or Glx are a generalized phenomenon of the at-risk state.
An MRS study in depressed patients at different illness stages reported that Glx levels in the hippocampus were similar to controls in first episodes of depression but then declined over the course of recurrent illness (de Diego-Adelino et al. Reference di Diego-Adelino, Portella, Gomez-Anson, Lopez-Moruelo, Serra, Vives, Puigdemont, Perez-Egea, Alvarez and Perez2013). This raises the possibility that a state of vulnerability to depression might be marked by relatively higher glutamate levels compared with normal, and that these elevated levels then decline with the onset of depression, decreasing further during the course of chronic illness. This is consistent with proposals that increased glutamate levels act as a precursor of neurotoxicity in the hippocampus in depression through a biochemical cascade that inhibits synaptic plasticity and neuronal survival (see MacQueen & Frodl, Reference MacQueen and Frodl2011). Thus, initial high levels of glutamate in the high-risk subject might decline over time with a corresponding loss of hippocampal neurones. Clearly, however, this notion is speculative and needs empirical support from longitudinal studies of high-risk patients.
Conclusions
In conclusion, we found no deficits in hippocampal structure in young people at increased familial risk of depression. However we did find evidence of abnormally increased neural activity in hippocampal-dependent memory networks during an encoding task as well as increased levels of a composite measure of glutamate and glutamine in the hippocampus. In this respect our data add to other studies suggesting abnormal activity of the anterior cingulate cortex in young FH+ subjects (Mannie et al. Reference Mannie, Norbury, Murphy, Inkster, Harmer and Cowen2008; McCabe et al. Reference McCabe, Wiffindale, Harmer and Cowen2012). Further longitudinal studies are need both to confirm these changes and identify their possible value in predicting illness onset and progression.
Supplementary material
For supplementary material accompanying this paper visit http://dx.doi.org/10.1017/S0033291714000580.
Acknowledgements
The study was sponsored by the UK Medical Research Council (grant no. G0900576). N.F. is funded by the HDH Wills 1965 Charitable Trust. C.E.M. received support from the National Institute for Health Research (NIHR) Oxford Biomedical Research Centre. The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
Declaration of Interest
P.J.C. is a member of a paid advisory board for Lundbeck.








