Introduction
Interferon-α (IFN-α) is an endogenous cytokine that, when given as an exogenous therapy, potently activates the pro-inflammatory cytokine network to produce antiviral and antiproliferative effects (Capuron & Miller, Reference Capuron and Miller2004). Until recently, IFN-α was the most widely used treatment for hepatitis C virus (HCV) and was usually administered together with the oral polymerase inhibitor, ribavirin, for up to 48 weeks (Baraldi et al. Reference Baraldi, Hepgul, Mondelli and Pariante2012).
Despite its efficacy as an immunotherapy, IFN-α is strongly associated with the onset of neuropsychiatric symptoms, including anxiety, sleep alterations, fatigue and depressive symptomatology in 30–50% of patients (Musselman et al. Reference Musselman, Lawson, Gumnick, Manatunga, Penna and Goodkin2001; Hauser et al. Reference Hauser, Khosla, Aurora, Laurin, Kling and Hill2002; Schaefer et al. Reference Schaefer, Engelbrecht, Gut, Fiebich, Bauer and Schmidt2002). The clinical observation that IFN-α is capable of inducing symptoms that are strikingly similar to major depression is important evidence for the proposed role of endogenous pro-inflammatory cytokines in the development of depressive states more generally (Raison et al. Reference Raison, Capuron and Miller2006; Zunsain et al. Reference Zunsain, Hepgul and Pariante2013).
Several neurobiological mechanisms have been proposed as mediators of the ability of IFN-α to trigger depressive symptomatology. For example, it has been proposed that IFN-α-induced changes in tryptophan metabolism may lead to lowered brain serotonin levels and increased production of neurotoxic metabolites, such as quinolinic acid (Capuron et al. Reference Capuron, Ravaud, Neveu, Miller, Maes and Dantzer2002; Miller, Reference Miller2009). There is also preliminary evidence from magnetic resonance spectroscopy that IFN-α-induced alterations in glutamine and glutamate levels in the anterior cingulate cortex may correlate with depressive symptomatology (Haroon et al. Reference Haroon, Woolwine, Chen, Pace, Parekh and Spivey2014; Taylor et al. Reference Taylor, Godlewska, Near, Christmas, Potokar and Collier2014). However, there has been little work to date on the neuropsychological mechanisms that may be involved in the mood-lowering effects of IFN-α.
Cognitive biases in emotional processing are thought to play a central role in the onset and maintenance of depression. For example, depression is associated with increased recognition, memory and attention to negative v. positive stimuli, thereby effectively exposing the patient to greater effects of any stressor, life event or negative social interaction. There is also robust evidence demonstrating that pharmacological modulation can modify emotional processing biases, as measured in psychological tasks, for example, following serotonergic antidepressant treatments (Harmer & Cowen, Reference Harmer and Cowen2013). Importantly, during antidepressant treatment, changes in emotional processing occur prior to changes in subjective mood suggesting that they might be mediating mechanisms (Harmer et al. Reference Harmer, Goodwin and Cowen2009a , Reference Harmer, O'Sullivan, Favaron, Massey-Chase, Ayres and Reinecke b ). A recent study also found that emotional processing bias in depression predicted subsequent changes in mood (Lewis et al. Reference Lewis, Kounali, Button, Duffy, Wiles, Munafo, Harmer and Lewis2017). This study therefore aimed to investigate the effects of IFN-α administration on emotional processing, with a view to improving understanding of its action at a neuropsychological level and to assess whether deterioration in mood during IFN-α treatment is associated with changes in emotional processing. It was hypothesised that IFN-α treatment would induce negative biases in emotional processing reminiscent of a depressed state.
Method
Participants and study design
We recruited 21 participants who were scheduled to receive pegylated IFN-α plus ribavirin treatment as part of their routine clinical care for chronic HCV. Four participants did not return for the second study visit; therefore, data presented are from 17 participants (13 males, 4 females; mean age 39.7, s.d. 10.3, range 22–60 years). Participants were referred from a National Health Service hepatology clinic at the John Radcliffe Hospital (Oxford, UK). They were screened using the Structured Clinical Interview for DSM-IV (SCID; Spitzer et al. Reference Spitzer, Williams, Gibbon and First1995) to be free of current Axis I mood and anxiety disorder; however, past psychiatric history of these disorders were not exclusion criteria. Participants taking non-stable psychotropic medication were excluded from the study, as were participants with clinical or biochemical evidence of cirrhosis. Concomitant medication was maintained at a stable dose throughout the study with the following medications being taken by participants: metformin and insulin (n = 1), ventolin inhaler (n = 1), lansoprazole (n = 1), omeprazole (n = 1), three patients were on stable doses of methadone and two patients were taking stable doses of the antidepressant mirtazapine. The study received full ethical approval by the Oxfordshire Research Ethics Committee A, and all participants gave written informed consent prior to study procedures being performed.
Participants attended two study visits; one pre-treatment with IFN and one after 6–8 weeks of IFN-α treatment. Mood state was assessed at baseline (pre-treatment) and 6–8 weeks after IFN-α administration using the Hamilton Depression Rating Scale (HAM-D; Hamilton, Reference Hamilton1960), Beck Depression Inventory (BDI; Beck et al. Reference Beck, Ward, Mendelson, Mock and Erbaugh1961), state component of the State Trait Anxiety Inventory (Spielberger et al. Reference Spielberger, Gorsuch and Lushene1970) and the Chalder Fatigue Scale (CFS; Chalder et al. Reference Chalder, Berelowitz, Pawlikowska, Watts, Wessely and Wright1993). Additionally, participants completed the above questionnaires (except the HAM-D) weekly for 6 weeks after the start of treatment to assess changes over time.
Psychological tasks
Facial expression recognition
This task featured facial expressions of six basic emotions (happiness, surprise, sadness, fear, anger and disgust) taken from the Pictures of Affect Series (Ekman, Reference Ekman1976). Faces were morphed between each prototype and neutral to portray varying intensities of each emotion, in 10% steps from 0% (neutral) to 100% (full intensity of emotion). Four examples of each emotion at each intensity were displayed, in addition to 10 neutral stimuli, giving a total of 250 stimuli. Facial expressions were presented in random order for 500 ms, followed by a blank screen. Participants were asked to classify facial expressions as quickly and accurately as possible by pressing one of seven labelled keys on the keyboard. Accuracy (the number of correctly identified faces of a particular emotion divided by the total number of faces containing that emotion), reaction times for correct responses and misclassifications of emotional faces were calculated.
Emotional categorisation task
Sixty personality characteristic words selected to be disagreeable (e.g. domineering, hostile) or agreeable (e.g. optimistic, honest; taken from Anderson, Reference Anderson1968) were presented in a random order on screen for 500 ms. Words were matched in terms of length, ratings of frequency and meaningfulness, and equal numbers of positive and negative words were included. Participants were asked to imagine whether they would be pleased or upset if they were to overhear someone else describing them in this way, so that the judgement was self-referential in nature. Responses were made by pressing a labelled button according to whether they would ‘like’ or ‘dislike’ to be described as possessing that characteristic. Subjects were asked to respond as quickly and accurately as possible. Accuracy of categorisation and reaction times for correct identifications were calculated in this task.
Dot probe attentional task
Stimuli were pairs of facial expressions (Matsumoto & Ekman, Reference Matsumoto and Ekman1988), each comprising one emotional and one neutral expression of the same individual or two neutral expressions of the same individual (described previously by Murphy et al. Reference Murphy, Downham, Cowen and Harmer2008). This meant that there were three types of face pairs: neutral–neutral, fearful–neutral and happy–neutral. Fearful and happy faces were presented with equal frequency. A central fixation cross began each trial, followed by pairs of faces, one at the top and one at the bottom of the screen. In the unmasked condition, each face pair was presented for 100 ms, immediately followed by a probe, which appeared in the location of one of the preceding facial expressions. The probe was two dots in a vertical (:) or horizontal (..) orientation. Participants were asked to identify the orientation of the dots by pressing the correspondingly labelled key on a keyboard. The probe remained on the screen until the participant had responded, and participants were asked to respond as quickly and accurately as possible. The masked condition consisted of the same sequence of events, except that the face pair was displayed for 16 ms followed by a mask (scrambled face), which was displayed for 84 ms. Each of the three face pair conditions were presented 32 times in the masked condition and 32 times in the unmasked condition, thus there were 192 trials in total. There were eight blocks of both the masked and unmasked conditions, which were presented in an alternating order. Position of an emotional face, probe position and type were fully counterbalanced. The task design therefore involved congruent trials (where the probe appears in the position of the emotional face) and incongruent trials (where the probe appears in the position of the neutral face while an emotional face is present).
Attentional vigilance scores were calculated for each participant by subtracting the median reaction time in congruent trials from incongruent trials. Positive values reflect attention towards the emotional face (vigilance), whereas negative values reflect attention away from the emotional face (avoidance).
Statistical analysis
Statistical analyses were carried out using IBM SPSS Statistics (version 22). Questionnaire data [HAM-D, BDI, state anxiety and Chalder Fatigue Questionnaire (CFQ)] were compared before and after IFN-α treatment using paired sample t tests. To assess changes in subjective mood over the 6-week period after the start of treatment, repeated measures analyses of variance (ANOVAs) were performed with time as a within-subject factor. The facial expression recognition, emotional categorisation and dot probe tasks were all analysed using repeated measures ANOVA, with time (representing pre-treatment and treatment with IFN-α) and emotion as within-subject factors. In the dot probe task, masking was added as an additional within-subject factor. Post hoc analyses using paired sample t tests were performed to follow-up interactions observed. Where assumptions of equality of variances were broken, Greenhouse–Geisser procedure was used to correct the degrees of freedom. Extreme outlying data (data lying at more than three times the participants’ interquartile range above their third or below their first quartile) were removed from all psychological tasks. This resulted in minimal loss of data on any task (<2%). Data from each task were analysed separately, without correcting for multiple comparisons across tasks.
To assess the relationship between depressed mood and behavioural measures, Pearson's correlation coefficient was computed between depression severity scores (measured by the BDI) and neuropsychological task performance. Pearson's correlation coefficients were also examined between pre-treatment and post-treatment HAM-D and subjective mood scores, to assess whether pre-treatment mood was an indicator of depressed mood following IFN-α administration in this cohort.
Results
Clinical rating scales
IFN-α treatment significantly increased HAM-D scores [t(16) = −4.93, p < 0.001]. Furthermore, all subjective state measures of negative affect included in the study were significantly elevated after IFN-α treatment, as shown in Table 1. Mood, anxiety and fatigue scores recorded weekly for 6 weeks following the start of treatment increased in a linear manner with time; therefore, results presented here are from baseline and 6–8 weeks only.
Table 1. Mood state changes over time

HAM-D, Hamilton Depression Rating Scale; BDI, Beck Depression Inventory; CFQ, Chalder Fatigue Questionnaire.
Values are ratings at baseline (pre-treatment) and 6–8 weeks after IFN-α treatment (n = 17). Means (standard deviations).
Significant correlations between pre- and post-IFN-α depressed mood scores were found, suggesting that mood state at baseline predicted later depressed mood scores following IFN-α administration (HAM-D r = 0.56, p = 0.02; BDI r = 0.73, p = 0.001). State anxiety at baseline also correlated with elevated anxiety ratings following IFN-α therapy (r = 0.76, p < 0.001), although this was not significantly correlated with later depressed mood score (p > 0.1).
Facial expression recognition
There were significant main effects of emotion [F(5,70) = 14.03, p < 0.001] and treatment [F(1,14) = 10.95, p = 0.005] on accuracy in this task, as well as a significant interaction between emotion and treatment [F(3.07,43.0) = 3.41, p = 0.025]. Post hoc analysis revealed that this was driven by significantly increased accurate recognition of disgust after IFN-α treatment [t(15) = −4.71, p < 0.001; see Fig. 1]. No significant effects were observed for other valences (anger p = 0.82, fear p = 0.25, happy p = 0.71, sad p = 0.38, surprise p = 0.77); hence, this appears to be a specific effect on disgust recognition.
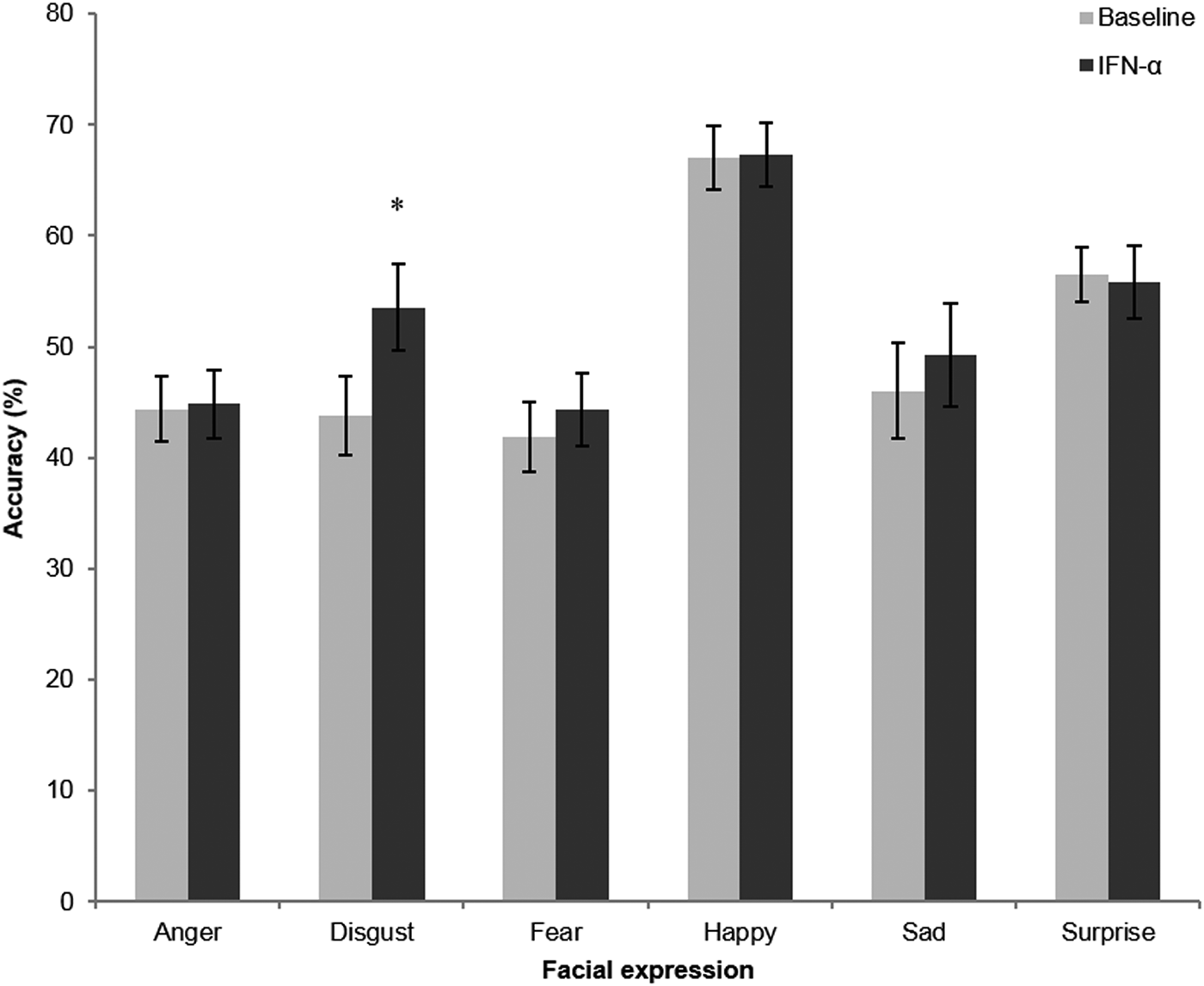
Fig. 1. Performance in the facial expression recognition task before (light bars) and following 6–8 weeks IFN-α treatment. Values represent the mean percentage correct for each of the six basic emotions summed over the different intensity levels used in this task, with error bars representing standard error of the mean. *p < 0.001.
For emotion discrimination (d′), which controls for differences in response criteria, the results were similar to those seen for accuracy above; there was a significant main effect of emotion [F(5,70) = 15.06, p < 0.001] and of treatment [F(1,14) = 12.16, p = 0.004]. In addition, there was a significant emotion x treatment interaction [F(3.06,42.81) = 3.05, p = 0.038]. Again, there was a marked increase for disgust sensitivity after IFN-α treatment compared with baseline; post hoc analysis revealed that this difference was highly significant [paired t test: t(15) = −5.20, p < 0.001]. There was a trend for increased sensitivity to fearful faces (p = 0.062), but no significant effects on other emotions (p > 0.2).
There were no significant correlations between depression rating and accuracy or reaction times in the facial expression recognition task either pre-treatment or following IFN-α administration (all p > 0.1).
Emotional categorisation task
There was a significant main effect of emotion [F(1,14) = 8.74, p = 0.01] and of treatment [F(1,14) = 9.6, p = 0.02] on accuracy in categorising self-referent personality characteristics, largely driven by increased accuracy in categorising negative characteristics [t(14) = −2.70, p = 0.017, Fig. 2]. However, there was no interaction between emotion and treatment [F(1,14) = 4.27, p = 0.24], so this should be interpreted with caution.
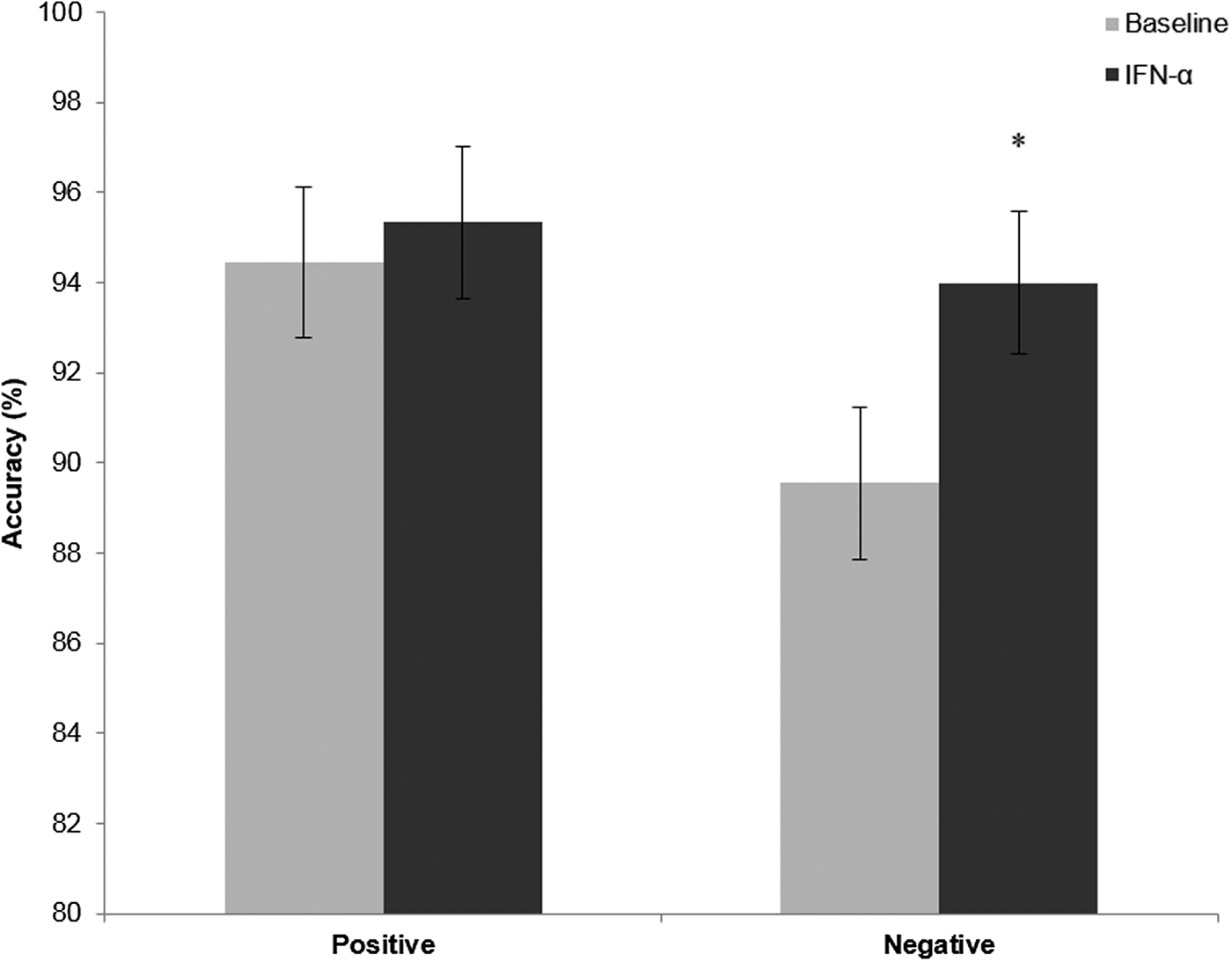
Fig. 2. Accuracy in the emotional categorisation task before (light bars) and after IFN-α administration (dark bars). Values represent the mean percentage correct categorisation of positive and negative personality characteristic words used in this task, with error bars representing standard error of the mean. *p = 0.017.
There were no treatment effects on reaction times to categorise self-referent personality characteristics in terms of a main effect of treatment [F(1,14) = 0.84, p = 0.38] or emotion x treatment interaction [F(1,14) = 2.04, p = 0.18], though there was a main effect of emotion [F(1,14) = 10.17, p = 0.007]. There were no correlations between depression score and accuracy or reaction times in the emotional categorisation task (all p > 0.1).
Dot probe attentional vigilance
There was a significant interaction between emotion, mask and treatment [F(1,16) = 8.32, p = 0.01], where mask was included as an additional within-subject factor (see Fig. 3). When masked and unmasked trials were considered separately, there was a significant emotion x treatment interaction in the unmasked [F(1,16) = 5.46, p = 0.03], but not masked [F(1,16) = 1.08, p = 0.31], condition. Post hoc analysis revealed that this was driven by a significant reduction in vigilance scores for happy faces in the unmasked condition following IFN-α treatment [t(16) = 2.10, p = 0.05]. There were no general effects of IFN-α on reaction times in this task (all p > 0.1).

Fig. 3. Effects of IFN-α on attentional vigilance for happy and fearful facial expressions in the masked condition (a) and unmasked condition (b) of the attentional dot probe task. Values are attentional scores before (baseline) and after IFN-α treatment. Attentional vigilance scores were calculated by subtracting the median reaction time from congruent trials (when the probe appeared in the same position as the emotional face) from incongruent trials (when the probe appeared in the opposite position to the emotional face, i.e. in the position of the neutral face). A positive score indicates vigilance towards the emotional face, whereas a negative score reflects avoidance of the emotional face. Error bars show the standard error of the mean. *p = 0.05.
Correlations between psychological ratings and emotional processing tasks
Depressed mood scores measured by the BDI and HAM-D either at baseline or at 6 weeks were not significantly correlated with emotional processing task performance in the current study, for example, depressed mood was not associated with disgust recognition. There was also no significant correlation between change in depressed mood and change in disgust recognition (p > 0.4), emotional recognition (p>0.1) or dot probe performance (p⩾0.1). Finally, performance on the emotional processing tasks at baseline did not predict later depression scores at 6 weeks (p⩾0.1).
Discussion
Subjective ratings
IFN-α significantly increased depression severity ratings, as well as subjective ratings of state anxiety and fatigue by 6–8 weeks, which is in keeping with numerous previous reports of induction of depressive symptoms by this treatment (Musselman et al. Reference Musselman, Lawson, Gumnick, Manatunga, Penna and Goodkin2001; Hauser et al. Reference Hauser, Khosla, Aurora, Laurin, Kling and Hill2002; Schaefer et al. Reference Schaefer, Engelbrecht, Gut, Fiebich, Bauer and Schmidt2002; Capuron & Miller, Reference Capuron and Miller2004; Maddock et al. Reference Maddock, Landau, Barry, Maulayah, Hotopf and Cleare2005; Fontana et al. Reference Fontana, Kronfol, Lindsay, Bieliauskas, Padmanabhan and Back-Madruga2008). Pre-treatment depressed mood scores were positively correlated with depressed mood following IFN-α, which also supports previous observations suggesting that severity of depressive symptoms during IFN-α treatment is predicted by baseline depression scores (Capuron & Ravaud, Reference Capuron and Ravaud1999; Musselman et al. Reference Musselman, Lawson, Gumnick, Manatunga, Penna and Goodkin2001). However, neither depressed mood scores, nor change in scores over treatment measured by the BDI and HAM-D were significantly correlated with emotional processing task performance in the current study. This suggests that changes in emotional processing described here are not simply a consequence of change in mood.
Previous studies suggest that there is a time lag between cognitive bias and changes in symptoms of depression (Lewis et al. Reference Lewis, Kounali, Button, Duffy, Wiles, Munafo, Harmer and Lewis2017), and therefore measuring mood and bias at the same time points may have obscured a mediating relationship, that is, that cognitive biases may affect mood over time via interaction with environmental inputs, such as life events, stressors and social interactions. As such we cannot rule out that early change in cognitive biases (e.g. after 1 week of treatment) may be associated with week 6 depression symptoms. It is also possible that the lack of association between change in cognitive bias and depression ratings at 6–8 weeks reflects a lack of power in our study.
Facial expression recognition
The present preliminary study showed highly specific effects of IFN-α treatment on sensitivity to disgusted faces, including enhanced ability to discriminate disgusted faces, paired with reduced threshold of disgust detection. There were no effects of treatment on processing of other key emotions, and elevated disgust accuracy was not accompanied by enhancement of mislabelling other facial expressions as disgust.
Several previous studies have reported that increased disgust recognition might indicate a negative perceptual bias that is relevant to mood disorders, for example, elevated discrimination of disgusted facial expressions has been observed in euthymic medicated patients with bipolar disorder (Harmer et al. Reference Harmer, Grayson and Goodwin2002). Hayward et al. (Reference Hayward, Goodwin, Cowen and Harmer2005) also observed enhanced recognition of disgusted faces in a recovered depressed sample following tryptophan depletion. Similarly, tryptophan depletion in remitted depressed individuals speeded the recognition of disgust faces (Merens et al. Reference Merens, Booij, Haffmans and van der Does2008). In contrast, tryptophan supplementation, as opposed to depletion, reduced recognition of disgusted faces in healthy female volunteers (Murphy et al. Reference Murphy, Longhitano, Ayres, Cowen and Harmer2006).
Despite considerable evidence of neural substrates involved in disgust processing and growing appreciation of disgust as an important feature in depression (Surguladze et al. Reference Surguladze, El-Hage, Dalgleish, Radua, Gohier and Phillips2010), interpretation of the role of disgust processing in depressive disorders lacks clarity. Emotional processing biases in disgust processing have been suggested to serve an evolutionary purpose of behavioural modification to avoid threats of infectious disease (Curtis et al. Reference Curtis, Aunger and Rabie2004), which could be related to the evidence that suggests that environmental and social disgusted stimuli are attended to and appraised for more time than fearful faces (Zhang et al. Reference Zhang, Liu, Wang, Chen and Luo2014). Enhanced disgust recognition may also be associated with visceral symptoms, such as nausea (Anderson et al. Reference Anderson, Del-Ben, McKie, Richardson, Williams, Elliott and Deakin2007). For example, increased recognition of disgusted faces was seen following duloxetine treatment, which induced nausea (Harmer et a l. Reference Harmer, Heinzen, O'Sullivan, Ayres and Cowen2008). There are suggestions, however, that disgust processing can extend into the social domain, and that heightened disgust sensitivity might reflect an emotional processing bias that constitutes social and self-related disgust, feelings of social rejection (Rozin et al. Reference Rozin, Lowery and Ebert1994), as well as feelings of shame and guilt (Surguladze et al. Reference Surguladze, El-Hage, Dalgleish, Radua, Gohier and Phillips2010; Giner-Sorolla & Espinosa, Reference Giner-Sorolla and Espinosa2011), all of which are highly relevant to depression. Emotional responses to inflammation are also relevant to evolutionary biological theories linking inflammation and depression in which depressive behaviours, regulated by the immune system, play a role in dealing with infection both at the individual level and that of the social group (Anders et al. Reference Anders, Tanaka and Kinney2013).
It should be noted that although discussion here has focussed on enhanced disgust sensitivity, some studies report no difference in disgust processing in depression (Bediou et al. Reference Bediou, Krolak-Salmon, Saoud, Henaff, Burt and Dalery2005), while others have reported impaired disgust recognition (Douglas & Porter, Reference Douglas and Porter2010). When consideration is given to the heterogeneity of symptomatology in depression, in addition to differing compensatory adaptations, perhaps this variability in disgust processing is not surprising.
Such divergent evidence makes it difficult to reliably conclude the meaning of enhanced disgust recognition following immune system activation in the current study. It seems conceivable, however, that effects on disgust recognition observed could represent a complex interplay between inflammatory pathways and neurocircuits that result in modified information processing of aversive disgusted facial emotions, which may in part be modulated by serotonin function (Anderson et al. Reference Anderson, Del-Ben, McKie, Richardson, Williams, Elliott and Deakin2007). Indeed, recent fMRI neuroimaging studies support a role for the insula in the effects of inflammatory challenge (Harrison et al. Reference Harrison, Voon, Cercignani, Cooper, Pessiglipone and Critchley2016), and the insula is a key node in processing cues of disgust (Surguladze et al. Reference Surguladze, El-Hage, Dalgleish, Radua, Gohier and Phillips2010). A previous study also reported increased activity within subgenual anterior cingulate cortex and reduced connectivity to amygdala, medial prefrontal cortex and the nucleus accumbens during emotional face processing following an inflammatory challenge (Harrison et al. Reference Harrison, Voon, Cercignani, Cooper, Pessiglipone and Critchley2016). Thus, the current pattern may represent a cognitive correlate of the underlying neurocircuits emerging as important for the effects of inflammation in depression (Byrne et al. Reference Byrne, Whittle and Allen2016).
Emotional categorisation
The increased accurate categorisation of negative self-referent personality words observed following IFN-α therapy is suggestive of a negative bias in emotional processing, though it should be interpreted with caution given the lack of a statistically significant interaction with valence in the full ANOVA. Negative biases in emotional categorisation have previously been reported in depressed patients, reversal of which has been associated with later antidepressant response. For example, slower reaction times to positive trait words were found in depressed patients compared with healthy controls; however, after a single dose of reboxetine, reaction times to positive adjectives were faster (Harmer et al. Reference Harmer, O'Sullivan, Favaron, Massey-Chase, Ayres and Reinecke2009b ). Further studies have reported similar antidepressant effects on emotional categorisation with reboxetine (Harmer et al. Reference Harmer, Shelley, Cowen and Goodwin2004), citalopram (Harmer et al. Reference Harmer, Shelley, Cowen and Goodwin2004) and mirtazapine (Arnone et al. Reference Arnone, Horder, Cowen and Harmer2009).
Attentional dot probe
The current findings in the attentional dot probe task suggest that administration of IFN-α produces negative biases in attentional vigilance, particularly by increasing attentional avoidance of happy facial expressions in the unmasked condition. Negative biases in emotional processing include both vigilance towards negative stimuli and away from positive stimuli (Disner et al. Reference Disner, Beevers, Haigh and Beck2011). Depressed patients have previously been shown to display an attentional bias away from positive (happy) faces and towards negative (sad) faces (Gotlib et al. Reference Gotlib, Krasnoperova, Yue and Joormann2004; Fritzsche et al. Reference Fritzsche, Dahme, Gotlib, Joormann and Magnussen2010) and negative words (Bradley et al. Reference Bradley, Mogg, Millar, Bonham-Carter, Fergusson and Jenkins1997).
It has been previously proposed that in the neutral–happy face pair presented in this task, the neutral face signifies the more threatening facial stimuli when compared with the happy face. Therefore, avoidance of the happy face in the current study indicates attention towards the neutral face, which may be relevant to threat-related processing biases (Cooper & Langton, Reference Cooper and Langton2006). This would also be in keeping with the (non-significant) observation of enhanced vigilance to threatening fearful faces with IFN-α treatment.
Tianeptine, an antidepressant drug with suggested properties of reducing serotonin function early in treatment was also shown to significantly enhance avoidance to happy faces (Cooper et al. Reference Cooper, Whiting, Cowen and Harmer2015) in a very similar manner to effects seen here with IFN-α. Tryptophan supplementation, in contrast, has been shown to reduce vigilance to negative words in healthy female volunteers (Murphy et al. Reference Murphy, Longhitano, Ayres, Cowen and Harmer2006). It is too soon to conclude the way in which IFN-α may be exerting this effect, though it is intriguing to note that once again, as in the increased disgust recognition after IFN-α, the literature would be consistent with a reduction in serotonin availability as a possible mechanism.
Limitations
There are some important limitations in the present study. Firstly, a sample size of 17 participants is relatively small, particularly given known variance in mood and behavioural response to IFN-α treatment as not all patients become depressed. The sample size is also limiting since it does not allow reliable stratification of those patients who did exhibit depressive symptoms, compared with those who did not. This would be helpful to investigate whether baseline neuropsychological task performance is able to predict mood response to treatment, and thereby identify those at elevated risk of developing IFN-α-associated depression. Further limitations of this study include the lack of control subjects with HCV receiving placebo and not IFN-α treatment, which would have helped to examine cause and effect in relation to depression symptoms, as well as learning effects on task performance. However, previous work examining the test–retest effects in this same battery have shown limited learning effects of repeat testing in the absence of any intervention (Thomas et al. Reference Thomas, Higgs and Dourish2016). Future work should nonetheless examine the specificity of the negative bias induction seen here relative to placebo treatment in this patient group.
The lack of accurate accounts of somatic symptoms on testing days could have also been useful with regards to accounting for visceral symptoms, such as nausea (Anderson et al. Reference Anderson, Del-Ben, McKie, Richardson, Williams, Elliott and Deakin2007), which can unfortunately be a side effect of IFN-α treatment and, as discussed above, may be involved in altered disgust recognition. It is also possible that the changes in emotional processing we have identified are secondary to the mood changed produced by IFN-α. Against this, we could not find any correlation between depression scores and emotional processing.
The present study does not have any biological markers, including inflammatory cytokines or tryptophan and its metabolites, which could have provided insight into the biological mechanisms associated with behavioural effects observed. Furthermore, mediation analysis factoring in inflammatory cytokines measures could have facilitated assessment of the mediator of psychological effects observed and potentially informed the lack of correlation between mood scores and behavioural findings.
Conclusions
The present study provides intriguing evidence that IFN-α, either directly or perhaps indirectly via pro-inflammatory cytokine pathways, is capable of producing negative emotional processing biases that are widely thought to be important in the onset and maintenance of depression. As such, these results highlight that exposure to inflammation by immune system activation may alter affective information processing, and that this could play a role in the development of depression over time in subgroups of patients. While there are many similarities between the present findings and the depression literature, there are some interesting differences, particularly the highly specific enhancement of disgust recognition. Although this has previously been seen in mood disorders, it could represent an effect that is more specific to inflammatory pathways.
Acknowledgements
The authors thank Victoria Black, Denise O'Donnell, Jane Phillips, Elizabeth Sims and Elizabeth Stafford for nursing care, and the patients for their participation in this study. This study was supported by the Medical Research Council (MR/K022202/1). Additional support was received from the Oxford NIHR Biomedical Research Centre.
Declaration of Interest
In the last three years, Dr Cowen has acted as a consultant for Lundbeck. Dr Harmer has acted as a consultant for P1vital and has taken part in educational activities for Pfizer and Lundbeck. None of the other authors report any conflict of interest.






