Introduction
Lysergic acid diethylamide (LSD) is a potent serotonergic hallucinogen or ‘psychedelic’ that alters consciousness in a marked and unusual way. The drug was first intentionally consumed by the Swiss chemist Albert Hofmann in 1943 in a self-experiment in which he ingested 250 µg (a high dose) in his laboratory before travelling home. In a detailed report of his experience, written a few days later, Hofmann describes an initially unpleasant experience, characterized by altered perception, fear and paranoia: his next-door neighbour transformed into a ‘malevolent, insidious witch with a coloured mask’, he sensed a ‘disintegration of the outer world’, a ‘dissolution of [his] ego’ and was ‘seized by a dreadful fear of going insane’ (Hofmann, Reference Hofmann1980).
From this account, it would be reasonable to suspect that Dr Hofmann was negatively affected by this experience but his description of his mental state the next day suggests otherwise: ‘I then slept, to awake the next morning with a clear head… A sensation of wellbeing and renewed life flowed through me. Breakfast tasted delicious and gave me extraordinary pleasure. When I later walked out into the garden, in which the sun shone now after a spring rain, everything glistened and sparkled in a fresh light. The world was as if newly created.’(Hofmann, Reference Hofmann1980)
When LSD was first distributed by Sandoz pharmaceuticals in 1948, product guidelines stipulated two main applications: (1) analytical psychotherapy and (2) experimental studies on psychoses. The rationale for the former was that LSD could ‘elicit [the] release of repressed material and provide mental relaxation for anxiety and obsessional neuroses’, and, for the latter, that it could model aspects of psychosis and facilitate an understanding of its nature and pathogenesis (Hofmann, Reference Hofmann1980). These two properties formed the basis of a large number of research projects with LSD in the 1950s and 1960s. However, the apparent paradox by which the same compound can be both a model of, and yet a treatment for, psychopathology has never been properly addressed.
In the early years of research with LSD, its remarkable potency (LSD is psychoactive in doses of 25 µg or lower; Hintzen & Passie, Reference Hintzen and Passie2010) led psychiatrists to speculate about the existence of an endogenous LSD-like ‘schizotoxin’ in the brains of patients with schizophrenia (Osmond & Smythies, Reference Osmond and Smythies1952). In subsequent years, however, focus shifted more onto therapeutic applications, such as treating alcohol dependence, mood disorders and anxiety related to dying. Human research with LSD was brought to a halt in the late 1960s due to political pressure, motivated in part by reports of adverse psychological reactions among people using the drug improperly. Ironically, however, at the same time, reports of therapeutic success in the treatment of various psychiatric disorders were beginning to amount (Grinspoon & Bakalar, Reference Grinspoon and Bakalar1979). In the last 18 months, five new reports on clinical research with LSD have appeared in the scientific press (Carhart-Harris et al. Reference Carhart-Harris, Kaelen, Whalley, Bolstridge, Feilding and Nutt2014a ; Gasser et al. Reference Gasser, Holstein, Michel, Doblin, Yazar-Klosinski, Passie and Brenneisen2014; Schmid et al. Reference Schmid, Enzler, Gasser, Grouzmann, Preller, Vollenweider, Brenneisen, Muller, Borgwardt and Liechti2014; Dolder et al. Reference Dolder, Schmid, Haschke, Rentsch and Liechti2015; Kaelen et al. Reference Kaelen, Barrett, Roseman, Lorenz, Family, Bolstridge, Curran, Feilding, Nutt and Carhart-Harris2015), one of which focused on the drug's therapeutic effects (Gasser et al. Reference Gasser, Holstein, Michel, Doblin, Yazar-Klosinski, Passie and Brenneisen2014) and, another, its psychotomimetic effects (Schmid et al. Reference Schmid, Enzler, Gasser, Grouzmann, Preller, Vollenweider, Brenneisen, Muller, Borgwardt and Liechti2014).
Clinical research with psychedelics is currently undergoing a major revival and modern studies are documenting the same paradoxical properties that were historically described with LSD. For example, in a controlled study in healthy volunteers, high-dose psilocybin produced strong or extreme fear in 30% of healthy volunteers and yet 80% reported improvements in wellbeing after the experience, with none reporting any decreases (Griffiths et al. Reference Griffiths, Richards, McCann and Jesse2006). Remarkably, in follow-up of the same sample, 65% reported improved wellbeing 14 months after their (single) psilocybin experience (Griffiths et al. Reference Griffiths, Richards, Johnson, McCann and Jesse2008) and significant increases in the personality trait openness were also evident (MacLean et al. Reference Maclean, Johnson and Griffiths2011). These finding in healthy volunteers are supplemented by an increasing number of patient studies. Clinical improvements have been observed with psilocybin-assisted psychotherapy for the treatment of tobacco (Johnson et al. Reference Johnson, Garcia-Romeu, Cosimano and Griffiths2014) and alcohol addiction (Bogenschutz et al. Reference Bogenschutz, Forcehimes, Pommy, Wilcox, Barbosa and Strassman2015), obsessive–compulsive disorder (Moreno et al. Reference Moreno, Wiegand, Taitano and Delgado2006) and anxiety related to dying (Grob et al. Reference Grob, Danforth, Chopra, Hagerty, McKay, Halberstadt and Greer2011). Many of these reports mention some psychological discomfort during the acute experience; yet, the therapeutic benefits have been impressive and enduring.
Case reports of persistent psychological problems apparently precipitated by a psychedelic have considerable potential to excite alarm (Reich & Hepps, Reference Reich and Hepps1972). However, such cases are rare and largely restricted to recreational use. Evidence does not support the view that psychedelics are harmful to mental health (Hendricks et al. Reference Hendricks, Thorne, Clark, Coombs and Johnson2015). Indeed, to the contrary, two recent population studies found decreased rates of suicidality and psychological distress among persons reporting previous use of psychedelics (Hendricks et al. Reference Hendricks, Thorne, Clark, Coombs and Johnson2015) and no evidence of any increased rates of mental health problems (Krebs & Johansen, Reference Krebs and Johansen2013). Similarly, large meta-analyses of controlled research have found that cases of mental health complications following exposure to a psychedelic are extremely rare (i.e. <0.1%), even in vulnerable populations (i.e. <0.2%), and are rarer still if volunteers are properly screened (Cohen & Ditman, Reference Cohen and Ditman1962; Studerus et al. Reference Studerus, Kometer, Hasler and Vollenweider2011).
The main aim of the present study was to investigate the acute and ‘mid-term’ (i.e. 2 weeks after the acute experience) psychological effects of LSD in a placebo-controlled study in healthy volunteers. Validated measures of personality, optimism and psychotic symptoms were collected at baseline and 2 weeks post-LSD/placebo and measures of mood, cognition and psychotomimetic states were collected at the end of each dosing day. It was predicted that LSD would induce emotional lability and psychosis-like symptoms acutely but increase psychological wellbeing and openness in the longer term.
Method
Experimental design
This was a placebo-controlled, within-subjects/cross-over study, with a balanced-order design. A total of 20 healthy volunteers were recruited to the study via word of mouth. The study received a favourable opinion from NRES Committee London – West London and was conducted in accordance with Good Clinical Practice guidelines, NHS Research Governance Framework and complied with the ethical standards of the declaration of Helsinki (1975, revised 2008). Imperial College London sponsored the research and a Home Office license was obtained for research with schedule one drugs. All volunteers were sent a study information sheet and asked to read it before their screening visit.
Volunteers made three study visits: screening, dosing session 1 and dosing session 2. Dosing sessions were separated by at least 2 weeks in every case and the order of receipt of LSD was balanced, i.e. half of the volunteers received LSD in dosing session 1 and half in dosing session 2. Volunteers were blind to the dosing order but the research team was not. Dosing order was determined sequentially so that all odd-numbered volunteers (i.e. S1 was the first volunteer recruited and S20 was the last) received LSD in dosing session 1, whereas all even-numbered volunteers received it in dosing session 2. Subjective ratings were completed electronically and remotely soon after screening (baseline) and 2 weeks after a dosing session and sent to the researchers via email. Volunteers were instructed to complete the questionnaires in a quiet space without rushing. Two questionnaires pertaining to the acute drug experience were completed electronically within the research centre at the close of dosing days.
Screening
Prior to study enrolment, volunteers attended a screening visit at the Imperial Clinical Research Facility (ICRF) at the Hammersmith Hospital in West London. The study design, procedures and psychological effects of LSD were explained and signed informed consent taken. Key exclusion criteria were: <21 years of age, personal history of diagnosed psychiatric illness, immediate family history of a psychotic disorder, an absence of previous experience with a classic psychedelic drug (e.g. LSD, mescaline, psilocybin/magic mushrooms or dimethyltryptamine/ayahuasca), pregnancy, problematic alcohol use (assessed via psychiatric interview and reported weekly units), or a medically significant condition rendering the volunteer unsuitable for the study. The decision to recruit only individuals with prior experience with psychedelics was motivated by safety considerations, i.e. to minimize the risk of an adverse response to the drug. Screening involved routine blood tests, electrocardiogram, heart rate, blood pressure and a brief neurological examination. All enrolled participants were deemed physically and mentally healthy by the study psychiatrist, and none had histories of drug or alcohol dependence or diagnosed psychiatric disorder. Volunteers were asked to remain abstinent from alcohol the evening before a dosing day and to refrain from using other recreational drugs for the duration of the study.
Study procedures
Participants were asked to arrive at the study centre (Cardiff University's Brain Research Imaging Centre, CUBRIC) at a specific time at or before 09.00 hours. A urine test for drugs of abuse and pregnancy (where relevant) was carried out. Participants were re-briefed about the procedures for the day and any recent drug and alcohol use was documented. The study physician inserted a cannula into a vein in the antecubital fossa in preparation for intravenous (i.v.) dosing and the volunteer was encouraged to relax prior to drug/placebo administration. The dose of LSD was 75 µg (by mouth; p.o.) in 10 ml saline. Previous research has found this dose to produce robust psychological effects that are generally well tolerated (Carhart-Harris et al. 2014a). Placebo was 10 ml saline (i.v.). Both solutions were infused over 2 min.
After dosing, volunteers completed a functional neuroimaging protocol. This aspect of the study will be covered in detail in separate publications. In brief, subjects spent a period habituating to a scanner environment in a mock magnetic resonance imaging (MRI) scanner before entering a real MRI scanner (1 h post-dosing). The MRI scanning session lasted for 1 h after which a structured interview was performed and some ratings completed. Participants entered a magnetoencephalography (MEG) scanner approximately 3.5 h post-dosing. MEG scanning lasted for just over 1 h. Participants were then interviewed and completed a battery of cognitive and behavioural tests (these will be detailed in a separate publication). Tasks were completed 6–7 h post-dosing.
The subjective effects of LSD were detected approximately 10 min post-infusion, peaked approximately 120 min post-infusion, and subsided to a negligible level approximately 7–8 h post-infusion. Participants were discharged by the study physician when they were considered to be functioning normally. Volunteers were either picked up by a friend or partner, ordered a taxi, or accompanied home by the research team as far as was feasible. Volunteers were asked to contact a researcher via telephone or text message once they had arrived home safely. A study psychiatrist was present for the duration of each dosing session and one researcher was allocated to each participant to assist them throughout the day. For each participant, the same researcher was present for both dosing days.
Main outcomes and measures
Acute outcomes
Participants completed two questionnaires at the end of each study day before being discharged by the study physician. These questionnaires enquired about different aspects of the subjective experience. The first was the Altered States of Consciousness questionnaire (ASC), a well validated and widely used tool for defining different altered states of consciousness that has been usefully revised in recent years (Studerus et al. Reference Studerus, Gamma and Vollenweider2010) and the second was the Psychotomimetic States Inventory (PSI), a tool developed to probe the psychotomimetic effects of different drugs (Mason et al. Reference Mason, Morgan, Stefanovic and Curran2008). Participants were asked to complete the questionnaires with reference to the peak subjective effects of LSD (i.e. when the effects were most intense) or with reference to how they generally felt throughout the day – e.g. if they did not notice any effects.
Mid-term outcomes
Mid-term outcomes were completed 2 weeks after each dosing session (as well as at baseline) and these included: the Revised Life Orientation Test (LOT-R; Glaesmer et al. Reference Glaesmer, Rief, Martin, Mewes, Brahler, Zenger and Hinz2012), the Revised NEO Personality Inventory (NEO PI-R; Costa & McCrae, Reference Costa and McCrae1995) and Peters’ Delusions Inventory (PDI; Peters et al. Reference Peters, Joseph and Garety1999). The LOT-R was chosen as it is a well-validated measure of trait or dispositional optimism (Scheier et al. Reference Scheier, Carver and Bridges1994), the NEO PI-R was chosen as it is well-validated and previous research has shown its sensitivity to the enduring effects of psychedelics (MacLean et al. Reference Maclean, Johnson and Griffiths2011), and the PDI was chosen as it is a well-validated measure of delusional thinking that has shown to be sensitive to psychotic-like symptoms in the general population (Peters et al. Reference Peters, Joseph and Garety1999).
Additional measures
Additional questionnaires completed at baseline included the Beck Depression Inventory (BDI; Beck et al. Reference Beck, Ward, Mendelson, Mock and Erbaugh1961), the Quick Inventory of Depressive Symptomatology (QIDS; Rush et al. Reference Rush, Trivedi, Ibrahim, Carmody, Arnow, Klein, Markowitz, Ninan, Kornstein, Manber, Thase, Kocsis and Keller2003), the State–Trait Anxiety Inventory (STAI; Hodgues & Spielberger, Reference Hodgues and Spielberger1969), the Ruminative Response Scale (RRS; Roelofs et al. Reference Roelofs, Muris, Huibers, Peeters and Arntz2006), the Dysfunctional Attitudes Scale (DAS; Floyd et al. Reference Floyd, Scogin and Chaplin2004) and the modified version of the Tellegen Absorption Scale (MODTAS; Tellegen & Atkinson, Reference Tellegen and Atkinson1974).
Data analysis
Repeated-measures analyses of variance (ANOVAs) were used to test between-condition differences in the ASC, PSI and PDI, which have multiple factors. However, since a strong prior hypothesis was held that the personality trait ‘openness’ would be significantly increased post-LSD (MacLean et al. Reference Maclean, Johnson and Griffiths2011), this was analysed using a paired t test rather than an ANOVA. The remaining four personality dimensions in the NEO PI-R were analysed using paired t tests with Bonferonni correction for multiple comparisons. A paired t test was used to analyse changes in the LOT-R, as this has only one dimension. To test for order confounds in relation to primary outcomes, order was included as a variable in significant tests, and to further test for the influence of placebo/participation, baseline versus 2 weeks post-placebo differences in outcomes were tested for those who received placebo in their first dosing session (potential carry-over effects of LSD precludes the inclusion of those who received placebo in their second dosing session). Effect sizes (Cohen's d) were calculated for primary outcomes. For correlational analyses, Pearson product-moment coefficients were calculated and two-tailed hypotheses tested. The problem of multiple comparisons was accounted for by Bonferonni correction.
Results
Participants
A total of 20 healthy volunteers participated in the study (four females, mean age = 30.9, s.d. = 7.8, range = 22–47 years). All had at least one previous experience with a classic psychedelic drug (mean estimated LSD uses = 14, s.d. = 17.8, range = 0–70) but not within 14 days of the first dosing session (mean last use of LSD = 899.3, s.d. = 1363, range = 14–5400 days). Self-estimates of other drug use were as follows: mean weekly alcohol units = 10.3 (s.d. = 9); mean number of daily cigarettes = 0.3 (s.d. = 1.1, range = 0–5); mean number of cannabis uses = 705 (s.d. = 639, range = 30–2000); mean number of 3,4-methylenedioxy-methamphetamine (MDMA) uses = 27 (s.d. = 24, range = 2–100); mean number of psilocybin/magic mushroom uses = 9.4 (s.d. = 7.8, range = 1–35); mean number of ketamine uses = 3.6 (s.d. = 5, range = 0–20); mean number of cocaine uses = 9.6 (s.d. = 9.4, range = 0–30). Other mean baseline scores were as follows: BDI = 0.6 (s.d. = 0.9, range = 0–3); QIDS = 4.2 (s.d. = 3.1, range = 0–10); DAS = 105.5 (s.d. = 23, range = 55–146); RRS = 35.4 (s.d. = 7.9, range = 24–52); STAI = 29.2 (s.d. = 4.6, range = 20–38); MODTAS = 58.3 (s.d. = 19.8, range = 19–89).
Acute subjective effects of LSD
A repeated-measures ANOVA revealed a significant main effect of drug (F 1,19 = 82.6, p < 0.001) and a significant drug × ASC interaction (F 1,19 = 10.3, p < 0.001). Post-hoc t tests revealed that all 11 dimensions or factors of the ASC were rated significantly higher after LSD than placebo (p < 0.01). Mean scores for each factor are displayed in the radar chart in Fig. 1. It is notable that although the LSD experience was dominated by changes in visual perception (i.e. elementary imagery, complex imagery and audio-visual synaesthesia), the factor ‘blissful state’ was markedly elevated under the drug (+0.37, s.d. = 0.28, Cohen's d = 1.65). Although still significantly increased, the factor ‘anxiety’ was the least elevated under LSD (+0.15, s.d. = 0.2, Cohen's d = 1.03). These results indicate a marked increase in emotional arousal and lability under LSD but with a distinct bias towards positive affect. Reinforcing this, a t test revealed that increases in ‘blissful state’ under LSD were significantly greater than increases in ‘anxiety’ [t = −3.7, degrees of freedom (df) = 19, p < 0.01, Cohen's d = 0.91].

Fig. 1. Acute effects of lysergic acid diethylamide (LSD) measured via the Altered States of Consciousness questionnaire (ASC). Displayed are the mean scores on each of the 11 dimensions of the ASC for the LSD and placebo conditions.
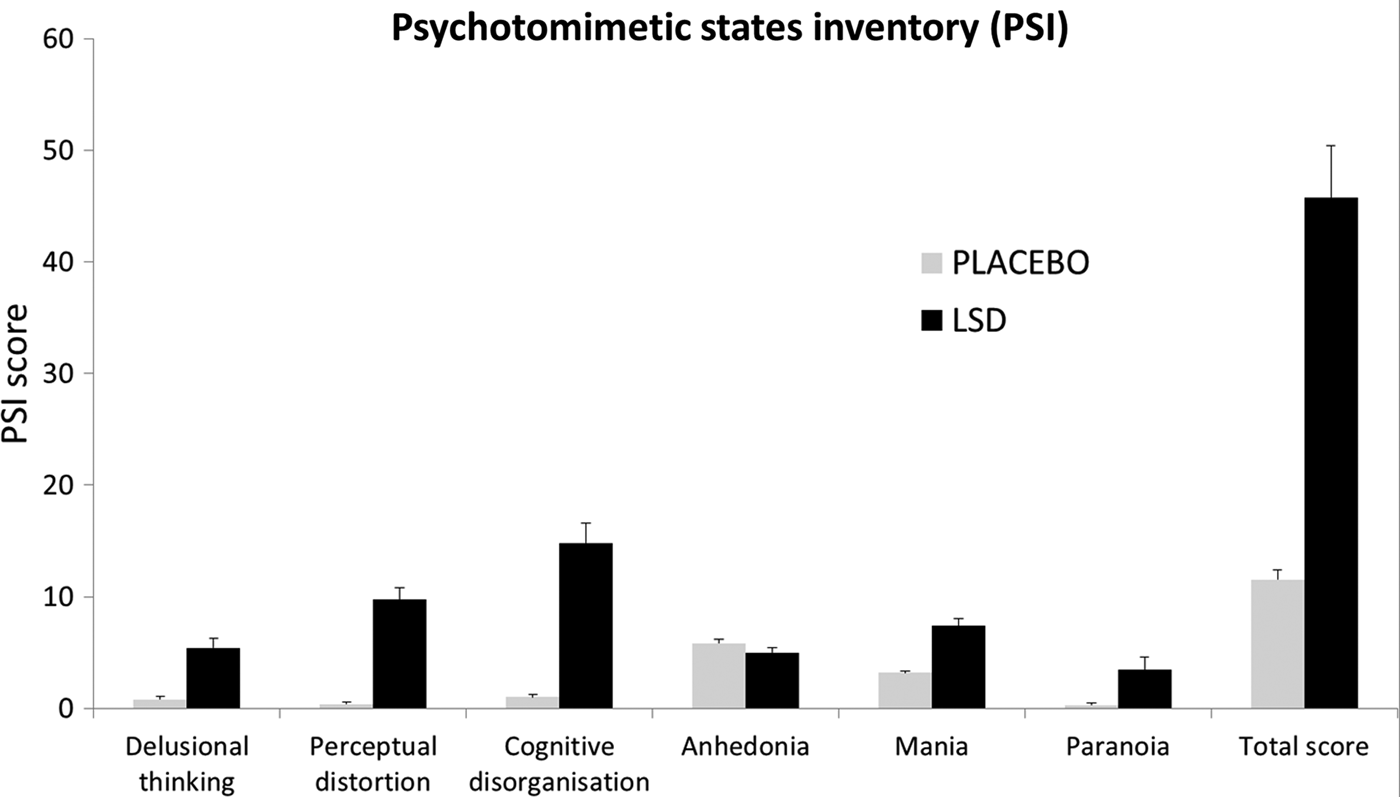
A repeated-measures ANOVA revealed a significant main effect of condition on the PSI (F 1,19 = 65.19, p < 0.001) and a significant condition × PSI interaction (F 5,95 = 35.2, p < 0.001). Mean scores for each factor of the PSI are shown in the bar chart in Fig. 2. Post-hoc t tests revealed that all factors, except ‘anhedonia’, were significantly increased under the drug (p < 0.01). The most marked effect was on ‘cognitive disorganization’ (Cohen's d = 2.37) which is related to thought disorder in psychosis, but other highly characteristic aspects of psychosis such as ‘delusional thinking’ (Cohen's d = 1.63) and ‘paranoia’ (Cohen's d = 0.83) were also markedly increased under the drug.
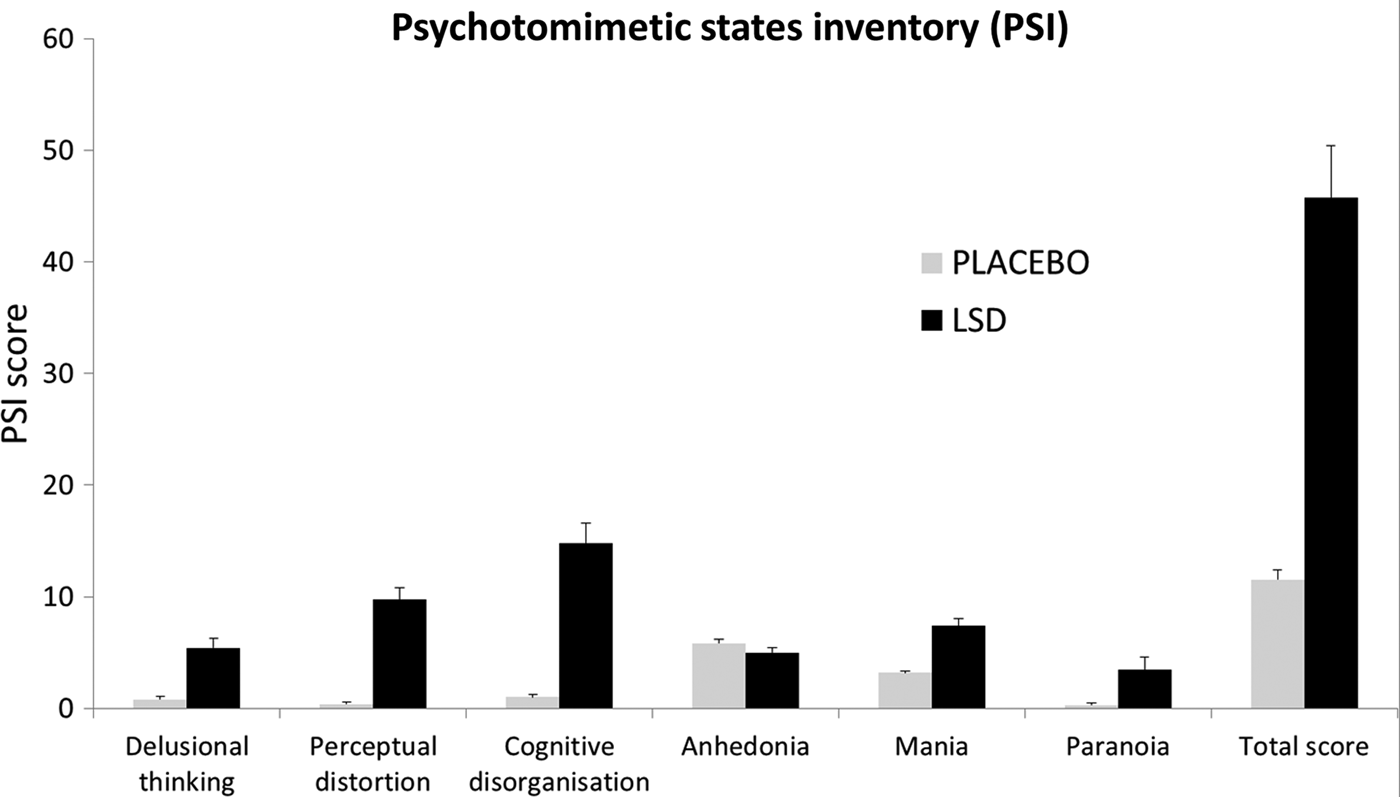
Fig. 2. Acute effects measured of lysergic acid diethylamide (LSD) measured via the Psychotomimetic States Inventory (PSI). Values are the mean scores plus the positive standard errors of the mean (s.e.m.) for each of the six factors of the PSI, plus the total score (which is a sum of the scores of the six factors). All factors, except for anhedonia, were scored significantly higher under LSD than placebo.
Mid-term subjective effects of LSD
Optimism was significantly increased 2 weeks after LSD (t = 2.91, df = 18, p = 0.005, Cohen's d = 0.56), as was trait openness (t = 1.95, df = 19, p = 0.03, Cohen's d = 0.16) (Fig. 3), and there were no such changes in optimism or personality post-placebo. In exploratory t tests, there was a trend towards an increase in trait agreeableness post-LSD (t = 2.2, df = 19, p = 0.038, Cohen's d = 0.21); however, this did not survive correction for multiple comparisons [corrected p = 0.15].

Fig. 3. Mid-term effects of lysergic acid diethylamide (LSD). These charts display paired data points for optimism (a) and openness (b) scores at baseline and 2 weeks after LSD for each participant. Also displayed on each chart are the mean scores (horizontal bars) and the positive standard errors of the mean (s.e.m.). Both optimism and openness were significantly increased after LSD. There was no effect of order of receipt of LSD nor were there any changes in optimism or personality 2 weeks after receipt of placebo. LOT-R, Revised Life Orientation Test; NEO PI-R, Revised NEO Personality Inventory.
Repeated-measures ANOVA found no change in delusional thinking (PDI scores) 2 weeks after LSD (p > 0.05). In fact, exploratory t tests suggested a trend towards less distress (t = 1.92, df = 19, p = 0.068) and preoccupation with ‘delusional thoughts’ (t = 1.92, df = 19, p = 0.07) but these trends were not significant, even before correction for multiple comparisons. There were no changes in PDI scores post-placebo.
Predictors of the mid-term effects of LSD
Regarding baseline predictors of the mid-term effects of LSD, focus was placed on personality (as measured by the NEO PI-R), depression (measured by the QIDS) and anxiety (measured by the STAI). A negative correlation was found between baseline agreeableness and increases in optimism post-LSD (r = −0.56, r 2 = 0.31, p = 0.014) and positive correlations were found between baseline depression (r = 0.53, r 2 = 0.28, p = 0.024) and anxiety (r = 0.49, r 2 = 0.24, p = 0.04) and the post-LSD increases in openness. However, none of these survived correction for multiple comparisons (revised p = 0.05/7 = 0.007).
Regarding acute predictors of the mid-term effects of LSD, there was a negative correlation between acute anxiety (ASC) and post-LSD increases in optimism (r = −0.47, r 2 = 0.22, n = 19, p = 0.04) and positive correlations between impaired/disorganized cognition scores on the ASC and PSI and post-LSD increases in openness (ASC: r = 0.44, r 2 = 0.2, n = 20, p = 0.049; PSI: r = 0.52, r 2 = 0.26, n = 20, p = 0.019) but these correlations also failed to survive correction for multiple comparisons (revised p = 0.5/17 = 0.003).
Discussion
The primary aim of this study was to investigate the acute and mid-term subjective effects of LSD in a controlled study in order to advance our understanding of its psychological effects. It was hypothesized that LSD would increase emotional lability and psychosis-like symptoms acutely, and increase optimism and openness 2 weeks later, and these hypotheses were supported by the data.
Focusing first on LSD's acute effects, scores on the PSI reinforce the view that LSD is a potent psychotomimetic. Previous studies have examined PSI scores after sleep deprivation (Petrovsky et al. Reference Petrovsky, Ettinger, Hill, Frenzel, Meyhofer, Wagner, Backhaus and Kumari2014), dreaming (Mason & Wakerley, Reference Mason and Wakerley2012), cannabis (Mason et al. Reference Mason, Morgan, Stefanovic and Curran2008), tetrahydrocannabinol (THC) (Stokes et al. Reference Stokes, Mehta, Curran, Breen and Grasby2009) and ketamine intoxication (Mason et al. Reference Mason, Morgan, Stefanovic and Curran2008). Although both dreaming and sleep deprivation elevated subscales of the PSI, only the aforementioned drugs produced appreciable psychosis-like symptoms – and still not to the same extent as LSD in the present study. For example, mean total scores on the PSI were 24 (s.d. = 10.9) post-ketamine infusion (150 mg/ml plasma), 15.9 (s.d. = 11) post-THC (10 mg, p.o.) and 33.3 (s.d. = 19.5) post-cannabis use (smoked, quantity unknown), whereas the mean total PSI score in the present study was 45.8 (s.d. = 21). To our knowledge, only cannabis in highly schizotypal individuals (Mason et al. Reference Mason, Morgan, Dhiman, Patel, Parti and Curran2009) has produced PSI scores of an equivalent magnitude to those seen here with LSD.
More generally, relatively strong subjective effects were produced by the present dose of LSD (i.e. 75 µg, i.v.) as indexed by the ASC. In previous studies, psilocybin (115–350 µg/kg, p.o.), MDMA (1.5–1.7 mg/kg, p.o.) and ketamine (6–12 µg/kg per min, i.v.) produced effects of a lower magnitude than those seen here (Studerus et al. Reference Studerus, Gamma and Vollenweider2010) but in a recent study with 200 µg LSD (p.o.), ASC scores were of a similar magnitude to those observed here (Schmid et al. Reference Schmid, Enzler, Gasser, Grouzmann, Preller, Vollenweider, Brenneisen, Muller, Borgwardt and Liechti2014).
With the PSI results in mind, it is worth briefly discussing the relative merits of different drug models of psychosis. Ketamine is often described as an especially meritorious model of psychosis because it can induce both positive and negative symptoms (Krystal et al. Reference Krystal, Karper, Seibyl, Freeman, Delaney, Bremner, Heninger, Bowers and Charney1994). However, the premise that negative symptoms can be modelled by an acute drug state is questionable (Carhart-Harris et al. Reference Carhart-Harris, Brugger, Nutt and Stone2013). For example, it has been argued that negative symptoms are non-specific for psychosis (i.e. they are also prevalent in depression; Kaiser et al. Reference Kaiser, Heekeren and Simon2011; Carhart-Harris et al. Reference Carhart-Harris, Brugger, Nutt and Stone2013). Positive symptoms are specific for psychosis, but comparison studies have suggested that these are better modelled by classic psychedelics than ketamine (Gouzoulis-Mayfrank et al. Reference Gouzoulis-Mayfrank, Heekeren, Neukirch, Stoll, Stock, Obradovic and Kovar2005; Carhart-Harris et al. Reference Carhart-Harris, Brugger, Nutt and Stone2013). Others have shown that THC is a particularly potent inducer of positive psychotic symptoms (Morrison et al. Reference Morrison, Zois, Mckeown, Lee, Holt, Powell, Kapur and Murray2009). Thus, future studies might compare the psychotomimetic properties of THC, ketamine and a classic psychedelic in a controlled, cross-over design to systematically assess the relative value of these different models.
Based on the PSI results, one might infer that participants’ acute LSD experiences were dominated by unpleasant psychosis-like phenomena; however, this was not the case. Some volunteers did show frank psychotic phenomena during their LSD experiences (e.g. paranoid and delusional thinking) but at the group level, positive mood was more common. For example, scores on the (positively valenced) ‘blissful experience’ dimension of the ASC were significantly higher than scores on the (negatively valenced) ‘anxiety’ dimension. Consistent with the view that LSD is more likely to produce positive than negative mood, a separate study with LSD (200 µg, p.o.) reported marked positive mood effects, described as ‘MDMA-like’ (Schmid et al. Reference Schmid, Enzler, Gasser, Grouzmann, Preller, Vollenweider, Brenneisen, Muller, Borgwardt and Liechti2014); an important caveat, however, is that both the Schmid et al. study and the present one recruited volunteers with previous experience with psychedelics. Thus, positive prior experiences and positive regard towards LSD may have biased outcomes.
The findings that optimism and openness are increased 2 weeks after LSD are consistent with previous findings (Griffiths et al. Reference Griffiths, Richards, McCann and Jesse2006, Reference Griffiths, Richards, Johnson, McCann and Jesse2008; MacLean et al. Reference Maclean, Johnson and Griffiths2011), suggesting that improved psychological wellbeing and increased openness are relatively reliable mid- to long-term effects of psychedelics. That there were no increases in psychotic symptomatology at the 2 week end point is also consistent with reports of preserved or even improved mental health among populations of people that have used psychedelic drugs (Bouso et al. Reference Bouso, González, Fondevila, Cutchet, Fernández, Ribeiro Barbosa, Alcázar-Córcoles, Araújo, Barbanoj, Fábregas and Riba2012; Krebs & Johansen, Reference Krebs and Johansen2013; Hendricks et al. Reference Hendricks, Thorne, Clark, Coombs and Johnson2015). These results are also consistent with previous findings that psychedelics can be useful in treating certain psychiatric disorders (Moreno et al. Reference Moreno, Wiegand, Taitano and Delgado2006; Grob et al. Reference Grob, Danforth, Chopra, Hagerty, McKay, Halberstadt and Greer2011; Gasser et al. Reference Gasser, Holstein, Michel, Doblin, Yazar-Klosinski, Passie and Brenneisen2014; Johnson et al. Reference Johnson, Garcia-Romeu, Cosimano and Griffiths2014; Bogenschutz et al. Reference Bogenschutz, Forcehimes, Pommy, Wilcox, Barbosa and Strassman2015) as well as the notion that they may have therapeutic potential in the treatment of mood disorders such as depression (Carhart-Harris et al. Reference Carhart-Harris, Leech, Hellyer, Shanahan, Feilding, Tagliazucchi, Chialvo and Nutt2014b ). High dispositional optimism is associated with a range of positive health and socio-economic outcomes (Carver & Scheier, Reference Carver and Scheier2014); thus, the increases in optimism observed here may be treated as further evidence of the therapeutic potential of psychedelic drugs.
A limitation of the present study was the relatively short duration of the follow-up period (i.e. 2 weeks); however, longer-term follow-up is planned and 2 weeks is still relevant when considering potential therapeutic applications (Zarate et al. Reference Zarate, Singh, Carlson, Brutsche, Ameli, Luckenbaugh, Charney and Manji2006). Another limitation is the single-blind design; however, the classic double-blind model can feel contrived in the context of controlled studies with psychoactive compounds such as LSD, since the blind is almost universally ineffective. A single-blind design allowed us to introduce some uncertainty, however, and so was considered better than an entirely open-label design. The inclusion of an ‘active placebo’ condition might improve the ineffectiveness of the blind in studies with psychedelics; however, an inert placebo was required in the present study in order to provide a valid baseline for the neuroimaging contrasts. Related to this, a final limitation is that the LSD and placebo sessions involved MRI and MEG scans that each lasted for over 60 min. Brain imaging environments can be demanding for some individuals, particularly under the influence of psychedelics (Studerus et al. Reference Studerus, Kometer, Hasler and Vollenweider2011). Individuals are known to be especially sensitive to the environment in which they experience the effects of a psychedelic (Johnson et al. Reference Johnson, Richards and Griffiths2008), and so the drug plus scanner combination may have contributed to the especially high PSI scores observed here with LSD.
With these caveats entered, it is important to attempt an explanation of how LSD can be both acutely psychotomimetic and yet psychologically beneficial in the mid to long term, and insights from neurobiology may be helpful in this regard. There is a consensus that serotonin 2A receptor (5-HT2AR) agonism is central to the psychopharmacology of psychedelics, e.g. affinity for the 5-HT2AR correlates strongly with their potencies (Glennon et al. Reference Glennon, Titeler and Mckenney1984), and pre-treatment with a 5-HT2AR antagonist effectively abolishes the classic ‘psychedelic’ effects of psilocybin (Vollenweider et al. Reference Vollenweider, Vollenweider-Scherpenhuyzen, Babler, Vogel and Hell1998). The principal psychological purpose or function of the 5-HT2AR is poorly understood; however, it is known that 5-HT2AR stimulation promotes certain aspects of learning (Harvey, Reference Harvey2003; Harvey et al. Reference Harvey, Quinn, Liu, Aloyo and Romano2004; Romano et al. Reference Romano, Quinn, Li, Dave, Schindler, Aloyo and Harvey2010) and cognition (King et al. Reference King, Martin and Seymour1972; Williams et al. Reference Williams, Rao and Goldman-Rakic2002). Specifically, 5-HT2AR stimulation enhances the flexibility of cognition (Boulougouris et al. Reference Boulougouris, Glennon and Robbins2008), which may be related to reports of enhanced imagination (Carhart-Harris et al. Reference Carhart-Harris, Leech, Hellyer, Shanahan, Feilding, Tagliazucchi, Chialvo and Nutt2014a ) and creative thinking (Frecska et al. Reference Frecska, More, Vargha and Luna2012) with psychedelics. Interestingly, an association has been found between positive mood and flexible, creative thinking (Hirt et al. Reference Hirt, Devers and Mccrea2008; Lin et al. Reference Lin, Tsai, Lin and Chen2014) and this may explain why both are associated with psychedelics. Indeed, the positive mood effects of psilocybin (a direct 5-HT2AR agonist) and MDMA (a potent serotonin ‘releaser’) are significantly attenuated by pre-treatment with the 5-HT2AR antagonist ketanserin (Kometer et al. Reference Kometer, Schmidt, Bachmann, Studerus, Seifritz and Vollenweider2012; Van Wel et al. Reference Van Wel, Kuypers, Theunissen, Bosker, Bakker and Ramaekers2012).
Psychedelics also transiently impair certain aspects of cognition, however, such as the ability to focus and concentrate (Umbricht et al. Reference Umbricht, Vollenweider, Schmid, Grubel, Skrabo, Huber and Koller2003; Vollenweider et al. Reference Vollenweider, Csomor, Knappe, Geyer and Quednow2007), which is consistent with the high ratings of impaired cognition in the present study (Figs. 1 and 2). It is also important to acknowledge that while 5-HT2AR antagonism attenuates the positive mood effects of psilocybin (and MDMA), it also attenuates the positive psychotic symptoms that can be produced by the drug (Carter et al. Reference Carter, Hasler, Pettigrew, Wallis, Liu and Vollenweider2007) and 5-HT2AR agonism has been linked with anxiety-related behaviours in rodents (Weisstaub et al. Reference Weisstaub, Zhou, Lira, Lambe, Gonzalez-Maeso, Hornung, Sibille, Underwood, Itohara, Dauer, Ansorge, Morelli, Mann, Toth, Aghajanian, Sealfon, Hen and Gingrich2006).
Thus, 5-HT2AR stimulation has been associated with both positive and negative facets of the acute psychedelic state (i.e. positive mood but also anxiety and psychotic symptoms), so how can we reconcile these things with each other? Is there a more fundamental action of psychedelics that can explain both effects? As reported above, 5-HT2AR stimulation has been associated with enhanced cognitive flexibility, and inspired by recent neuroimaging findings (Carhart-Harris et al. Reference Carhart-Harris, Erritzoe, Williams, Stone, Reed, Colasanti, Tyacke, Leech, Malizia, Murphy, Hobden, Evans, Feilding, Wise and Nutt2012; Muthukumaraswamy et al. Reference Muthukumaraswamy, Carhart-Harris, Moran, Brookes, Williams, Errtizoe, Sessa, Papadopoulos, Bolstridge, Singh, Feilding, Friston and Nutt2013; Petri et al. Reference Petri, Expert, Turkheimer, Carhart-Harris, Nutt, Hellyer and Vaccarino2014; Roseman et al. Reference Roseman, Leech, Nutt, Feilding and Carhart-Harris2014; Tagliazucchi et al. Reference Tagliazucchi, Carhart-Harris, Leech, Nutt and Chialvo2014), the psychedelic state has been characterized as an ‘entropic’ state in which the mind/brain operates outside of its normal, optimal level of order, in a realm of relative disorder (Carhart-Harris et al. Reference Carhart-Harris, Leech, Hellyer, Shanahan, Feilding, Tagliazucchi, Chialvo and Nutt2014b ). It may be that what underlies both facets of the psychedelic state and can resolve the ‘valence paradox’ therefore, is this principle of increased cognitive entropy (see Fig. 4). Accordingly, we predict that disordered or entropic cognition is a more fundamental characteristic of the psychedelic state than either positive or negative mood. This hypothesis could be tested by carrying out a principal components analysis of the ASC data; we would predict that valence non-specific items and particularly those related to ‘entropic cognition’ (such as loss of self/ego, loss of ego-boundaries, altered meaning and muddled thinking) would load more heavily onto the first principal component than valence-specific items (such as feeling euphoric or sad) – e.g. see Lebedev et al. (Reference Lebedev, Lovden, Rosenthal, Feilding, Nutt and Carhart-Harris2015). Acute entropic processes may also be useful predictors of the mid/long-terms effects of psychedelics, and the (trend-level) relationship between impaired/disordered cognition and subsequent increases in trait openness might be suggestive of such an association.

Fig. 4. Action of psychedelics on the mind and brain: This empirically informed model illustrates the hypothesized relationship between three different neurobiological or physiological states and their psychological counterparts. Specifically, it is predicted that deficient serotonin 2A receptor (5-HT2AR) stimulation has a stultifying influence on cognition, promoting pessimism, neuroticism and rigid thinking (phase 1). Informed by neuroimaging studies with psychedelics (Carhart-Harris et al. Reference Carhart-Harris, Leech, Hellyer, Shanahan, Feilding, Tagliazucchi, Chialvo and Nutt2014b ), 5-HT2AR stimulation is associated with unconstrained brain network dynamics and the characteristic ‘entropic’ quality of cognition in the psychedelic state (phase 2). Finally, it is hypothesized that an acute ‘onslaught’ or ‘blast’ of 5-HT2AR stimulation, via the action of a psychedelic, has a residual influence on brain network dynamics and associated cognition (phase 3). 5-HT2AR stimulation is described as having a ‘loosening’ or ‘lubricating’ influence on cognition and this is hypothesized to be conducive to improved psychological wellbeing. * The long-term effects of psychedelics on brain network dynamics have yet to be formally investigated.
Before concluding, it is worth considering one final important matter. The discussion so far has focused mainly on the paradoxical acute psychological effects of psychedelics. However, as the present results have demonstrated, the acute effects of psychedelics can be quite different to their longer-term effects, and it is arguably the latter that are more clinically relevant. Previous studies have shown that trait characteristics, such as personality and outlook, can be significantly altered by psychedelics (Griffiths et al. Reference Griffiths, Richards, Johnson, McCann and Jesse2008; MacLean et al. Reference Maclean, Johnson and Griffiths2011). Moreover, associations have been found between certain psychological traits and the 5-HT2AR (Turecki et al. Reference Turecki, Briere, Dewar, Antonetti, Lesage, Seguin, Chawky, Vanier, Alda, Joober, Benkelfat and Rouleau1999; Meyer et al. Reference Meyer, McMain, Kennedy, Korman, Brown, Dasilva, Wilson, Blak, Eynan-Harvey, Goulding, Houle and Links2003; Ott et al. Reference Ott, Reuter, Hennig and Vaitl2005; Bhagwagar et al. Reference Bhagwagar, Hinz, Taylor, Fancy, Cowen and Grasby2006; Frokjaer et al. Reference Frokjaer, Mortensen, Nielsen, Haugbol, Pinborg, Adams, Svarer, Hasselbalch, Holm, Paulson and Knudsen2008). Specifically, deficient 5-HT2AR stimulation has been linked with depression-related behaviours such as suicide (Turecki et al. Reference Turecki, Briere, Dewar, Antonetti, Lesage, Seguin, Chawky, Vanier, Alda, Joober, Benkelfat and Rouleau1999), dysfunctional or excessively pessimistic attitudes (Meyer et al. Reference Meyer, McMain, Kennedy, Korman, Brown, Dasilva, Wilson, Blak, Eynan-Harvey, Goulding, Houle and Links2003; Bhagwagar et al. Reference Bhagwagar, Hinz, Taylor, Fancy, Cowen and Grasby2006) and neuroticism (Frokjaer et al. Reference Frokjaer, Mortensen, Nielsen, Haugbol, Pinborg, Adams, Svarer, Hasselbalch, Holm, Paulson and Knudsen2008). In this context, increased psychological wellbeing (Griffiths et al. Reference Griffiths, Richards, Johnson, McCann and Jesse2008), openness (MacLean et al. Reference Maclean, Johnson and Griffiths2011), decreased suicidality (Hendricks et al. Reference Hendricks, Thorne, Clark, Coombs and Johnson2015) and now increased optimism after a ‘blast’ of 5-HT2AR stimulation may begin to make functional sense. We predict that deficient 5-HT2AR stimulation causes cognition to ‘stultify’, whereas 5-HT2AR stimulation loosens cognition and associated brain dynamics, serving as a metaphorical ‘lubricant’ for the mind and brain. We predict that this loosening effect persists beyond the acute intoxication phase and can potentially explain the mid- to long-term psychological effects of psychedelics. Recent reports of enduring brain changes with 5-HT2AR stimulation and psychedelic drug-use (Bouso et al. Reference Bouso, Palhano-Fontes, Rodríguez-Fornells, Ribeiro, Sanches, Crippa, Hallak, De Araujo and Riba2015), as well as speculations on the function of serotonin in the brain (Branchi, Reference Branchi2011), may herald the beginnings of an understanding of this important matter. We intend to detail the acute brain effects of LSD in forthcoming neuroimaging papers and to investigate the long-term psychological and brain effects of psychedelics in future studies.
In conclusion, this study sought to investigate the paradoxical psychological effects of LSD in a controlled study in healthy volunteers. LSD produced robust acute psychological effects that were characterized by psychosis-like symptoms but also positive mood. Mid-term effects included significant increases in optimism and the personality trait openness and no increases in delusional thinking. A mechanistic explanation for these findings was proposed based on the ‘entropic brain’ hypothesis (Carhart-Harris et al. Reference Carhart-Harris, Leech, Hellyer, Shanahan, Feilding, Tagliazucchi, Chialvo and Nutt2014b ).
Acknowledgements
This research received financial and intellectual support from the Beckley Foundation and was conducted as part of a wider Beckley–Imperial research programme. The researchers would also like to thank supporters of the Walacea.com crowd-funding campaign who played a crucial role in securing the completion of this study. The report presents independent research carried out at the National Institute of Health Research (NIHR)/Wellcome Trust Imperial Clinical Research Facility.
Declaration of Interest
None.