Introduction
Major depressive disorder (MDD) is commonly associated with impairments in social functioning, including reductions in the quantity and quality of interpersonal interactions (Segrin and Abramson, Reference Segrin and Abramson1994; Hirschfeld et al., Reference Hirschfeld, Montgomery, Keller, Kasper, Schatzberg, Möller, Healy, Baldwin, Humble, Versiani, Montenegro and Bourgeois2000; Segrin, Reference Segrin2000; Rottenberg and Gotlib, Reference Rottenberg and Gotlib2004; Kupferberg et al., Reference Kupferberg, Bicks and Hasler2016). For instance, children who tend to withdraw from social situations have a heightened chance of developing depression as adults (Katz et al., Reference Katz, Conway, Hammen, Brennan and Najman2011), depressed individuals display impaired social skills and have fewer close relationships than controls (Lewinsohn, Reference Lewinsohn, Friedman and Katz1974; Youngren and Lewinsohn, Reference Youngren and Lewinsohn1980; Brim et al., Reference Brim, Witcoff and Wetzel1982; Gotlib and Lee, Reference Gotlib and Lee1989; Segrin, Reference Segrin2000), and deficits in social functioning persist even after recovery from MDD (Gotlib and Lee, Reference Gotlib and Lee1989; Rhebergen et al., Reference Rhebergen, Beekman, de Graaf, Nolen, Spijker, Hoogendijk and Penninx2010; Ladegaard et al., Reference Ladegaard, Videbech, Lysaker and Larsen2016).
It has been proposed that depressed subjects may demonstrate a reduced quantity of social engagement (i.e. social withdrawal), because they experience anhedonic or negatively biased responses to interpersonal encounters (Rottenberg and Gotlib, Reference Rottenberg and Gotlib2004; Kupferberg et al., Reference Kupferberg, Bicks and Hasler2016). In line with this suggestion, MDD patients have been found to derive less pleasure from peer approval than controls (Davey et al., Reference Davey, Allen, Harrison and Ycel2011; Dedovic et al., Reference Dedovic, Slavich, Muscatell, Irwin and Eisenberger2016), and an association between heightened depression severity and diminished pleasure responses to social acceptance feedback has been observed (Caouette and Guyer, Reference Caouette and Guyer2016). Additionally, MDD symptoms have been related to increased expectancies of negative peer evaluation (Caouette and Guyer, Reference Caouette and Guyer2016), as well as to anticipation of more negative responses to social situations (Setterfield et al., Reference Setterfield, Walsh, Frey and McCabe2016). Importantly, both anhedonia (Silvia and Kwapil, Reference Silvia and Kwapil2011) and elevated negative expectancies (Zimmer-Gembeck et al., Reference Zimmer-Gembeck, Nesdale, Webb, Khatibi and Downey2016) have been linked to social withdrawal.
In addition, depressed subjects demonstrate decreases in the quality of interpersonal interactions, with experience sampling studies showing that individuals with MDD symptoms encounter fewer positive (Peeters et al., Reference Peeters, Nicolson, Berkhof, Delespaul and De Vries2003; Bylsma et al., Reference Bylsma, Taylor-Clift and Rottenberg2011; van Roekel et al., Reference van Roekel, Bennik, Bastiaansen, Verhagen, Ormel, Engels and Oldehinkel2016) and more negative (Bylsma et al., Reference Bylsma, Taylor-Clift and Rottenberg2011) social and non-social events than controls. It is obvious that anhedonic or negatively biased processing of pleasant and unpleasant outcomes is likely to contribute to these findings. However, it is equally plausible that impaired learning from social feedback may play a role in the diminished quality of interpersonal experiences in MDD. Specifically, deficits in learning may diminish depressed individuals' ability to appropriately adjust their behavior in response to social feedback, which, in turn, may bring about the experience of more unpleasant interpersonal encounters. Additionally, impaired learning may result in increased uncertainty, which, due to depressed subjects' tendency to regard uncertainty as negative (Carleton et al., Reference Carleton, Mulvogue, Thibodeau, McCabe, Antony and Asmundson2012), may give rise to more negatively perceived social interactions.
Surprisingly, despite the importance of social stimuli in everyday life, research on learning from social outcomes in depression is lacking. One exception is a signal detection study which found a reduction in social reward biases in remitted MDD patients (Pechtel et al., Reference Pechtel, Dutra, Goetz and Pizzagalli2013). However, in this study, subjects were aware that the ‘social’ outcomes – the words ‘Well done!’ displayed on the screen – were computer-generated. It is thus questionable whether this feedback can be regarded as truly social.
While there is little evidence regarding social learning in depression, a range of studies have observed abnormalities in learning from non-social feedback. For instance, in signal detection tasks with monetary outcomes, participants with or at risk for MDD fail to develop reward biases (Pizzagalli et al., Reference Pizzagalli, Iosifescu, Hallett, Ratner and Fava2008; Pechtel et al., Reference Pechtel, Dutra, Goetz and Pizzagalli2013; Vrieze et al., Reference Vrieze, Pizzagalli, Demyttenaere, Hompes, Sienaert, De Boer, Schmidt and Claes2013; Fletcher et al., Reference Fletcher, Parker, Paterson, Fava, Iosifescu and Pizzagalli2015; Liu et al., Reference Liu, Roiser, Wang, Zhu, Huang, Neumann, Shum, Cheung and Chan2016). Moreover, depressed subjects demonstrate impaired reward maximization, but, interestingly, enhanced punishment minimization, in a variety of decision-making task (Kunisato et al., Reference Kunisato, Okamoto, Ueda, Onoda, Okada, Yoshimura, Suzuki, Samejima and Yamawaki2012; Maddox et al., Reference Maddox, Gorlick, Worthy and Beevers2012; Beevers et al., Reference Beevers, Worthy, Gorlick, Nix, Chotibut and Todd Maddox2013; Blanco et al., Reference Blanco, Otto, Maddox, Beevers and Love2013; Herzallah et al., Reference Herzallah, Moustafa, Natsheh, Abdellatif, Taha, Tayem, Sehwail, Amleh, Petrides, Myers and Gluck2013; Cooper et al., Reference Cooper, Arulpragasam and Treadway2018; Kumar et al., Reference Kumar, Goer, Murray, Dillon, Beltzer, Cohen, Brooks and Pizzagalli2018).
The above observations have been refined with the use of computational models, in which value representations of stimulus–action pairs are formed and updated using the difference between expected and actual outcomes (i.e. prediction errors). Fitting these models to participants' choice behavior by adjusting model parameters allows for the assessment of group differences in various aspects of the learning process. Using this approach, it has been found that depressed participants are less sensitive to rewarding outcomes (Huys et al., Reference Huys, Pizzagalli, Bogdan and Dayan2013) but more responsive to punishments (Byrne et al., Reference Byrne, Norris and Worthy2016; Mkrtchian et al., Reference Mkrtchian, Aylward, Dayan, Roiser and Robinson2017) than controls. Additionally, depression has been associated with abnormalities in learning rate (Chase et al., Reference Chase, Frank, Michael, Bullmore, Sahakian and Robbins2010; Dombrovski et al., Reference Dombrovski, Clark, Siegle, Butters, Ichikawa, Sahakian and Szanto2010; Beevers et al., Reference Beevers, Worthy, Gorlick, Nix, Chotibut and Todd Maddox2013; Cooper et al., Reference Cooper, Gorlick, Denny, Worthy, Beevers and Todd Maddox2014) and exploration (Kunisato et al., Reference Kunisato, Okamoto, Ueda, Onoda, Okada, Yoshimura, Suzuki, Samejima and Yamawaki2012; Beevers et al., Reference Beevers, Worthy, Gorlick, Nix, Chotibut and Todd Maddox2013; Rupprechter et al., Reference Rupprechter, Stankevicius, Huys, Steele and Seriès2018) parameters, with results suggesting that depressed individuals show suboptimal reward learning parameters.
The above findings indicate that depression is associated with abnormalities during learning from non-social outcomes. However, it is not clear whether these deficits extend to the social domain, and, if so, how they relate to everyday social experiences. The current study aimed to address this question. For this purpose, participants with high and low depression scores completed a learning task with two other people. During the task, subjects made choices between party decoration items for which they received positive, neutral, or negative feedback. In the social condition, participants were told that the feedback came from the other two people, whereas, in reality, it was computer-generated. A non-social condition with monetary outcomes was also added to assess the specificity of potential findings. In both conditions, subjects' learning performance, as well as their negative expectancy biases, was measured. Additionally, participants completed questionnaires assessing their social anhedonia and depression severity and answered several questions about the quantity and quality of their daily interpersonal interactions. A computational modeling approach was used to examine group differences in the mechanisms underlying the learning process, and model parameters were linked to real-life measures.
It was hypothesized that, compared to controls, subjects with high depression scores would show deficits in learning from social outcomes. As described above, reduced social learning could lead to higher, negatively-perceived uncertainty about social outcomes or to suboptimal interpersonal behavior that elicits more negative feedback from others. Thus, it was expected that impairments in social learning in the current study may be associated with decreases in the quality of real-life interpersonal encounters. Additionally, it was predicted that increased social anhedonia scores and negative social expectancy biases would be linked to reductions in the reported quantity of social engagement, based on the abovementioned relation between these constructs and social withdrawal.
Methods and materials
Participants
Ninety-two volunteers between the age of 18 and 45 years were recruited: 40 participants with high (HD; Beck Depression Inventory scores ⩾17; Beck et al., Reference Beck, Steer and Brown1996) and 52 subjects with low (LD; BDI scores ⩽7) levels of depressive symptomatology. Some participants were tested at the university (N HD = 20, N LD = 30), while others performed the experiment online (N HD = 20, N LD = 22). This allowed for the collection of data from volunteers in different geographical locations with diverse backgrounds (see online Supplementary Material).
All participants were screened using an online version of the structured clinical interview for DSM-IV (SCID; adapted from First et al., Reference First, Spitzer, Gibbon and Williams1996). Given that the current study was focused more generally on individuals with depression symptoms, rather than specifically on those with clinical levels of MDD, the SCID was not used for diagnostic purposes, but merely to determine if any exclusion criteria were met. Specifically, volunteers were excluded if they had used any recreational drugs in the past 3 months, had taken psychiatric medication in the past year, or had ever experienced potentially clinical levels of symptoms of any Axis I disorder (besides depression or low levels of anxiety for HD subjects, with anxiety symptoms being secondary to depression).
Ethical approval for the study was obtained from the University of Reading Ethics Committee (2016-152-CM). All participants provided informed consent and received a reimbursement of £15.
Procedure
After the screening, eligible participants filled in the following questionnaires online: Temporal Experience of Pleasure Scale (TEPS; Gard et al., Reference Gard, Gard, Kring and John2006), Revised Social Anhedonia Scale (RSAS; Eckblad et al., Reference Eckblad, Chapman, Chapman and Mishlove1982), Social Anxiety Questionnaire (SAQ; Caballo et al., Reference Caballo, Salazar, Irurtia, Arias and Hofmann2010), and a demographics form. Additionally, subjects answered a number of questions about their real-life social experiences, reporting how many friends they have and how much time they spend engaged in pleasant (e.g. going for dinner with friends or listening to music) and unpleasant (e.g. having a disagreement or doing chores) social and non-social activities. Subsequently, participants performed the learning task described below with two other people (see online Supplementary Material) and were then given a debrief sheet which clearly justified the deception involved in the study.
Learning task
During the task, participants' aim was to make choices that maximized positive and minimized negative outcomes. On each trial, subjects were asked to choose one of two party decoration items. Subsequently, they received positive, neutral, or negative feedback and rated their emotional response to the outcome (see Fig. 1 and online Supplementary Material).
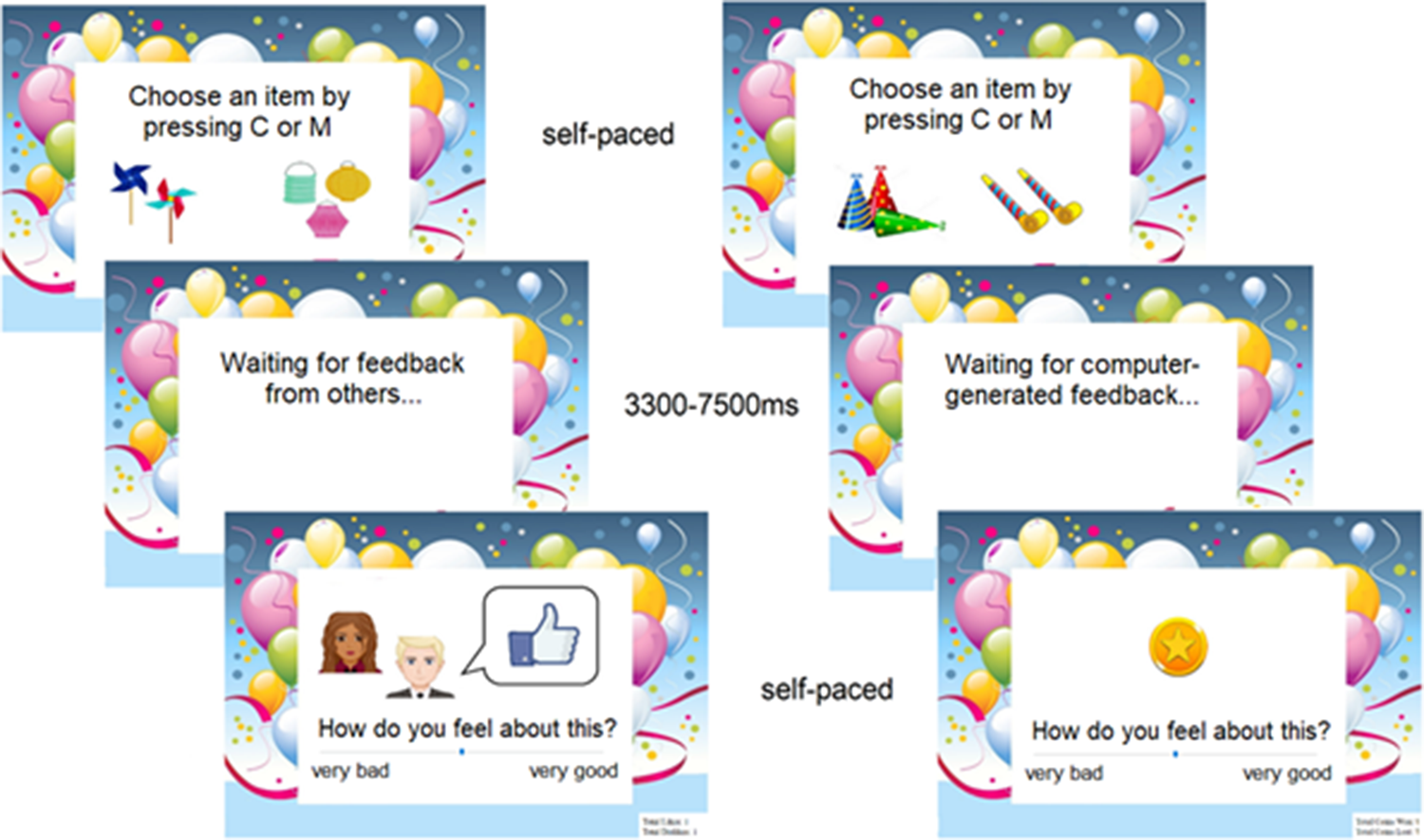
Fig. 1. Illustration of the social (left) and non-social (right) conditions of the learning task (see text for details).
The task consisted of a social and a non-social condition, which were completed in counterbalanced order. That is to say, about half of the HD and LD participants completed the social condition first, while the other half performed the non-social condition first. In the social condition, the feedback consisted of ‘like’, ‘neural’, and ‘dislike’ signs as used on social media (thumb up, horizontal, or down). Participants were told that the feedback came from the other two people with whom they were completing the task, while, in reality, it was computer-generated. In the non-social condition, the feedback consisted of winning 5 pence, no outcome, or losing 5 pence, represented by an image of a golden coin, a circle, or a crossed-out coin, respectively. Subjects were informed that the money they won during the task would be added to their reimbursement.
Eight party decoration items were used during the task, half of which were randomly allocated to the social and non-social condition, respectively. Items were paired in all possible combinations, with each of the pairs being repeated 12 times. Moreover, for each condition, each item was randomly assigned to one of the following outcome contingencies: 75% (item 1) or 25% (item 2) chance of yielding positive rather than neutral feedback, or 75% (item 3) or 25% (item 4) chance of yielding negative rather than neutral feedback.
Explicit feedback expectancies were assessed in the middle and at the end of each condition by asking participants to rate each item twice: once on how likely they thought selecting this item would yield positive feedback, and once on how likely they thought choosing this item would yield negative feedback. Ratings were made on a visual analogue scale ranging from ‘very unlikely – 0%’ to ‘very likely – 100%’.
Moreover, after each condition, participants were asked to rate how emotionally arousing they found the feedback, and, at the end of the testing session, subjects indicated how sure they were that the social feedback came from other people (1 = very doubtful; 10 = very sure).
Analysis
To examine participants' reward and ‘punishment’ learning performance, the frequencies of selecting the most rewarded item and of avoiding the most ‘punished’ item were calculated for the social and non-social conditions for each subject.
Moreover, reaction times (RTs) were log transformed due to a positive skew, and participants' negative bias scores were calculated based on their positive v. negative feedback expectancy ratings (see online Supplementary Material for details).
Task data were analyzed using mixed-measure ANCOVAs, in which the testing location (online or at the university) was added as a control variable. Where the sphericity assumption was violated, Greenhouse–Geisser corrected results are reported. The data of one online LD participant were removed from all task analyses because their performance was substantially below chance, indicating that they may have misunderstood the task.
Mann–Whitney U tests were performed on the questionnaire measures and on participants' ratings of how sure they were that the social feedback came from other people (see online Supplementary Material for results of the latter analysis).
Additionally, a multiple regression analysis was conducted, predicting the amount of time spent with friends from RSAS and negative bias scores, while controlling for BDI and SAQ measures. Given that for the raw data, the assumption of normally distributed residuals was not met, the regression was performed on rank-transformed data. As suggested by Thomas et al. (Reference Thomas, Nelson and Thomas1999), F-statistics were thus converted to L-statistics (N−1 × r 2), degrees of freedom were obtained by multiplying the number of independent variables with the number of dependent variables, and p-values were derived by evaluating the L-statistic on the χ2 table.
Computational modeling
A range of Q-learning models were fit separately to the social and non-social data. The models contained between two and seven free parameters, including learning rates for positive (αG) and negative (αL) prediction errors, as well as parameters accounting for choice biases (φ; i.e. for repeated item choices independent of the outcome), choice bias decay (γ), memory decay (ω; i.e. forgetting while making item ratings in the middle of the task), outcome valuation (d; i.e. the impact of rewards and ‘punishments’ is in relation to the initial outcome expectation), and amounts of exploration v. exploitation (using a temperature parameter, τ; see online Supplementary Material).
The models were fit to the data using maximum likelihood estimation and compared based on Akaike's Information Criterion weights (see Wagenmakers and Farrell, Reference Wagenmakers and Farrell2004). Additionally, data simulation and parameter recovery were performed for model validation (see online Supplementary Material).
Parameter values from the best-fitting (as well as from a similarly well-fitting) model were compared between groups and entered into a regression analysis predicting the reported amount of time spent in pleasant and unpleasant social situations. Again, the assumption of normally distributed residuals was not met; thus, L-statistics are reported.
Results
Demographic and questionnaire measures
No group differences in age or (non-social) consummatory anhedonia were observed. However, as expected, HD participants showed significantly higher levels of (non-social) anticipatory anhedonia, social anhedonia, social anxiety, and depression (see Table 1).
Table 1. Demographic data and questionnaire scores for participants with low (LD) and high (HD) depressive symptomatology
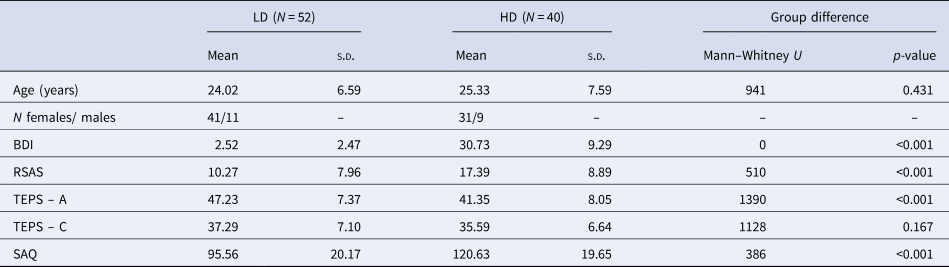
HD, high depressive symptomatology; LD, low depressive symptomatology; s.d., standard deviation; BDI, Beck Depression Inventory; RSAS, Revised Social Anhedonia Scale; TEPS, Temporal Experience of Pleasure Scale (C, consummatory; A, anticipatory); SAQ, Social Anxiety Questionnaire.
Real-life social interactions
Compared to controls, HD participants reported having significantly fewer friends (U = 1232, p = 0.007; see Fig. 2a) and indicated spending significantly more time in unpleasantly perceived social (U = 486, p < 0.001) and non-social (U = 575, p = 0.005) situations. By contrast, no significant group differences were observed in the reported amount of time spent in pleasantly perceived social (U = 1047, p = 0.173) or non-social (U = 998, p = 0.351) situations (see Fig. 2b and see online Supplementary Material for further results).
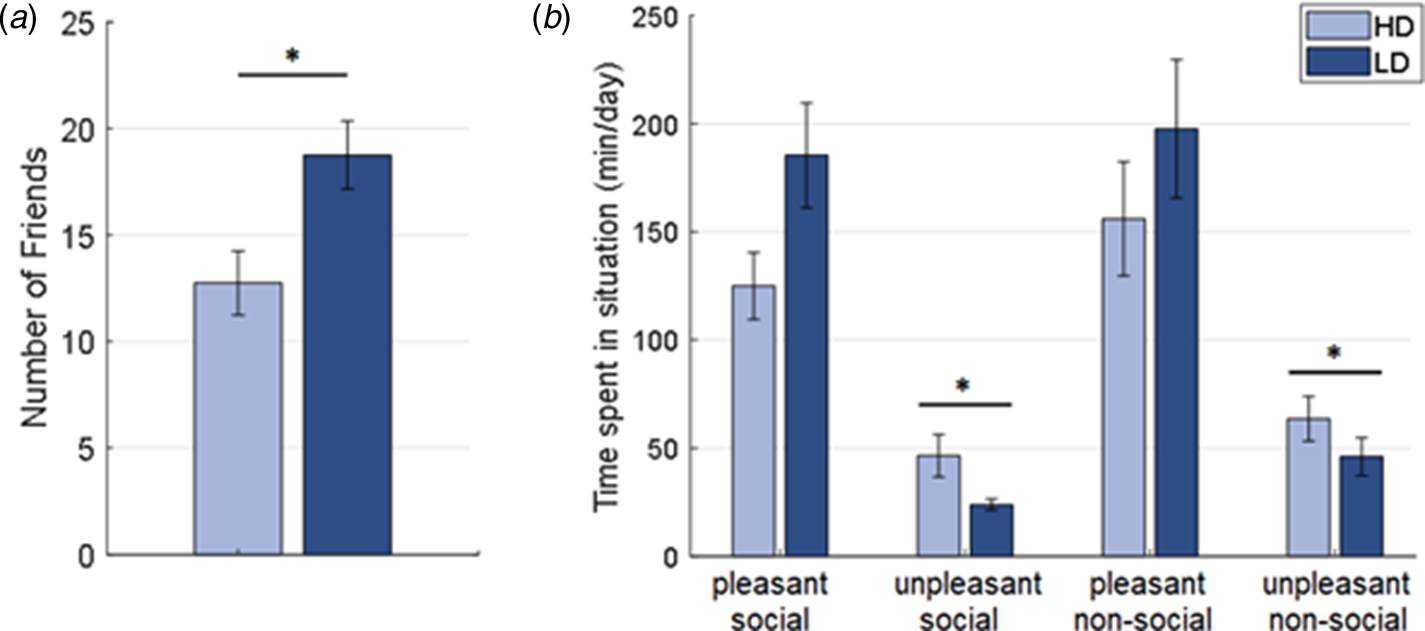
Fig. 2. (a) Number of friends and (b) reported time spent in pleasant and unpleasant social and non-social situations for participants with high (HD) and low (LD) depressive symptomatology.
Learning task performance
A mixed-measure ANCOVA (group × condition × valence, controlling for testing location) on participants' arousal ratings for positive, neutral, and negative feedback revealed a significant group by condition by valence interaction [F (2, 166) = 5.47, p = 0.005; see online Supplementary Material for main effects]. Follow-up one-way ANCOVAs showed that HD subjects reported significantly higher arousal to negative social feedback than LD participants [F (1, 85) = 4.84, p = 0.030], with no significant group differences for any other feedback type (all F < 1.6). Moreover, no significant group effects or interactions were observed for participants' ratings of their emotional responses to the feedback (see online Supplementary Material).
Furthermore, a mixed-measure ANCOVA (group × valence × condition, controlling for testing location) of the learning performance revealed a significant main effect of valence [F (1, 88) = 4.13, p = 0.045], with participants demonstrating better reward than punishment learning. Moreover, a trend for a group effect was observed [F (1, 88) = 3.50, p = 0.065], as HD individuals' learning performance tended to be worse than that of LD subjects. None of the other main effects or interactions is significant (all F < 2.2).
A mixed-measure ANCOVA (group × condition, controlling for testing location) performed on the log-transformed RTs demonstrated no significant main effects of group or condition, nor a significant interaction (all F < 0.60). It should be noted that, regardless of the depression group, participants tested online showed slower RTs than those tested at the university [F (1, 87) = 5.86, p = 0.017]. None of the other learning task measures showed significant differences between testing locations.
For the negative feedback expectancy biases, a mixed-measure ANCOVA (group × condition, controlling for testing location) showed a significant main effect of the group [F (1, 88) = 5.33, p = 0.023], as HD participants' bias scores were significantly higher than those of LD subjects across both conditions. This group effect remained significant when controlling for the difference in the overall amounts of negative and positive feedback actually received throughout the task [F (1, 88) = 5.70, p = 0.019].
Moreover, a regression analysis revealed that higher RSAS social anhedonia scores (β = −0.62, p < 0.001), as well as (marginally) more negative expectancy biases (β = −0.17, p = 0.063), predicted decreased reported amounts of time spent with friends [while controlling for SAQ social anxiety, β = 0.17, p = 0.127, and BDI depression, β = 0.13, p = 0.254, scores; L(4) = 25.70, p < 0.001, R 2 = 0.31; see online Supplementary Material for further analyses].
Computational modeling
For the social condition, the best-fitting model for both groups included one learning rate (α; αG = αL), as well as the outcome valuation (d), memory decay (ω), and temperature parameters (τ; model Q16 in online Supplementary Table S1). For the non-social condition, the best-fitting model for both groups contained one learning rate, and the outcome valuation, choice bias (φ), and temperature parameters (model Q5 in online Supplementary Table S1, see online Supplementary Material for model validation). The involvement of a ‘sticky’ choice bias parameter in the non-social but not the social condition may indicate that participants were more likely to find a strategy and stick with it in the non-social condition, potentially because they perceived the latter to be less volatile than the social condition.
Mann–Whitney U tests on parameters form the social condition found significantly lower learning rates (U = 1277, p = 0.040) in HD compared to LD subjects (see Fig. 3a). No group differences were observed for any of the other social learning parameters, nor for any of the parameters from the non-social condition (see Fig. 3b and online Supplementary Material). The same pattern of results was observed when assessing parameter group differences for models with a similarly good fit.
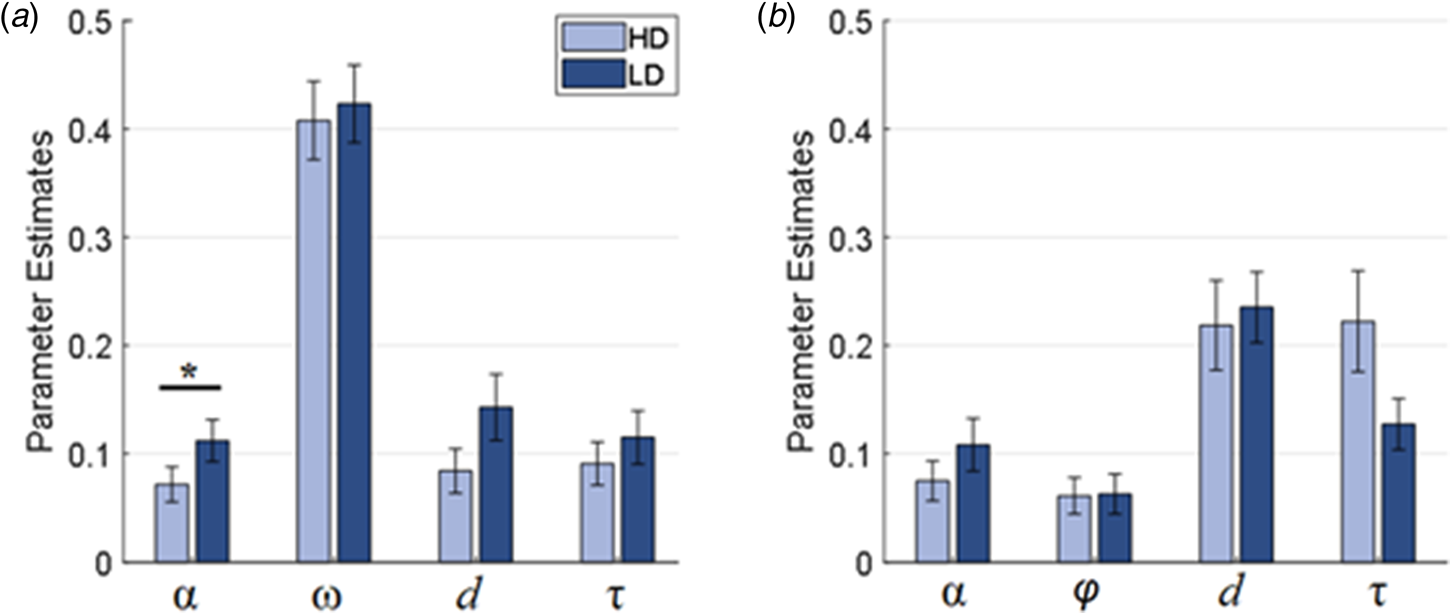
Fig. 3. Parameter estimates for the (a) social (Q16) and (b) non-social (Q5) condition for participants with high (HD) and low (LD) depressive symptomatology; α, learning rate; ω, memory decay; d, outcome valuation; τ; explore-exploit/temperature; φ, choice bias.
Moreover, a regression analysis revealed that social model parameters and questionnaire measures significantly predicted the reported amount of time spent in unpleasantly perceived social situations [L(6) = 16.21, p = 0.013, R 2 = 0.19]. This relation was driven by outcome valuation (β = 0.31, p = 0.016) and learning rate (β = −0.45, p = 0.046; see Fig. 4) parameter values, as well as BDI scores (β = 0.28, p = 0.018). By contrast, SAQ social anxiety scores (β = 0.15, p = 0.188), negative biases (β = 0.03, p = 0.949), and temperature parameter values (β = 0.23, p = 0.252) had no significant effect. Thus, participants with higher outcome valuation parameters (i.e. with diminished responsiveness to rewards and increased sensitivity to punishments), lower learning rates, and higher BDI scores reported spending more time in unpleasantly perceived social situations. Highly similar results were obtained when using parameters from a similarly well-fitting model (see online Supplementary Material), providing evidence for the robustness of these findings.

Fig. 4. Association between learning rate parameters and time spent in unpleasant social situations (shown data is rank-transformed).
In addition, the abovementioned measures also significantly predicted the reported amount of time spent in pleasantly perceived social situations [L(5) = 18.06, p = 0.003, R 2 = 0.22]. This association was driven by RSAS social anhedonia scores (β = −0.49, p < 0.001), with temperature parameters only marginally contributed to this relation (β = 0.34, p = 0.065). By contrast learning rates (β = −0.37, p = 0.091), outcome valuation parameters (β = 0.01, p = 0.944) and BDI scores (β = −0.01, p = 0.896) had no significant effect. Note that using parameters from a similarly well-fitting model led to a somewhat different pattern of results (see online Supplementary Material), indicating that the parameter-related findings are not robust and should thus be interpreted with caution.
Discussion
Addressing a lack of research on social learning in depression, the current study assessed the performance of participants with high (HD) and low (LD) depression scores in a learning task with social and non-social feedback. Additionally, measures of participants' everyday interpersonal interactions were collected, which allowed for an examination of the relation between task-based social learning performance and real-life social experiences.
Learning from social feedback predicts the quality of social experiences
In the task, it was found that HD participants tended to demonstrate reduced learning across all trials compared to LD controls. Due to the lack of interaction, it was not possible to ascertain whether this effect may have been driven by reward or ‘punishment’ learning deficits in the social or non-social condition. Nevertheless, the finding is consistent with previous reports of impaired learning in depression (Herzallah et al., Reference Herzallah, Moustafa, Natsheh, Abdellatif, Taha, Tayem, Sehwail, Amleh, Petrides, Myers and Gluck2013; Kumar et al., Reference Kumar, Goer, Murray, Dillon, Beltzer, Cohen, Brooks and Pizzagalli2018; Kunisato et al., Reference Kunisato, Okamoto, Ueda, Onoda, Okada, Yoshimura, Suzuki, Samejima and Yamawaki2012; Maddox et al., Reference Maddox, Gorlick, Worthy and Beevers2012; Robinson et al., Reference Robinson, Cools, Carlisi, Sahakian and Drevets2012; Pechtel et al., Reference Pechtel, Dutra, Goetz and Pizzagalli2013).
To examine which learning mechanisms may be affected in HD individuals, computational modeling was performed. This approach revealed that, in the social (but not the non-social) condition of the task, HD subjects demonstrated significantly lower learning rates than LD participants. Hence, HD subjects made smaller updates to their value representations based on social outcomes than controls. This result is in line with the previous observations of abnormal learning rates in depressed individuals (Chase et al., Reference Chase, Frank, Michael, Bullmore, Sahakian and Robbins2010; Dombrovski et al., Reference Dombrovski, Clark, Siegle, Butters, Ichikawa, Sahakian and Szanto2010; Beevers et al., Reference Beevers, Worthy, Gorlick, Nix, Chotibut and Todd Maddox2013; Cooper et al., Reference Cooper, Gorlick, Denny, Worthy, Beevers and Todd Maddox2014) and extends these findings to the social domain.
Interestingly, the current study further observed that social learning parameters predict real-life interpersonal experiences. Specifically, it was found that, across all participants, spending more time in unpleasant social situations was associated with higher outcome valuation parameters, i.e. with enhanced sensitivity to negative and diminished responsivity to positive feedback relative to initial expectations (of an outcome value of 0). Thus, participants with elevated outcome valuation parameters may subjectively perceive more social interactions as unpleasant, resulting in increased reporting of negative encounters.
Additionally, an increased amount of time spent in unpleasant social situations was also associated with lower learning rate values. This may partly be the case because reduced updating of outcome predictions based on social feedback may give rise to enhanced uncertainty about what to expect from social interactions. Considering that uncertainty can be regarded as negative (e.g. in depressed individuals; Carleton et al., Reference Carleton, Mulvogue, Thibodeau, McCabe, Antony and Asmundson2012), heightened uncertainty may result in more social encounters being subjectively perceived as unpleasant. Alternatively, it is possible that individuals with low learning rates objectively experience more unpleasant social encounters, because their impaired ability to use social feedback to appropriately update future actions may lead to suboptimal interpersonal behavior (see below).
The current study further found that the reported amount of time spent in pleasant social situations was predicted by social anhedonia scores. This finding could be a result of anhedonic participants' reduced motivation to engage in social activities or due to their tendency to experience and categorize fewer social encounters as pleasant. We did not observe a robust association between learning parameters and the everyday experience of pleasant social situations. However, this relation warrants further examination in light of previous research which found that, in a similar task, learning was related to heightened striatal dopamine release which was, in turn, linked to increased reward-oriented behavior in daily life (Kasanova et al., Reference Kasanova, Ceccarini, Frank, van Amelsvoort, Booij, Heinzel, Mottaghy and Myin-Germeys2017).
It is worth noting that, compared to LD controls, HD subjects showed heightened social anhedonia scores, as well as reduced learning rates, and reported spending a numerically lower and significantly higher amount of time in pleasantly and unpleasantly perceived social situations, respectively. Taken together with the above results, these findings suggest that depressed participants' increased levels of social anhedonia may reduce their experience of pleasant social encounters. Moreover, depressed subjects' deficits in updating outcome predictions based on social feedback may expose them to more unpleasant interpersonal experiences, potentially due to higher uncertainty about social outcomes or due to an impaired ability to appropriately adjust their behavior based on other people's responses.
The latter suggestion is in line with previous proposals that increased the experience of negative social encounters in depression may be the result of deficits in the (learned) ability to evoke pleasant responses from other people (Lewinsohn et al., Reference Lewinsohn, Sullivan and Grosscup1980; Carvalho and Hopko, Reference Carvalho and Hopko2011). This notion is partly supported by findings that depressed individuals show less appropriate behavior during social interactions, as they make less eye contact, smile less, time their responses less fittingly, and are less likely to offer help to others than controls (reviewed in Segrin, Reference Segrin2000; Rottenberg and Gotlib, Reference Rottenberg and Gotlib2004; see also Setterfield et al., Reference Setterfield, Walsh, Frey and McCabe2016). Importantly, inappropriate social behavior has been shown to elicit fewer positive responses to, and even rejection of, depressed subjects by their interlocutors (Segrin and Abramson, Reference Segrin and Abramson1994). Following on from our results, it would therefore be interesting to investigate whether the relation between learning performance and the (objective) frequency of negative social encounters is mediated by individuals' (learned) social skills.
Responses to social feedback predict the quantity of social engagement
We further observed that, in the learning task, HD participants reported heightened arousal to negative social outcomes, as well as enhanced negative feedback expectancy biases, compared to controls. It is possible that the elevated arousal experienced by HD subjects in response to negative social feedback may have made negative outcomes more salient than positive ones, which may have contributed to increases in negative expectancy biases. Alternatively, the latter may have been the consequence of a generalization from heightened levels of (actual or perceived) negative experiences in real life to the experimental setting. In either case, our findings are in line with past observations that depression symptoms are associated with enhanced expectancies of negative evaluations from others (Caouette and Guyer, Reference Caouette and Guyer2016).
Moreover, we found that higher social anhedonia scores and, marginally, negative social expectancy biases predicted a reduction in the quantity of social engagement. Notably, HD subjects showed increased anhedonia and negative bias scores. Thus, in agreement with previous proposals (Lewinsohn, Reference Lewinsohn, Friedman and Katz1974; Kupferberg et al., Reference Kupferberg, Bicks and Hasler2016), our findings suggest that HD individuals' reduced responsiveness to pleasant social interactions, as well as their increased expectancies of negative social outcomes, may result in withdrawal from close relationships. This disengagement, in turn, may prevent exposure to positive social experiences, thereby sustaining anhedonia levels and further biasing HD subjects' expectancies.
Limitations
It should be noted that excluding HD individuals based on medication use within the past year could have led to a mix of participants, some of whom were treatment resistant and had given up on medication, and others who had not yet, or only recently, been diagnosed with depression. However, as we know anti-depressant medications can effect reward processing (McCabe et al., Reference McCabe, Mishor, Cowen and Harmer2010) thus we thought it best to exclude. Further, although those who did the study online and those in the lab both did the experiment, the environment around them may have been quite different, and although we did control for this in our analysis, there may still have been effects on the results related to the different contexts that we are unaware off.
In future studies, it would be interesting to assess whether potential early social learning impairments in depression predict later social withdrawal, possibly via abnormal expectancies and impaired social skills. Additionally, an examination of the effects of Behavioral Activation therapy with a social skills training component (Lewinsohn, Reference Lewinsohn, Friedman and Katz1974; Barth et al., Reference Barth, Munder, Gerger, Nüesch, Trelle, Znoj, Jüni and Cuijpers2013) on the abovementioned social deficits in depression would be of interest.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0033291719003222.
Acknowledgements
We would like to thank Baisha Copeman and Shauna Cooney (University of Reading) for their help with the data collection. Additionally, we thank the members of the Laboratory of Neural Computation and Cognition (Director: Michael Frank; Brown University) for providing computational modeling training.
Financial support
This research was supported by the Medical Research Council PhD studentship of Anna-Lena Frey.
Conflict of interest
Ciara McCabe and Anna-Lena Frey report no potential conflicts of interest. Michael Frank is a consultant for Hoffman La Roche Pharmaceuticals. The current experiment was not related to any consulting activity.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.








