Background
Anorexia nervosa (AN) is a serious eating disorder marked by significantly low body weight accompanied by restrictive eating, weight and/or body shape disturbance, fear of weight gain, and significant impairment in physical, social, and psychological functioning (APA, 2013). The lifetime prevalence of AN in Europe is 1.0 to 4.0% for females and 0.3 to 0.7% for males (Keski-Rahkonen & Mustelin, Reference Keski-Rahkonen and Mustelin2016). AN onset mostly occurs in adolescence, and it is estimated that 10–20% of people report chronic, persistent AN (Keski-Rahkonen & Mustelin, Reference Keski-Rahkonen and Mustelin2016; Speciani et al., Reference Speciani, Barak, Damanhuri, De Ronchi, Panariello and Atti2021; Steinhausen, Reference Steinhausen2009; Udo & Grilo, Reference Udo and Grilo2018). Worldwide, incidence rates of AN have remained stable over the last 30 years, although they have increased among certain subsets of the population (e.g. younger individuals aged <15 years) (van Eeden, van Hoeken, & Hoek, Reference van Eeden, van Hoeken and Hoek2021). AN ranks among the psychiatric disorders with strongest association with premature relative mortality, surpassed only by opioid use disorder (Arcelus, Mitchell, Wales, & Nielsen, Reference Arcelus, Mitchell, Wales and Nielsen2011; Chesney, Goodwin, & Fazel, Reference Chesney, Goodwin and Fazel2014; Wonderlich, Bulik, Schmidt, Steiger, & Hoek, Reference Wonderlich, Bulik, Schmidt, Steiger and Hoek2020), with 5–6 times higher mortality rate than the general population (van Eeden et al., Reference van Eeden, van Hoeken and Hoek2021; van Hocken & Hoek Reference van Hoeken and Hoek2020; Wonderlich et al., Reference Wonderlich, Bulik, Schmidt, Steiger and Hoek2020).
Family and twin studies of AN provide evidence for notable heritability. For instance, females who have a relative with AN are 11 times more likely to develop AN than those who do not have a relative with AN (Strober, Freeman, Lampert, Diamond, & Kaye, Reference Strober, Freeman, Lampert, Diamond and Kaye2000). Twin studies report AN heritability estimates ranging from 0.48 to 0.74 (Yilmaz, Hardaway, & Bulik, Reference Yilmaz, Hardaway and Bulik2015). A total of four genome-wide association studies (GWAS) have been conducted examining risk loci for AN (Boraska et al., Reference Boraska, Franklin, Floyd, Thornton, Huckins, Southam and Bulik2014; Duncan et al., Reference Duncan, Yilmaz, Gaspar, Walters, Goldstein, Anttila and Bulik2017; Wang et al., Reference Wang, Zhang, Bloss, Duvvuri, Kaye, Schork and Hakonarson2011; Watson et al., Reference Watson, Palmos, Hunjan, Baker, Yilmaz and Davies2021), estimating ~11–17% of AN heritability to be attributed to common genetic variants and identifying eight genome-wide significant loci including four single-gene loci (CADM1, MGMT, FOXP1, and PTBP2) in AN etiology (Watson et al., Reference Watson, Yilmaz, Thornton, Hübel, Coleman, Gaspar and Slagboom2019b). Recent GWAS efforts with increased sample sizes have resulted in greater gene discovery in the field of complex trait genomics (Watson et al., Reference Watson, Palmos, Hunjan, Baker, Yilmaz and Davies2021). With each GWAS variant accounting for a small increase in underlying genetic risk factors for AN, a more powerful way to predict genomic risk is accomplished with polygenic risk scores (PRS: the sum of an individual's risk alleles weighted by effect size; Uffelmann et al., Reference Uffelmann, Huang, Munung, De Vries, Okada, Martin and Posthuma2021). Many studies to date have used PRS alone to predict complex traits and conditions. When combined with epidemiological risk factors, more comprehensive risk modeling is achieved (Kullo et al., Reference Kullo, Lewis, Inouye, Martin, Ripatti and Chatterjee2022; Lee et al., Reference Lee, Yang, Tyrer, Gentry-Maharaj, Ryan, Mavaddat and Antoniou2022). Additionally, the relationship among PRS, childhood exposure to nitrogen dioxide, and schizophrenia outcomes were examined in a Danish population cohort, where increased PRS for schizophrenia was positively associated with higher nitrogen dioxide exposure during childhood (Horsdal et al., Reference Horsdal, Agerbo, McGrath, Vilhjálmsson, Antonsen, Closter and Pedersen2019). These examples highlight the utility of combining PRS with epidemiological risk factors to gain greater insight into the etiology of psychiatric disorders. Similar to other common psychiatric disorders, AN is highly polygenic in nature (where individual genes of small effect contribute to its occurrence), and as such PRS is a promising tool for identifying AN genetic risk (Baker, Schaumberg, & Munn-Chernoff, Reference Baker, Schaumberg and Munn-Chernoff2017; Wray et al., Reference Wray, Lee, Mehta, Vinkhuyzen, Dudbridge and Middeldorp2014), although research evaluating their clinical utility and ethical issues related to their application is needed (Wray et al., Reference Wray, Lin, Austin, McGrath, Hickie, Murray and Visscher2021).
In addition to genetic factors, epidemiological factors also significantly contribute to the etiology of AN (Yao et al., Reference Yao, Larsson, Norring, Birgegård, Lichtenstein, D'Onofrio and Kuja-Halkola2021). Several types of risk factors for AN have been identified, including birth-related factors, early childhood adversities, and psychosocial risk factors. In the present study, we restricted our selection to factors presented in the literature found to be significantly associated with AN risk that were also available in the Danish registers. For example, higher parental educational attainment has been linked to increased risk for AN (Ahrén, Chiesa, af Klinteberg, & Koupil, Reference Ahrén, Chiesa, Af Klinteberg and Koupil2012; Goodman, Heshmati, & Koupil, Reference Goodman, Heshmati and Koupil2014; Koch et al., Reference Koch, Larsen, Plessen, Thornton, Bulik and Petersen2022; Sundquist, Ohlsson, Winkleby, Sundquist, & Crump, Reference Sundquist, Ohlsson, Winkleby, Sundquist and Crump2016). Additional mixed evidence has been identified regarding the relationship between parental income and risk for AN, with some studies finding a significant positive association (Koch et al., Reference Koch, Larsen, Plessen, Thornton, Bulik and Petersen2022; Lindberg & Hjern, Reference Lindberg and Hjern2003), and others failing to identify an association independent of parental education (Goodman et al., Reference Goodman, Heshmati and Koupil2014). Maternal genitourinary tract infection, higher maternal and paternal age at birth, multiple births, Cesarean section, congenital malformations of the mouth or digestive system, and preterm birth have been consistently linked to increased risk for AN (Larsen, Bulik, Thornton, Koch, & Petersen, Reference Larsen, Bulik, Thornton, Koch and Petersen2021; Lindberg & Hjern, Reference Lindberg and Hjern2003; Marzola et al., Reference Marzola, Cavallo, Panero, Porliod, Amodeo and Abbate-Daga2021). Smoking during pregnancy, birth weights above 4500 g, and birth after 42 weeks of gestation have been associated with significantly lower risk for AN (Ekblad, Gissler, Lehtonen, & Korkeila, Reference Ekblad, Gissler, Lehtonen and Korkeila2010; Larsen et al., Reference Larsen, Bulik, Thornton, Koch and Petersen2021; Marzola et al., Reference Marzola, Cavallo, Panero, Porliod, Amodeo and Abbate-Daga2021; Watson et al., Reference Watson, Diemer, Zerwas, Gustavson, Knudsen, Torgersen and Bulik2019a). Psychosocial and childhood adversity risk factors that have been identified include parental psychiatric history, intercountry adoption, and foster care before age 13 (Lindberg & Hjern, Reference Lindberg and Hjern2003). We included urbanicity – which is associated with lower AN risk in the case of living in a rural setting in Denmark (Koch et al., Reference Koch, Larsen, Plessen, Thornton, Bulik and Petersen2022; Larsen et al., Reference Larsen, Bulik, Thornton, Koch and Petersen2021) – which has been investigated in a Dutch study but was not replicated, possibly due to insufficient sample size (van Son, van Hoeken, Bartelds, van Furth, & Hoek, Reference van Son, van Hoeken, Bartelds, van Furth and Hoek2006). We also included childhood infections which – as is also in the case of other psychiatric disorders – are associated with higher AN risk (Breithaupt et al., Reference Breithaupt, Köhler-Forsberg, Larsen, Benros, Thornton, Bulik and Petersen2019). Genes and environment have been hypothesized to work together in the etiology of AN through gene-environment correlation and gene-by-environment interaction (Baker et al., Reference Baker, Schaumberg and Munn-Chernoff2017). Examination of both epidemiological risk factors and biology – while being mindful that the interaction will likely to be complex in nature, with epidemiological factors also being influenced by genetics – has the potential to facilitate more personalized risk prediction in individuals with AN.
The aim of the present study is to examine interplay between polygenetic liability and risk factors in the risk of AN, utilizing data obtained from Danish national registers. Risk factors examined included birth-related, somatic, and psychosocial factors. To accomplish this, associations, confounding, and interactions were estimated. Associations between AN PRS and each risk factor in the population were evaluated, with significant findings suggesting that AN PRS is associated with (or even affect) an individual's likelihood to be exposed to the risk factor. Next, AN PRS and parental psychiatric history were included as confounding variables when examining the association between the risk factors and AN incidence, with the results revealing to what extent the associations attenuate when adjusted for confounding from AN PRS and parental psychiatric history. Lastly, we determined the interaction between AN PRS and each risk factor on AN incidence, showing whether the association involving AN PRS for AN risk is stronger at one level compared to another level of the risk factors.
For the association analyses, we hypothesized that the AN PRS would be positively associated with the risk factors; i.e., the genetic load of AN risk was hypothesized to be higher for individuals exposed to the risk factor compared with unexposed individuals. Accordingly, part of the increased AN risk in people exposed to the risk factor could be attributable to higher AN genetic load. Consequently, we hypothesized that the increased AN risk associated with the risk factors would be attenuated after adjusting for AN PRS and family history in the confounding analyses. Based on the literature, the factors we hypothesized to have the strongest associations with AN risk were sex, maternal education, and familial psychiatric history, as these factors are known to be affected by genetics, and could, speculatively, show interactions with the PRS on AN risk. Potential interpretations could be that genetic load may play a stronger role at one level of a given risk factor than other levels of the risk factor, which could point to diverse pathways toward an AN diagnoses.
Methods
Sample
We used data from the iPSYCH consortium and its established population-based case-cohort sample (iPSYCH2015; Bybjerg-Grauholm et al., Reference Bybjerg-Grauholm, Pedersen, Bækvad-Hansen, Pedersen, Adamsen and Hansen2020), and the Danish arm of the Anorexia Nervosa Genetics Initiative (ANGI; Thornton et al., Reference Thornton, Munn-Chernoff, Baker, Jureus, Parker, Henders and Bulik2018) and Eating Disorders Genetics Initiative (EDGI; Bulik et al., Reference Bulik, Thornton, Parker, Kennedy, Baker, MacDermod and Martin2021b) cohorts, all of which are described in detail elsewhere. The samples were drawn from individuals born in Denmark between 1 May 1981 and 31 December 2008 and alive and living in Denmark on their first birthday (N = 1 768 952 in the background population). Genetic information was based in DNA extracted from neonatal dried blood spot samples kept in the Danish Newborn Screening Biobank. Retrieval of blood samples, genotyping, and quality control have been described elsewhere (Pedersen et al., Reference Pedersen, Bybjerg-Grauholm, Pedersen, Grove, Agerbo, Baekvad-Hansen and Mortensen2018). The analytic cohort included 45 458 randomly selected individuals and 7003 individuals with an AN diagnosis (ICD-10 F50.0 and F50.1) after age 1 in the Danish Psychiatric Central Research Register (DPCRR) or the Danish National Patient Register (DNPR), an electronic register that contains data on all psychiatric hospital admissions from 1969 to present as well as outpatient admissions since 1995 (Lynge, Sandegaard, & Rebolj, Reference Lynge, Sandegaard and Rebolj2011; Mors, Perto, & Mortensen, Reference Mors, Perto and Mortensen2011; Munk-Jorgensen et al., Reference Munk-Jørgensen and Mortensen1997). The case-cohort design has some advantages to case-control studies: the randomly selected cohort allows estimation of relative risk rather than odds ratios, and the cohort can be reused for comparison to different case groups. Presently, it is used in iPSYCH and for the AN case group. Out of the 45 458 individuals randomly selected, there were 229 who were registered with an AN diagnosis.
Risk factors
We examined several established risk factors available in the Danish registers, such as birth-related, somatic, and psychosocial factors. Demographic factors including sex, urbanicity at birth, and parental age at birth were extracted from The Danish Civil Registration System. Information on birth-related risk factors, including gestational age, birth weight, and method of delivery were extracted from the Danish Medical Birth Register (DMBR; Bliddal, Broe, Pottegård, Olsen, & Langhoff-Roos, Reference Bliddal, Broe, Pottegård, Olsen and Langhoff-Roos2018; Pedersen, Reference Pedersen2011). The DNPR was used to identify other birth-related and somatic factors, including maternal infections during pregnancy, and congenital malformations of the mouth and digestive system, and childhood infections (Lynge et al., Reference Lynge, Sandegaard and Rebolj2011). Maternal genitourinary infection during pregnancy, and any infections during pregnancy (including genitourinary infection) were both analyzed. Data on psychosocial factors such as out-of-home and in-home care were found in the Register of Support for Children and Adolescents. Data on other psychosocial risk factors, such as parental income and education were extracted from Denmark's socioeconomic registers (Petersson, Baadsgaard, & Thygesen, Reference Petersson, Baadsgaard and Thygesen2011). Finally, the DPCRR was used to examine parental psychiatric history (Mors et al., Reference Mors, Perto and Mortensen2011), the variables were categorized into parental eating disorders, other psychiatric diagnoses in mother or the father, and no psychiatric disorders in the parents.
Polygenic risk score calculation
AN PRS was calculated using LDpred2 (Privé, Arbel, & Vilhjálmsson, Reference Privé, Arbel and Vilhjálmsson2020) using summary statistics from the Psychiatric Genomics Consortium Eating Disorders Working Group (PGC-ED) Freeze 2 AN GWAS (Watson et al., Reference Watson, Yilmaz, Thornton, Hübel, Coleman, Gaspar and Slagboom2019b) after the removal of Danish AN cases and controls (discovery sample for PRS: 12 400 AN cases and 35 046 controls). After being standardized in the random sample, this comprised the application of AN PRS to estimate associations, confounding, and interactions with each risk factor in the target Danish sample.
The study was approved by the Danish Scientific Ethics Committee, the Danish Health Data Authority, the Danish Data Protection Agency, and Danish Newborn Screening Biobank Steering. The Danish Scientific Ethics Committee, in accordance with Danish legislation, has, for this study, waived the need for informed consent in biomedical research based on existing biobanks (Mortensen, Reference Mortensen2019; Pedersen et al., Reference Pedersen, Bybjerg-Grauholm, Pedersen, Grove, Agerbo, Baekvad-Hansen and Mortensen2018).
Statistical analyses
For the association analyses we included only the randomly selected participant. We used multinomial logistic regression with the included risk factors as outcomes and the PRS for AN as the exposure to estimate the association between the levels of the risk factors and the PRS. For the confounding and interaction effect case-cohort analyses were conducted using weighted Cox models, where the individuals were followed from age 6 until AN diagnosis or until death/emigration or 31 December 2016, whichever occurred first. The weights represented the inverse probability of a participant being selected; for the random part of the sample (size of main study population (1 768 952) divided by the number without AN selected, here 45 229), and for AN cases the weight was equal to 1, as all AN cases from the main study population were included. With these models, we investigated potential confounding from the PRS and parental psychiatric history for each risk factor for AN, unadjusted (model 1), adjusting for PRS (model 2), parental psychiatric history (model 3), and both (model 4) by including these variables as covariates in the model. Furthermore, we looked at potential interactions allowing differential linear effects of PRS across levels of the risk factors by including an interaction term between the PRS and the risk factor in the weighted Cox model. In all analyses we adjusted for the first 10 genetic principal components. For the confounding analyses, model 1 has specific hypotheses for each risk factor. The confounding analyses (model 2–4) were not tested by specific tests; instead the adjusted estimates are shown. For the association and interaction analyses we regard as multiple testing situations, accordingly in these analyses, we also included an adjusted p value using the Benjamini–Hochberg correction (adjustment was conducted for all analyses within each table). Missing values were handled by complete case analyses, as they were rare (see Table 1). We completed all analyses and generated plots using R version 4.0.4 and R Studio version 1.4.1106.
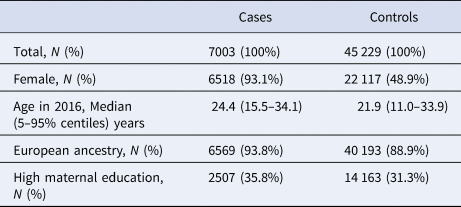
Table 1. Descriptive of cases and controls in our sample
Results
The full study population included 45 458 population-based cohort members (23 129 [50.9%] males; 22 329 [49.1%] females), plus the 7003 cases of AN (n = 485 [6.9%] males; n = 6518 [93.1%] females). The mean age at first AN diagnosis was 17.6 years in females and 16.0 years in males.
PRS and risk factor associations
Our analysis revealed that AN PRS was significantly positively associated with urbanicity, paternal age at birth, maternal age at birth, maternal genitourinary tract infection during pregnancy, paternal and maternal education, and both paternal and maternal income in the random sample of our study (excluding those only in the sample of individuals with AN) (see Fig. 1). AN PRS was not significantly associated with sex, paternal or maternal infection during pregnancy, congenital malformation of the mouth or digestive system, method of delivery, gestational age, birth weight, childhood infection, foster care/out-of-home care, in-home care, or parental psychiatric history (see Fig. 1 and online Supplementary Table S1).
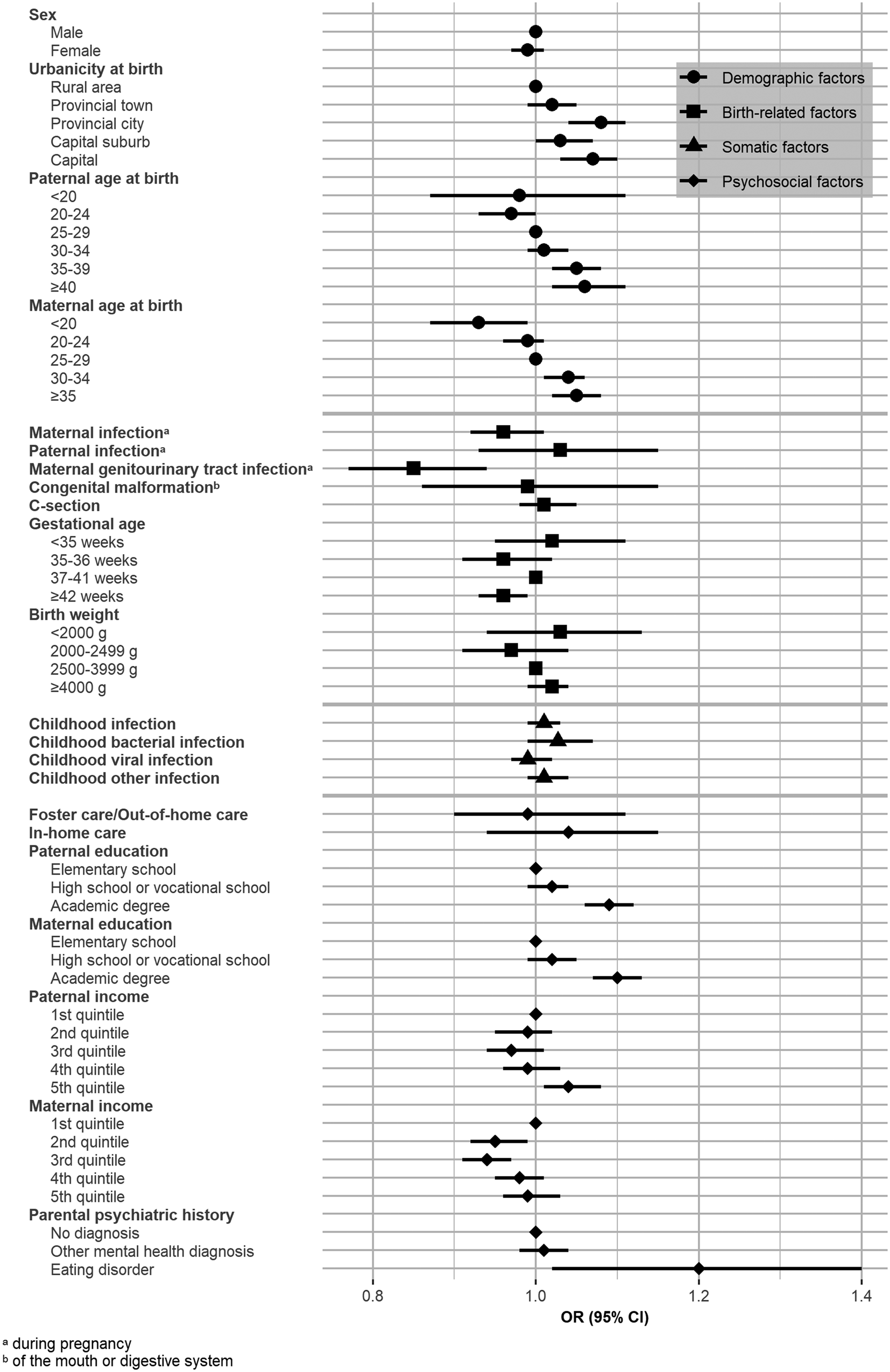
Figure 1. Association analysis: odds ratio per 1 standard deviation increase in anorexia nervosa polygenic risk score across levels of risk factors for anorexia nervosa.
Known AN risk factors and confounding analyses
Hazard ratios for AN were significantly higher among females and individuals who live in urban areas (capital cities, capital suburbs, provincial cities, and provincial towns) compared with rural areas (see Table 2). Parental age was also significantly associated with hazard ratios for AN, with paternal age > 30 years old and maternal age between 30–34 years old associated with increased risk for AN (compared with paternal and maternal ages 25–29), further, hazard ratios for AN were significantly lower among individuals born to a father 20–24 years old at birth (compared to 25–29 years old), and individuals born to a mother < 20 or 20–24 years old at birth (compared to 25–29 years old).
Table 2. Confounding analysis: hazard ratios of genetic and environmental risk factors for anorexia nervosa
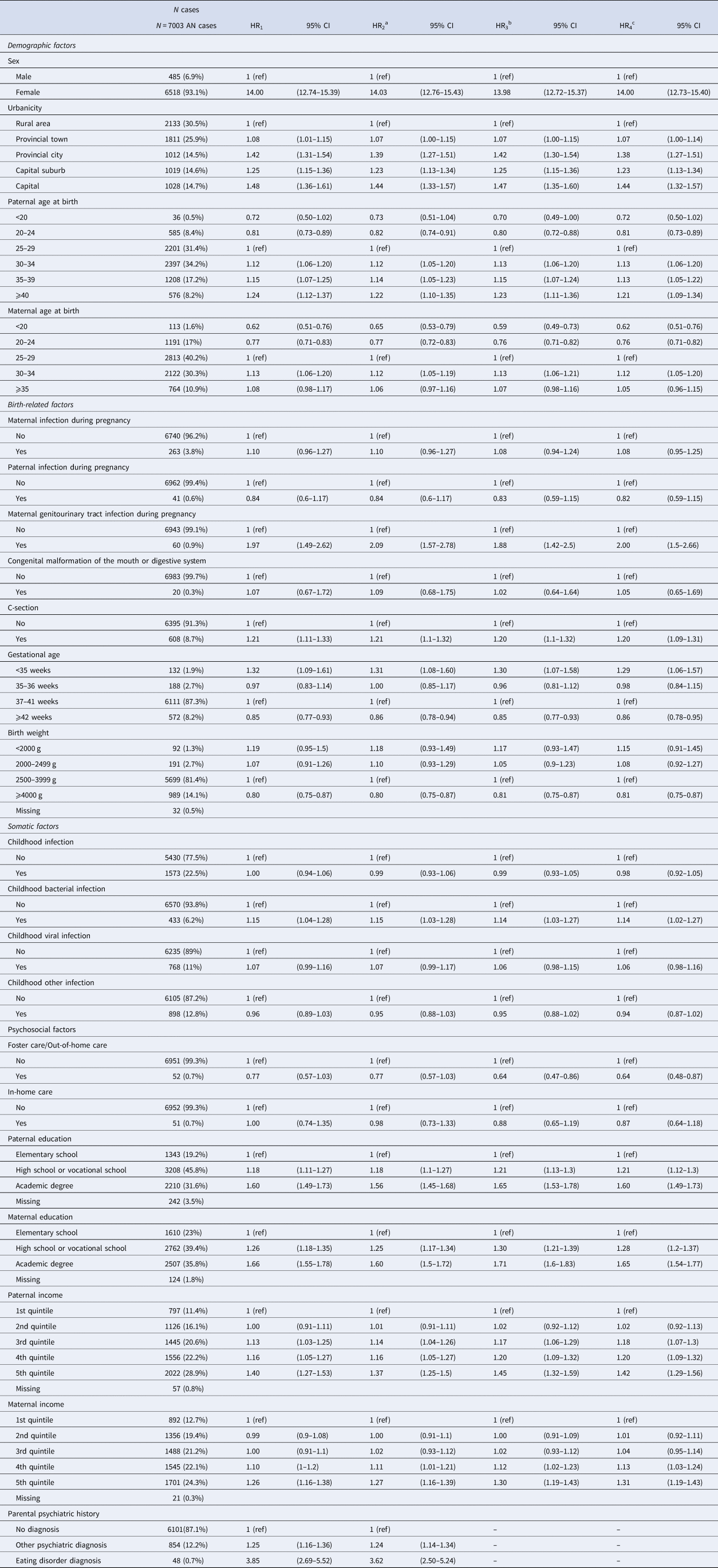
Abbreviations: N, sample size; HR, hazard ratio; CI, confidence interval.
a HR1 with additional adjustment for anorexia nervosa polygenic risk score.
b HR1 with additional adjustment for parental psychiatric history.
c HR1 with additional adjustment for anorexia nervosa polygenic risk score and parental psychiatric history.
The risk of AN was higher among individuals with mothers who experienced genitourinary tract infection during pregnancy (compared with mothers who did not), and individuals born via C-section (compared with vaginal birth). AN risk was also higher among individuals with gestational age < 35 weeks (compared to 37–41 weeks) and lower among individuals with gestational age ⩾ 42 weeks (compared to 37–41 weeks). Similarly, AN risk was inversely associated with birth weight, and significantly decreased for individuals with birth weight ⩾ 4000 g (compared to 2500–3999 g).
Individuals born to a father and/or mother with more than an elementary school education (compared with elementary school education only), individuals with fathers whose income was in the upper three quintiles (compared with the lowest quintile), and individuals with mothers whose income was in the highest quintile (compared with the lowest quintile) demonstrated elevated hazard ratios for AN. Finally, both general parental psychiatric history and parental history of eating disorders were associated with significantly elevated AN hazard ratios, compared with no parental psychiatric history.
To evaluate confounding, all the above risk factors were adjusted for AN PRS (model 2), parental psychiatric history (model 3), and both AN PRS and parental psychiatric history (model 4). All adjustments resulted in only minor changes to the associations between risk factors and AN.
Interaction analyses
Table 3 summarizes the Cox models including interaction terms between AN PRS and risk factors on AN risk. Significant interaction effects were identified for sex, maternal age at birth, maternal genitourinary tract infection, method of delivery, paternal education, maternal education, paternal income, and parental psychiatric history. Specifically, higher AN PRS had a larger effect on risk for AN diagnosis among females, individuals born to mothers younger than 20 and older than 25 years old, individuals born through cesarean section, individuals born when mother experienced genitourinary tract infection during pregnancy, individuals born to fathers or mothers with an academic degree, and individuals with fathers whose income was in the highest quintile.
Table 3. Interaction analysis: differential (linear) effect of anorexia nervosa polygenic risk score across levels of risk factors for anorexia nervosa
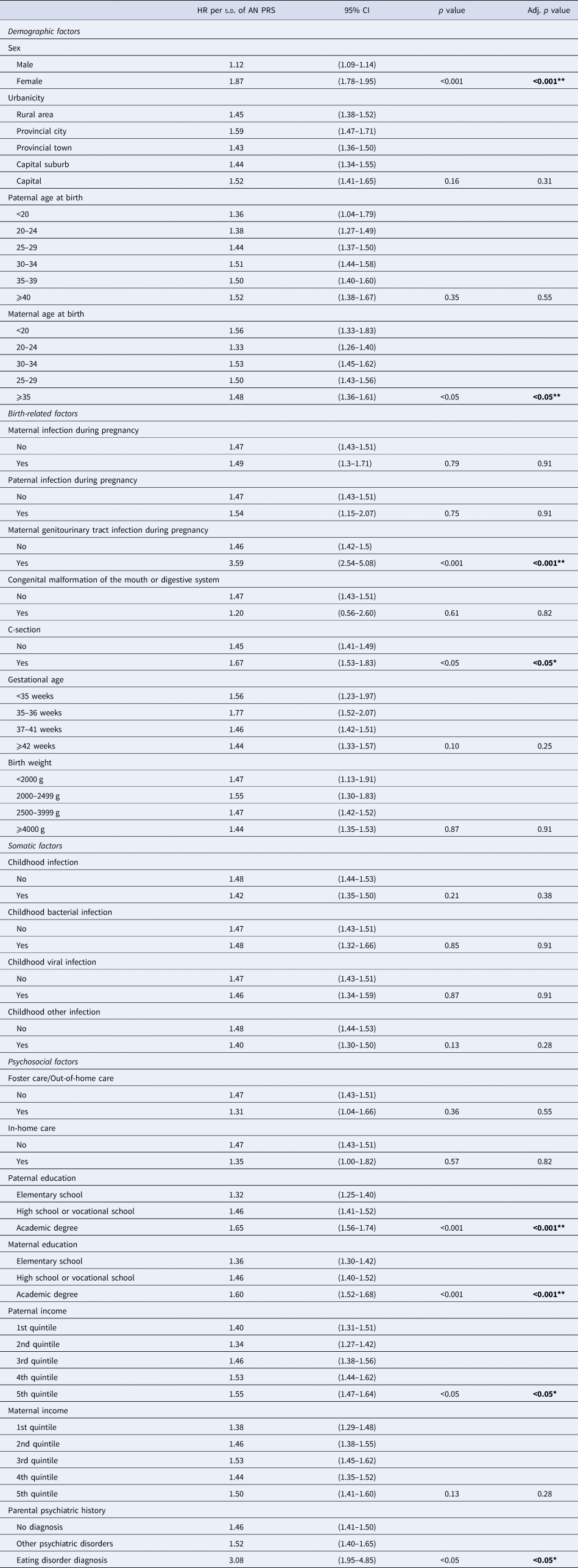
Abbreviations: HR, hazard ratio; CI, confidence interval. p values were adjusted using the Benjamini–Hochberg correction table-wise within the association analyses.
Note. Interaction using differential linear effect of anorexia nervosa polygenic risk score across the levels of the risk factors. * = significant at the p < 0.05 level. ** = significant at the p < 0.001 level.
Discussion
The present study investigated three types of interplay (association, confounding, and interaction) in AN using AN PRS and birth-related, somatic, and psychosocial risk factors. AN PRS was significantly associated with eight risk factors, including urbanicity, paternal and maternal age at birth, maternal genitourinary tract infection during pregnancy, paternal and maternal education, and paternal and maternal income. Despite these associations, AN PRS and parental psychiatric history had minimal confounding effects on the association between the risk factors investigated and a diagnosis of AN. In other words, polygenetic liability and parental psychiatric history may have little influence as confounders of the association between the investigated factors and AN risk. Previously published findings were confirmed (HR1 in Table 2) (Breithaupt et al., Reference Breithaupt, Köhler-Forsberg, Larsen, Benros, Thornton, Bulik and Petersen2019; Koch et al., Reference Koch, Larsen, Plessen, Thornton, Bulik and Petersen2022; Larsen et al., Reference Larsen, Munk-Olsen, Bulik, Thornton, Koch, Mortensen and Petersen2017, Reference Larsen, Bulik, Thornton, Koch and Petersen2021; Marzola et al., Reference Marzola, Cavallo, Panero, Porliod, Amodeo and Abbate-Daga2021; Yao et al., Reference Yao, Larsson, Norring, Birgegård, Lichtenstein, D'Onofrio and Kuja-Halkola2021). The interaction results provided preliminary evidence for PRS interactions with the risk factors: sex, maternal age at birth, maternal genitourinary tract infection during pregnancy, method of delivery, paternal and maternal education, paternal income, and parental psychiatric history.
This study was the first to examine the association between genetic underpinnings of AN and a series of risk factors in a random sample of 45 458 participants from the general population. We identified that in the general population, higher AN PRS was associated with several AN risk factors, offering preliminary evidence that associations exist between AN PRS and birth-related, somatic, and psychosocial risk factors. In particular, associations between AN PRS and urbanicity, paternal and maternal age at birth, maternal genitourinary tract infection during pregnancy, paternal and maternal education, and paternal and maternal income may suggest that these factors could be impacted by the same genetic mechanisms as AN. It is also plausible that there are environmental or lifestyle factors that influence both the development of AN and these other factors. Further, the risk of AN may be more pronounced in specific environments. For example, growing up in an urban setting or experiencing maternal genitourinary tract infection during pregnancy may increase the risk of AN, either directly or indirectly through other factors such as stress (Phillipou, Gurvich, Castle, & Rossell, Reference Phillipou, Gurvich, Castle and Rossell2022) or inflammation (Dalton et al., Reference Dalton, Campbell, Chung, Breen, Schmidt and Himmerich2018).
Our finding on the association between higher SES and increased AN risk could be an artifact of treatment seeking behavior. Though Denmark has a universal health care system and the diagnoses and admissions captured by the national registers are publicly funded, a greater awareness of treatment availability among those with higher SES cannot be excluded. However, this pattern is seen only among AN patients: other psychiatric disorders show the opposite pattern in Denmark, and other eating disorders show a pattern somewhere in the middle (Koch et al., Reference Koch, Larsen, Plessen, Thornton, Bulik and Petersen2022).
Our finding of only minor confounding effect from genetic liability to AN risk and parental history of psychiatric disorders suggest these factors act independently from the epidemiological risk factors accessed; however, future iterations of the AN PRS based on larger samples may change this.
Our findings suggest interactions between AN PRS and sex, maternal age at birth, maternal genitourinary infection during pregnancy, method of delivery, paternal and maternal education, paternal income, and parental psychiatric history of an eating disorder, identifying subgroups affected to a higher degree by their genetic liability to AN than others. Maternal antibodies in relation to in utero immune-related exposures have been demonstrated as potential risk factors for a number of psychiatric disorders (Braunschweig & Van de Water, Reference Braunschweig and Van de Water2012; Hall, Willis, Rodriguez, & Schwarz, Reference Hall, Willis, Rodriguez and Schwarz2023), including AN (Favaro et al., Reference Favaro, Tenconi, Ceschin, Zanetti, Bosello and Santonastaso2011; Lydholm et al., Reference Lydholm, Köhler-Forsberg, Nordentoft, Yolken, Mortensen, Petersen and Benros2019; Solmi et al., Reference Solmi, De Stavola, Khandaker, Bulik, Dalman and Lewis2020). Furthermore, maternal antibiotic use during pregnancy could also impact neonatal gut microbiome (Dierikx et al., Reference Dierikx, Visser, Benninga, van Kaam, de Boer, de Vries and de Meij2020; Qu, Liu, & Miao, Reference Qu, Liu and Miao2021), which has also been implicated in the development and maintenance of AN (Bulik, Carroll, & Mehler, Reference Bulik, Carroll and Mehler2021a). For psychosocial risk factors, these interactions may be due to the presence of personality features associated with the development of AN and high educational/income attainment, such as perfectionism (Cassin & von Ranson, Reference Cassin and von Ranson2005; Madigan, Reference Madigan2019), autonomy (i.e. independence, control, and need for achievement; Cassin & von Ranson, Reference Cassin and von Ranson2005; de la Fuente, Malpica-Chavarria, Garzon-Umerenkova, & Pachon-Basallo, Reference de la Fuente, Malpica-Chavarria, Garzon-Umerenkova and Pachon-Basallo2021; Shi & Qu, Reference Shi and Qu2021), neuroticism/anxiety (Kienngam et al. Reference Kienngam, Maneeton, Maneeton, Pojanapotha, Kawilapat and Damrongpanit2022; Marzola, Porliod, Panero, De-Bacco, & Abbate-Daga, Reference Marzola, Porliod, Panero, De-Bacco and Abbate-Daga2020; Tucker-Drob, Briley, Engelhardt, Mann, & Harden, Reference Tucker-Drob, Briley, Engelhardt, Mann and Harden2016), and harm avoidance (Cassin & von Ranson, Reference Cassin and von Ranson2005). The limited number of interactions observed in the present study should be interpreted in light of the challenges of using PRS to examine interactions: namely, limited statistical power and the potential that phenotypic PRS may not necessarily capture genetic variations associated with differential exposure to risk factors (Zhang & Belsky, Reference Zhang and Belsky2022).
The sizes of the HRs for the risk factors and AN risk were moderate, with only female sex and family eating disorder history standing out with larger relative risks. The association between AN PRS is overall 1.48 per standard deviation, and compared to epidemiological risk factors this is not negligible, though much of the variation AN risk has yet to be explained thus far. From the interaction analyses, the variation of AN PRS association with AN risk across risk factor levels can be considered moderate, with the steady increase of association between AN PRS from low education/income to higher levels of education and income standing out among our other findings.
Strengths
The present study has several strengths. This study is the first to examine the interplay between polygenic liability of AN and a broad spectrum of birth-related, somatic, and psychosocial risk factors for AN. The sample was large and representative of the Danish population. The use of Danish registers for measurement of AN diagnosis and risk factors allows for comprehensive detection of these factors within the sample.
Limitations
Present findings must also be considered in light of study limitations. First, we were limited to risk factors available in the Danish registers; accordingly, future studies should examine other factors that have been shown to show prospective associations with AN risk in past research (such as sociocultural factors (Weissman, Reference Weissman2019), involvement in competitive sports (Krentz & Warschburger, Reference Krentz and Warschburger2013; Stoyel, Slee, Meyer, & Serpell, Reference Stoyel, Slee, Meyer and Serpell2020), and premorbid low body mass index (Yilmaz, Gottfredson, Zerwas, Bulik, & Micali, Reference Yilmaz, Gottfredson, Zerwas, Bulik and Micali2019).). Second, AN diagnoses were determined using ICD-10 F50.0 and F50.1 within individuals seeking eating disorder treatment; individuals with AN who did not seek treatment were not captured. The robustness of the PRS across sexes may potentially weaken our ability to reveal interactions since most of the cases and controls in the AN GWAS were female. As a result of this, we are unable to rule out the possibility that the finding may be different in men, which our present Danish sample does not provide the opportunity to explore more closely. Further, we were unable to examine differences in the interplay of risk factors between different subtypes of AN (restricting type and binge eating/purging type). Given the phenotypic differences observed across AN subtypes, it is plausible that specific risk factors could differentiate these subtypes. Although no differences were observed in the polygenic architecture of AN with binge eating v. AN without binge eating (Watson et al., Reference Watson, Yilmaz, Thornton, Hübel, Coleman, Gaspar and Slagboom2019b), future research should investigate differences in the interplay between AN polygenic liability with risk factors relative to the specific subtypes of AN. Despite use of a national registry to provide population estimates, another limitation of this study includes generalizability outside of Denmark, notably for more genetically diverse populations than Danish, which is predominantly European ancestry.
Finally, while we had > 7000 AN cases in our study population and used the largest AN GWAS published to date for calculating AN PRS, it is likely that statistical power may have been limited for detecting interactions of moderate effect sizes.
Conclusion
The present study elucidates significant interplay of AN polygenic liability genetic and epidemiological risk factors in AN risk. Findings provide evidence for interactions between AN PRS and certain risk factors, illustrating potential diverse AN risk pathways. Future work should replicate this study using populations with more admixed ancestry to determine differences across ancestry groups.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0033291724000175
Funding statement
The iPSYCH data were supported by grants from the Lundbeck Foundation (grant no. R102-A9118, R155-2014-1724, and R248-2017-2003) and the Universities and University Hospitals of Aarhus and Copenhagen. The Anorexia Nervosa Genetics Initiative (ANGI) was an initiative of the Klarman Family Foundation. Additional AN genotype data were supported by grants from NIMH (5R01MH120170-04) and the Lundbeck Foundation (grant no. R276-2018-4581). ZY acknowledges grant support from the National Institute of Mental Health (R01MH120170) and Independent Research Fund Denmark (DFF, Sapere Aude). EKP acknowledges grant funding from the National Institute of Mental Health (F31MH131262). CMB is supported by NIMH (R56MH129437; R01MH120170; R01MH124871; R01MH119084; R01MH118278; R01 MH124871); Swedish Research Council (Vetenskapsrådet, award: 538-2013-8864); Lundbeck Foundation (Grant no. R276-2018-4581). BJV acknowledges grants from the Independent Research Fund (2034-00241B), Lundbeck Foundation (R335-2019-2339), and Novo Nordisk Foundation (NNF21SA0072102).
Competing interests
BJV is a member of the scientific advisory board for Allelica. CM Bulik reports: Pearson (author, royalty recipient); Equip Health Inc. (Stakeholder Advisory Board). The remaining authors declare no conflicts of interest.







